Abstract
Historically, the role of the carotid bodies in ventilatory control has been understated, but the current view suggests that the carotid bodies (1) provide a tonic, facilitory input to the respiratory network, (2) serve as the major site of peripheral O2 chemoreception and minor contributor to CO2/H+ chemoreception, and (3) are required for ventilatory adaptation to high altitude. Each of these roles has been demonstrated in studies of ventilation in mammals after carotid body denervation. Following carotid body denervation, many of the compromised ventilatory “functions” show a time-dependent recovery plasticity that varies in the degree of recovery and time required for recovery. Respiratory plasticity following carotid body denervation is also dependent on species, with contributions from peripheral and central sites/mechanisms driving the respiratory plasticity. The purpose of this review is to provide a summary of the data pointing to peripheral and central mechanisms of plasticity following carotid body denervation. We speculate that after carotid body denervation there are altered excitatory and/or inhibitory neuromodulator mechanisms that contribute to the initial respiratory depression and the subsequent respiratory plasticity, and further suggest that the continued exploration of central effects of carotid body denervation might provide useful information regarding the capacity of the respiratory network for plasticity following neurologic injury in humans.
Keywords: control of breathing, chemoreception, carotid body, denervation, ventilation, plasticity
Abbreviations
CBD, carotid body denervation; NTS, nucleus of the solitary tract; PO2, partial pressure of O2; TH, tyrosine hydroxylase
INTRODUCTION
There are many forms and definitions of plasticity, particularly as it relates to the nervous system. As physiologists, we often define plasticity as a time-dependent recovery or return of function following experimental disruption of a component of a homeostatic control system. Our goal in this review is to discuss a form of plasticity in the context of the time-dependent changes in the fundamental mechanisms that govern the neural control of breathing following denervation of the carotid bodies (CBD), the major site of peripheral O2 chemoreception for breathing control. While the acute effects of CBD on eupneic (resting) ventilation, chemoreflexes, and ventilatory responses to exercise are fairly uniform across species, the plasticity or recovery of function following CBD is highly variable, including the degree of recovery of function and the time required for the full expression of the plasticity to occur. We will discuss these types of recovery of function in the context of potential sites of respiratory plasticity following CBD, including upregulation of other peripheral sites of chemosensitivity and the potential for altered central mechanisms that govern blood gas homeostasis and chemoreflexes.
THE NEURAL CONTROL OF BREATHING AND THE VARIOUS “DRIVES” TO BREATHE
Respiratory rhythm and pattern-generating neurons in the brainstem comprise a flexible oscillatory neural network that establishes the frequency of breathing and shapes coordinated activity of the respiratory pump and airway muscles. The output of this network is highly dependent upon tonic, excitatory “drives” that determine network excitability and ventilatory output. Based on the tight homeostatic control of resting arterial CO2 (eupneic PaCO2) and the effect of hypercapnia on ventilation, it was concluded by Haldane and Priestly that “CO2 is the factor that drives breathing”[1]. This suggested little or no role for the carotid bodies as a potential eupneic drive to breathe. As stated by Comroe and Schmidt in 1938, the “carotid body reflexes constitute an accessory mechanism, brought into action by emergencies such as foreign chemicals, anoxemia, and unusually large increases in CO2 tension in the blood”[2]. The findings of Fencl and others in awake goats that alveolar ventilation was linearly related to cerebrospinal fluid pH across several chronic acid base conditions certainly reinforced a minimal role for the carotid bodies[3].
This view has changed substantially, as it has now become apparent that the carotid chemoreceptors provide an important tonic excitatory “drive” to breathe[4,5], a contribution perhaps best shown by studies of CBD[6,7]. We also now appreciate the contributions of multiple neuromodulatory inputs into the respiratory network as additional sources of tonic, excitatory inputs to breathe, including serotonin (5-HT), norepinephrine, substance P, acetylcholine and others (Figure 1), which may be altered with changes in the awake or sleep state. Moreover, many of these neuromodulators of breathing might also provide substrates for recovery of function following CBD, as seen in other forms of respiratory plasticity such as long term facilitation following intermittent hypoxia[9,10]. Overall, there are several important contributory “drives” to breathe, including peripheral and central chemoreceptor inputs and neuromodulators.
Figure 1.
