Abstract
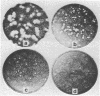
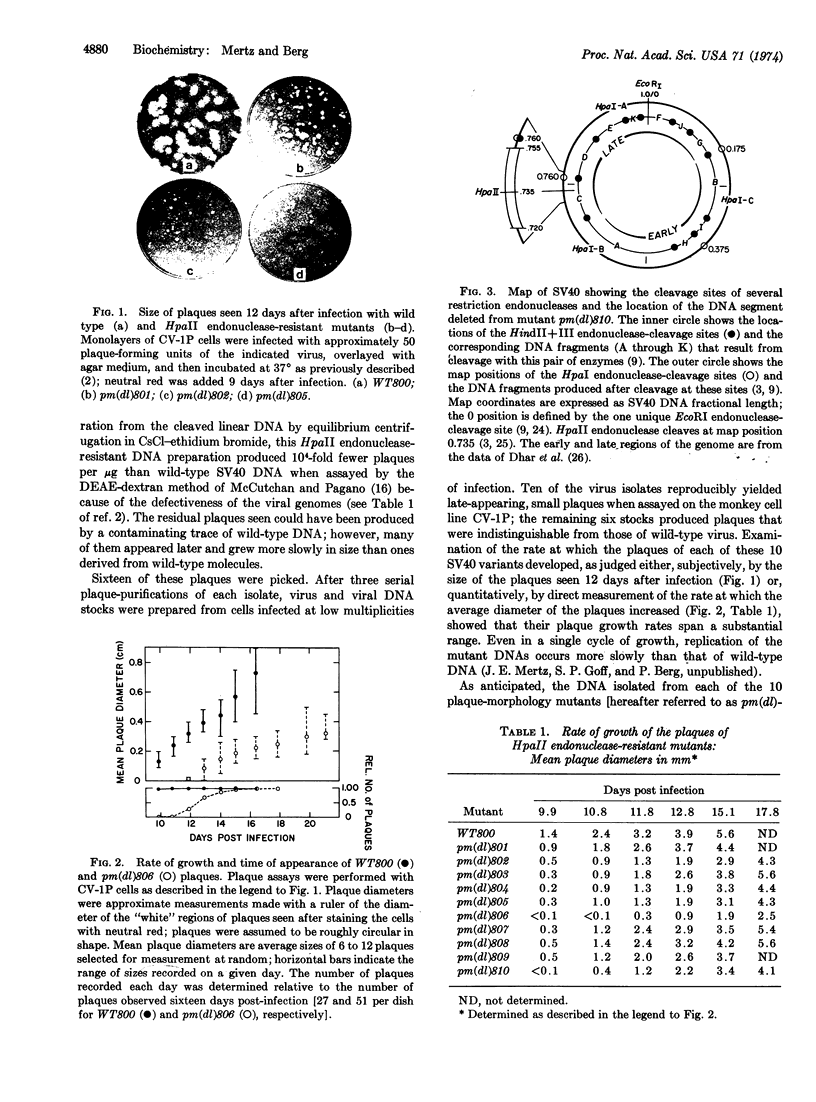
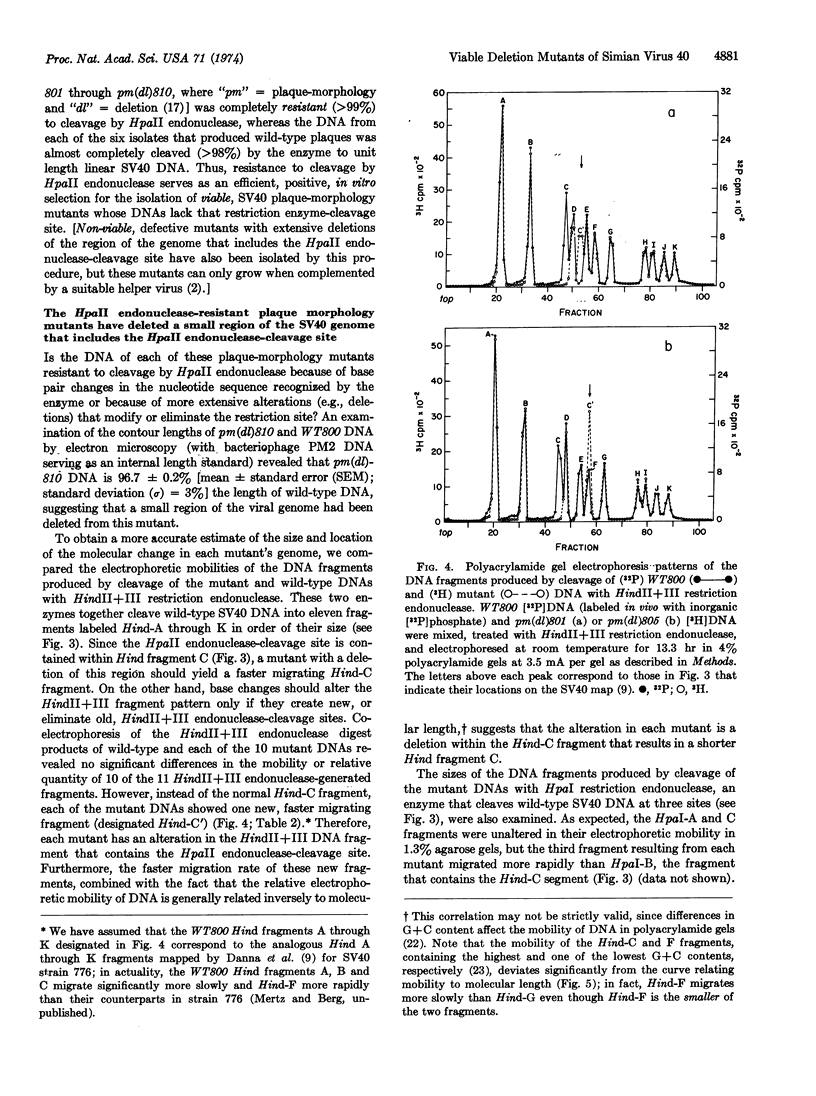
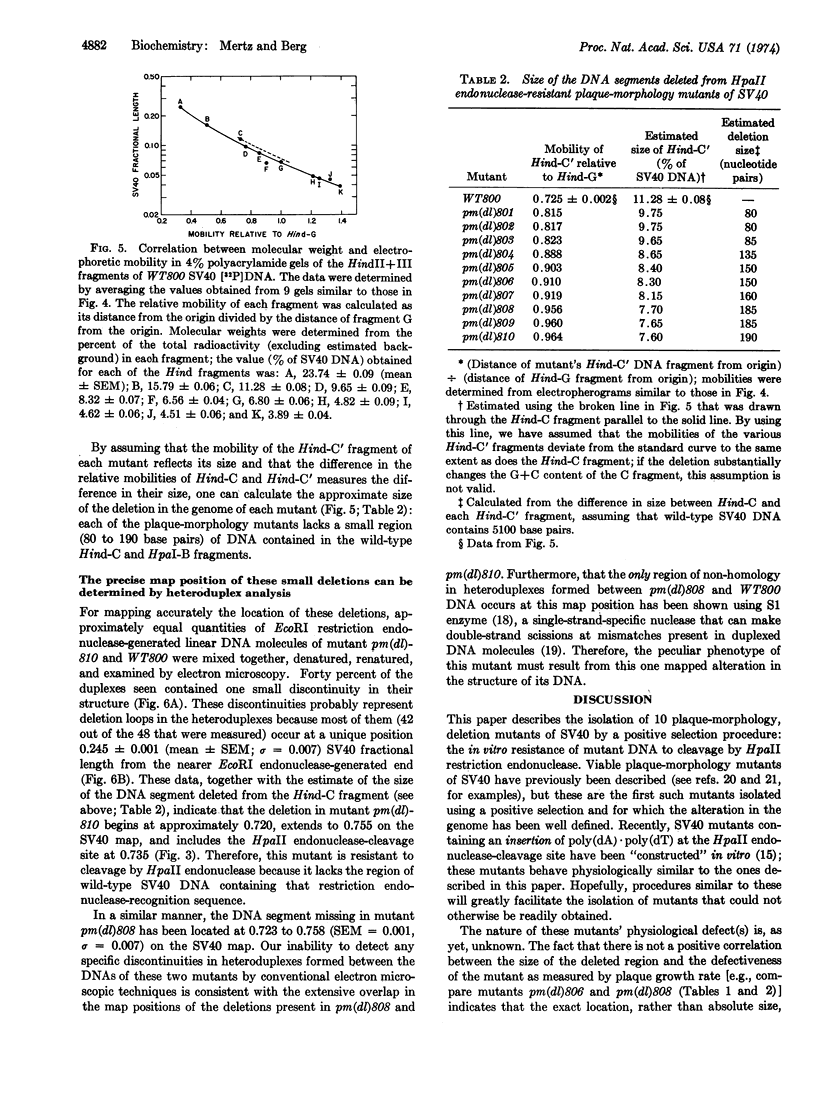
Resistance of simian virus 40 (SV40) DNA to cleavage by Hemophilus parainfluenzae II (HpaII) restriction endonuclease has been used as a positive, in vitro selection for mutants lacking the one HpaII endonuclease-cleavage site of wild-type SV40 DNA. Each of 10 viable mutants isolated by this procedure multiplies significantly more slowly than wild-type virus and contains a small deletion (80 to 190 base pairs in size) of the region of the genome that includes the HpaII endonuclease-recognition sequence. These well-defined mutants, having a selective disadvantage for growth, would not have been readily obtained by conventional methods used to screen for viral mutants. Therefore, in certain circumstances, restriction endonucleases are effective reagents for the selection of new classes of mutants. Because these small deletions can be visualized in heteroduplexes, these mutants provide internal markers for mapping other alterations or features of the simian virus 40 genome.
Keywords: Hpa II, plaque-morphology mutants, polyacrylamide gel electrophoresis, heteroduplex mapping