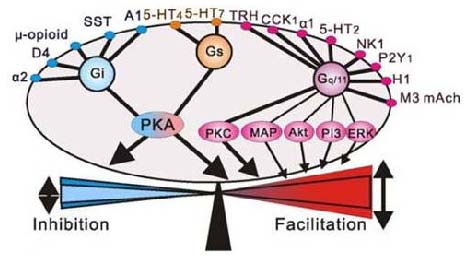
Multiple excitatory and inhibitory neuromodulators provide an additional respiratory “drive” through multiple g protein-coupled receptors. Adapted from[8].
PERIPHERAL CHEMORECEPTION: THE CAROTID BODIES
The goal of the respiratory control system is to provide the appropriate neuromuscular output for adequate alveolar gas exchange and maintenance of arterial blood gases. To achieve this objective, a network of brainstem nuclei generates respiratory rhythm and coordinated respiratory muscle activity patterns, with major chemical feedback from the peripheral O2 (carotid bodies) and central CO2/H+ chemoreceptors.
The carotid body chemoreceptors are located bilaterally at the bifurcation of the common carotid arteries. Afferent innervation of the carotid bodies is supplied via the carotid sinus nerve (a branch of the cranial nerve IX), which project to the nucleus of the solitary tract (NTS) via the petrosal ganglia. The carotid bodies are thought as the major (if not sole) site for respiratory O2 chemoreception, due to the presence of clusters of neuron-like type I cells termed glomus cells, which are thought to serve as the primary “sensors”. Indeed, it was shown by Lahiri and Delaney that the carotid bodies can directly “sense” O2, and to a lesser extent CO2[11]. Recording single nerve fibers from the carotid sinus, they were able to demonstrate that the discharge rate of the carotid bodies varied significantly by alterations in the partial pressure of O2 (PO2) at a constant PCO2, and to a lesser extent by alterations in PCO2 at constant levels of PO2[11]. It has been shown that the glomus cells also contain several neurotransmitters and receptors[12], transducing sensory information via calcium-dependent neurotransmission[13]. The carotid body glomus cells also express a complement of membrane channels particularly suited for their role as chemoreceptors. Taken together, the hypothesis remains that the carotid bodies contribute greatly to O2 chemoreception and to a lesser extent to CO2 chemoreception in part or totally through its intrinsic chemosensitivity.
In spite of their low intrinsic sensitivity to PaCO2, the carotid chemoreceptors are a major determinant of the hypercapnic ventilatory response through a positive interaction with central CO2/H+ chemoreceptors. This interaction was elegantly demonstrated in the studies of Blain and colleagues in an awake dog preparation with isolated carotid body perfusion. In these studies, when the carotid body activity was increased by perfusion with hypoxic blood central CO2 sensitivity was increased, while attenuating carotid body activity with hyperoxic and hypocapnic blood depressed central CO2 sensitivity[14]. These and other experiments have led to the novel concept of peripheral and central chemoreceptors interdependence[4], the nature of which remains to be determined.
In addition to their role as the acute “sensors” of hypoxia, the carotid chemoreceptors mediate another hypoxia-dependent physiologic phenomenon called high altitude ventilatory acclimatization. Eupneic ventilatory adaptation is multi-phasic for a native lowlander upon sojourn to high altitude, characterized by an initial stimulation of ventilation due to the hypoxemia, a ventilatory roll-off or depression due to a relative hyperventilation, and then an increase to an intermediate level of ventilation. It is the final phase of acclimatization that fails to occur in animals following CBD, implicating a key role for the carotid bodies in mediating the physiologic ventilatory adaptation to high altitude[15,16].
Finally, another important contribution of the carotid bodies is the breath to breath, or minute-to-minute stabilization of breathing. This is achieved by providing the ventilatory “error signal”, presumably through rapid transduction of chemoreceptor inputs through afferents. This mechanism is also thought to prevent deviations between the levels of ventilation and that required by metabolic rate[17].
CAROTID BODY DENERVATION (CBD): ACUTE EFFECTS
Denervation of the carotid bodies in most cases has dramatic effects on respiratory control. There are two distinct phases during which these physiologic disturbances occur: (1) an acute phase (days to weeks), and (2) a recovery phase (weeks to years). During the acute phase, CBD causes most adult mammals to hypoventilate (ventilation reduced relative to metabolic rate). Remarkably, the degree of hypoventilation (increased PaCO2) documented in the period immediately after CBD is fairly consistent across mammalian species, including ponies (12–15 mm Hg), goats (11 mm Hg), humans (~9 mm Hg) rats (8–9 mm Hg), and piglets (8-9 mm Hg)[6,7,18,19,20]. In addition, the hypoxic ventilatory response is severely attenuated, as indicated by the attenuated ventilatory responses to venous infusions of NaCN, which mimics hypoxia at sites of O2 chemoreception[6,19,20]. In addition, mammals do not acclimatize to high altitude following CBD. The hypercapnic ventilatory response, or CO2 sensitivity is also reduced following CBD[6,7,17], which is a striking effect in light of the supposedly minor role for the carotid bodies in the hypercapnic ventilatory response as suggested by several studies[21,22,23,24]. The denervation- induced hypoventilation has been also shown to persist during moderate exercise challenges. In summary, several mammalian species hypoventilate at rest and during moderate exercise, display reduced ventilatory responses to hypoxia and hypercapnia, and fail to acclimatize to high altitude sojourn after CBD[25].
IS THERE RESPIRATORY PLASTICITY FOLLOWING CBD?
In most species studied to date, the acute attenuation of ventilation and chemosensitivity after CBD diminish over time. However, degree of plasticity or recovery of function and the time required for this plasticity are highly variable (reviewed in[25]). For example, adult cats[26] and goats[6] have been shown to exhibit substantial recovery from CBD within 2-3 weeks, while other species like dogs[23], ponies[7,27], and humans[18] require months to years to demonstrate significant recovery in resting PaCO2. Moreover, there is variation in the degree of recovery observed in chemoreflexes and/or the ventilatory response to exercise. Some affected ventilatory control mechanisms recover more fully than others, perhaps suggesting more than one site/mechanism of plasticity following CBD.
Where does this form of respiratory plasticity originate, and by what mechanism(s)? The peripheral chemoreflex (as indicated by the ventilatory response to NaCN) and eupneic PaCO2 both recover to some degree in the months and years following CBD in ponies[25] (Figure 2).
Figure 2.
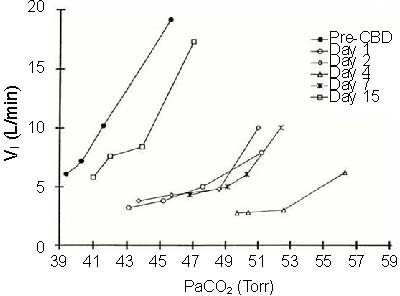
The relationship between ventilation (VI (L/min)) and PaCO2 (Torr) in an adult goat before carotid body denervation (pre-CBD) and on multiple days after CBD.
Note the hypoventilation (rightward shift in PaCO2, and the reduction in ventilation for a given level of PaCO2 following CBD. Note also the recovery of CO2 sensitivity by day 15 post-CBD. Adapted from[6].
However, there is far greater recovery toward pre-CBD levels in eupneic PaCO2, where the peripheral chemoreflex shows a significant, but minimal recovery. In these same ponies, further denervation of the aortic arch after this CBD-dependent plasticity has occurred eliminated the partially restored hypoxic ventilatory response, but had no effect on eupneic breathing[26]. This suggests that the recovery of the hypoxic ventilatory response following CBD depends upon aortic innervation, while the plasticity driving blood gases back toward “normal” may depend on a separate, potentially central mechanism. Similarly, CBD in neonatal piglets caused acute hypoventilation and attenuation of the hypoxic and NaCN responses, which showed varying degrees of recovery several days later[28]. Upon recovery, NaCN injections in the ascending aortic arch of CBD piglets elicited a hyperpnea, but the same injections in sham denervated piglets failed to increase ventilation, further suggesting that the aortic chemoreceptors become functional after CBD and may contribute to the recovery of ventilatory sensitivity to hypoxia. The area of the aorta with increased chemosensitivity was further shown to contain greater 5-HT content in CBD piglets compared to sham CBD piglets, and the aortic NaCN-dependent hyperpnea following CBD was shown to be attenuated by 5-HT receptor antagonists[29]. The sum of the data suggests that the plasticity following CBD may depend on: (1) multiple mechanisms at multiple sites, where the site of plasticity driving the normalization of eupneic ventilation may include the brainstem respiratory network, and (2) neuromodulators and their receptors, such as 5-HT.