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Dubbs D. R., Kit S. Isolation of defective lysogens from Simian virus 40-transformed mouse kidney cultures. J Virol. 1968 Nov;2(11):1272–1282. doi: 10.1128/jvi.2.11.1272-1282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R., Heywood J. Restriction and modification of DNA. Annu Rev Biochem. 1972;41:447–466. doi: 10.1146/annurev.bi.41.070172.002311. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Berg P., Kelly T. J., Jr, Lewis A. M., Jr Mapping of simian virus 40 early functions on the viral chromosome. J Virol. 1973 Sep;12(3):653–658. doi: 10.1128/jvi.12.3.653-658.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Location of the T4 gene 32 protein binding site on simian virus 40 DNA. J Virol. 1973 Dec;12(6):1631–1632. doi: 10.1128/jvi.12.6.1631-1632.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Mulder C. Relative locations of rearrangements in the DNA of defective simian virus 40 (SV40). Virology. 1974 Apr;58(2):424–438. doi: 10.1016/0042-6822(74)90077-4. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Tegtmeyer P., Martin R. G., Kit S. Proposal for a uniform nomenclature for simian virus 40 mutants. J Virol. 1972 Mar;9(3):562–563. doi: 10.1128/jvi.9.3.562-563.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Nathans D. Letter: A suggested nomenclature for bacterial host modification and restriction systems and their enzymes. J Mol Biol. 1973 Dec 15;81(3):419–423. doi: 10.1016/0022-2836(73)90152-6. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Griffith J., Geider K., Schaller H., Kornberg A. Initiation of deoxyribonucleic acid synthesis. VII. A unique location of the gap in the M13 replicative duplex synthesized in vitro. J Biol Chem. 1974 May 25;249(10):3049–3054. [PubMed] [Google Scholar]
- Tai H. T., Smith C. A., Sharp P. A., Vinograd J. Sequence heterogeneity in closed simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):317–325. doi: 10.1128/jvi.9.2.317-325.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Kirschstein R. L., Habel K. Mutants of simian virus 40 differing in plaque size, oncogenicity, and heat sensitivity. J Bacteriol. 1966 Oct;92(4):990–994. doi: 10.1128/jb.92.4.990-994.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiike K. Studies on DNA from low-density particles of SV40. I. Heterogeneous defective virions produced by successive undiluted passages. Virology. 1968 Mar;34(3):391–401. doi: 10.1016/0042-6822(68)90059-7. [DOI] [PubMed] [Google Scholar]
- Zeiger R. S., Salomon R., Dingman C. W., Peacock A. C. Role of base composition in the electrophoresis of microbial and crab DNA in polyacrylamide gels. Nat New Biol. 1972 Jul 19;238(81):65–69. doi: 10.1038/newbio238065a0. [DOI] [PubMed] [Google Scholar]