CENTRAL EFFECTS OF PERIPHERAL CHEMORECEPTOR DENERVATION
Clearly, CBD acutely causes hypoventilation at rest (PaCO2 + 8-15 mm Hg), and blood and brain acidosis, and decreases CO2 sensitivity[6,7]. This period of significant hypoventilation, increased PaCO2, and decreased CSF pH should provide an increased stimulus to central CO2/pH chemoreceptors. Specifically, increasing PaCO2 by 8 mm Hg (by increasing inspired CO2) increases ventilation from 5 to 20 L/min in goats prior to CBD[6] (Figure 3). However, PaCO2 increases 10 mmHg at rest due to decreased ventilation by day 4 after CBD, and further increases in PaCO2 by increasing inspired CO2 have far less of a stimulatory effect on ventilation. Why is breathing not stimulated under these conditions? One possibility is that the sensitivity of central chemoreceptors is reduced following CBD, as shown by a reduced ventilatory response to focal acidification of the medullary raphé nuclei in goats after bilateral CBD[30] (Figure 4).
Figure 3.
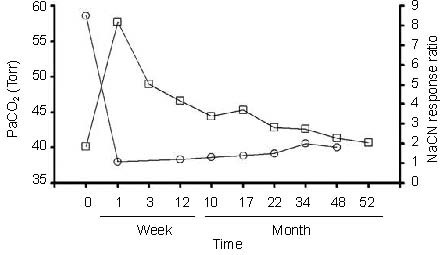
Chronic effects of carotid body denervation (CBD) on eupneic PaCO2 (Torr) and the NaCN response ratio (index of peripheral chemosensitivity to hypoxia) in ponies before and up to 52 months after CBD.
Note the recovery in resting PaCO2 is greater that the minimal, but significant recovery peripheral chemoreflex. Adapted from[25].
Figure 4.
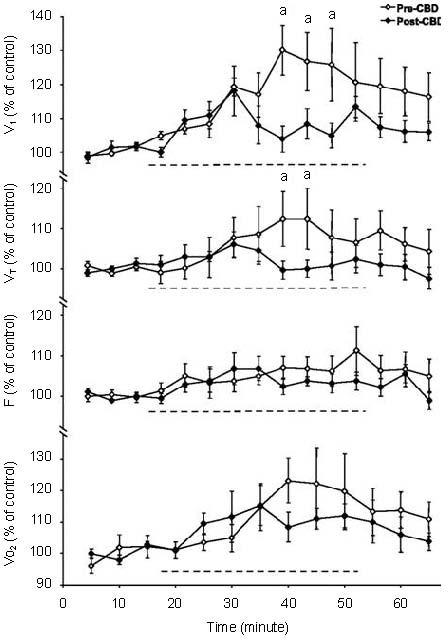
Ventilation, tidal volume (VT), breathing frequency (f) and O2 consumption (VO2), expressed as a percentage of control, before, during and after focal acidification in the medullary raphé in awake goats before and after bilateral carotid body denervation (CBD). “a” denotes significantly different from CBD (P < 0.05)
Note that the ventilatory response to raphé acidification is reduced following CBD. Adapted from[30].
Based on the hypothesis that the carotid bodies tonically “drive” the respiratory network, one might hypothesize generalized decreases in neuronal excitability or activity.
Indeed, cytochrome oxidase, which is a general marker of neuronal activity, has been shown to be decreased at a key site in the respiratory network, the pre-Bötzinger complex, following CBD in neonatal rats[31]. This reduced neuronal activity could result from a reduction in excitatory glutamatergic inputs, a concept consistent with the observations of Hoop et al[32]. who showed that the radiotracer CSF transfer rate of [13N] glutamine (a marker of glutamate bioavailability) was markedly reduced following CBD, but not during chronic hypoxia. If neuronal activity, and glutamatergic “drive” are reduced following CBD, a compensatory upregulation of excitatory receptors might occur. This may indeed be the case, as the ventilatory responses to raphé injections of the glutamate receptor-specific excitatory neurotoxin ibotenic acid was greatly enhanced following CBD (Figure 5;[30]).
Figure 5.
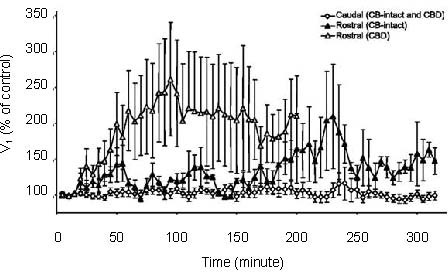
Ventilation (expressed as a percentage of control) for up to 5 hours after 10 µL injections of ibotenic acid into the caudal or rostral raphé in carotid body intact or carotid body denervation (CBD) goats.
Note the enhanced stimulatory effect of ibotenic acid in the rostral medullary raphé in goats that had prior CBD, suggesting altered raphé glutamate receptor activity. Adapted from[30].
Alternatively, the reduction in respiratory network excitability may result from alterations in excitatory neuromodulatory inputs. Roux et al. determined that tyrosine hydroxylase (TH) staining in the A1 (ventrolateral NTS) noradrenergic cell group was reduced (about 35%) 15 days after CBD, and increased in the A2 (129%) and A1 (ventrolateral medulla: 51%) 90 days post-CBD[33]. This reduction in TH could translate to a decrease in excitatory neuromodulatory input to the respiratory network. However, in a follow-up study, these investigators showed that chronic hypoxia alone was sufficient to enhance TH expression in noradrenergic cell groups independent of CBD, suggesting that the recovery from CBD may not be related to altered TH expression[34].
CBD eliminates excitatory afferent inputs from the carotid bodies to the NTS, which has numerous target nuclei, including the ventral respiratory group, retrotrapezoid nucleus[35,36] (RTN), and medullary raphé nuclei[37,38,39]. Thus, reduced NTS activity could result in reduced neuronal activity/excitability of RTN and/or 5-HT raphé neurons. Consistent with this hypothesis are the data demonstrating that generation of neuronal dysfunction of the ventrolateral medullary surface in an area that includes both serotonergic and glutamatergic RTN neurons by thermode cooling slows ventilation at rest during wakefulness in intact animals, but leads to sustained apnea after CBD[40]. Finally, as discussed previously focal acidification of the medullary raphé increases ventilation 30% in adult awake goats before CBD, but this response was significantly reduced after CBD (Figure 4). The sum of these data point to direct and indirect pathways by which peripheral chemoreceptors may modulate central neurons thought to serve as CO2/H+ chemoreceptor neurons.
SUMMARY AND CONCLUSIONS
The concepts that the input from the carotid bodies: (1) constitute a major excitatory drive to breathe, (2) serve as the major source of peripheral O2 chemoreception, and (3) significantly contribute to CO2/H+ chemoreception is exemplified by the major effects of CBD in multiple mammalian species. The respiratory plasticity, or restoration of function that occurs following CBD likely includes an upregulation of additional, normally quiescent sites of peripheral chemoreception potentially involving 5-HT. However, the data indicate that these conditional, peripheral sites cannot account for all of the plasticity in the mechanisms that govern eupneic breathing. Thus, we conclude that within the respiratory network there is an additional site/mechanism (s) of plasticity. Future work aiming to elucidate brainstem mechanisms of plasticity following peripheral lesions may prove useful harnessing and maximizing the capacity for the restoration of function in human disease and/or trauma.
Footnotes
Funding: NIH NHLBI HL097033 (MRH) and VA Merit Review 2885-02P (HVF)
Conflicts of interest: None declared
(Edited by Sheikh PB/Zhao LJ/Song LP)
REFERENCES
- 1.Haldane JS, Priestley JG. The regulation of the lung-ventilation. J Physiol. 1905;32:225–266. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comroe JH, Schmidt CM. The part played by reflexes from the carotid in the chemical regulation of the respiration in the dog. Am J Physiol. 1938;121:75–97. [Google Scholar]
- 3.Fencl V, Miller TB, Pappenheimer JR. Studies on the respiratory response to disturbances of acid-base balance, with deductions concerning the ionic composition of cerebral interstitial fluid. Am J Physiol. 1966;210:459–472. doi: 10.1152/ajplegacy.1966.210.3.459. [DOI] [PubMed] [Google Scholar]
- 4.Smith CA, Forster HV, Blain GM, et al. An interdependent model of central/peripheral chemoreception: evidence and implications for ventilatory control. Respir Physiol Neurobiol. 2010;173:288–297. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forster HV, Smith CA. Contributions of central and peripheral chemoreceptors to the ventilatory response to CO2/H+ J Appl Physiol. 2010;108:989–994. doi: 10.1152/japplphysiol.01059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan LG, Forster HV, Martino P, et al. Important role of carotid afferents in control of breathing. J Appl Physiol. 1998;85:1299–1306. doi: 10.1152/jappl.1998.85.4.1299. [DOI] [PubMed] [Google Scholar]
- 7.Bisgard GE, Forster HV, Orr JA, et al. Hypoventilation in ponies after carotid body denervation. J Appl Physiol. 1976;40:184–190. doi: 10.1152/jappl.1976.40.2.184. [DOI] [PubMed] [Google Scholar]
- 8.Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller DD, Zabka AG, Baker TL, et al. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. discussion 2000. [DOI] [PubMed] [Google Scholar]
- 11.Lahiri S, DeLaney RG. Stimulus interaction in the responses of carotid body chemoreceptor single afferent fibers. Respir Physiol. 1975;24:249–266. doi: 10.1016/0034-5687(75)90017-1. [DOI] [PubMed] [Google Scholar]
- 12.Ichikawa H. Innervation of the carotid body: Immunohistochemical, denervation, and retrograde tracing studies. Microsc Res Tech. 2002;59:188–195. doi: 10.1002/jemt.10193. [DOI] [PubMed] [Google Scholar]
- 13.Kumar P. Sensing hypoxia in the carotid body: from stimulus to response. Essays Biochem. 2007;43:43–60. doi: 10.1042/BSE0430043. [DOI] [PubMed] [Google Scholar]
- 14.Blain GM, Smith CA, Henderson KS, et al. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO(2) J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forster HV, Bisgard GE, Klein JP. Effect of peripheral chemoreceptor denervation on acclimatization of goats during hypoxia. J Appl Physiol. 1981;50:392–398. doi: 10.1152/jappl.1981.50.2.392. [DOI] [PubMed] [Google Scholar]
- 16.Forster HV, Bisgard GE, Rasmussen B, et al. Ventilatory control in peripheral chemoreceptor-denervated ponies during chronic hypoxemia. J Appl Physiol. 1976;41:878–885. doi: 10.1152/jappl.1976.41.6.878. [DOI] [PubMed] [Google Scholar]
- 17.Forster HV, Pan LG, Lowry TF, et al. Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol. 2000;119:199–208. doi: 10.1016/s0034-5687(99)00115-2. [DOI] [PubMed] [Google Scholar]
- 18.Dahan A, Nieuwenhuijs D, Teppema L. Plasticity of central chemoreceptors: effect of bilateral carotid body resection on central CO2 sensitivity. PLoS medicine. 2007;4:e239. doi: 10.1371/journal.pmed.0040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson EB, Vidruk EH, Dempsey JA. Carotid body excision significantly changes ventilatory control in awake rats. J Appl Physiol. 1988;64:666–671. doi: 10.1152/jappl.1988.64.2.666. [DOI] [PubMed] [Google Scholar]
- 20.Lowry TF, Forster HV, Pan LG, et al. Effects on breathing of carotid body denervation in neonatal piglets. J Appl Physiol. 1999;87:2128–2135. doi: 10.1152/jappl.1999.87.6.2128. [DOI] [PubMed] [Google Scholar]
- 21.Edelman NH, Epstein PE, Lahiri S, et al. Ventilatory responses to transient hypoxia and hypercapnia in man. Respir Physiol. 1973;17:302–314. doi: 10.1016/0034-5687(73)90005-4. [DOI] [PubMed] [Google Scholar]
- 22.Bellville JW, Whipp BJ, Kaufman RD, et al. Central and peripheral chemoreflex loop gain in normal and carotid body-resected subjects. J. Appl. Physiol. 1979;46:843–853. doi: 10.1152/jappl.1979.46.4.843. [DOI] [PubMed] [Google Scholar]
- 23.Rodman JR, Curran AK, Henderson KS, et al. Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol. 2001;91:328–335. doi: 10.1152/jappl.2001.91.1.328. [DOI] [PubMed] [Google Scholar]
- 24.Smith CA, Rodman JR, Chenuel BJ, et al. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol. 2006;100:13–19. doi: 10.1152/japplphysiol.00926.2005. [DOI] [PubMed] [Google Scholar]
- 25.Forster HV. Plasticity in the control of breathing following sensory denervation. J Appl Physiol. 2003;94:784–794. doi: 10.1152/japplphysiol.00602.2002. [DOI] [PubMed] [Google Scholar]
- 26.Smith PG, Mills E. Restoration of reflex ventilatory response to hypoxia after removal of carotid bodies in the cat. Neuroscience. 1980;5:573–580. doi: 10.1016/0306-4522(80)90054-8. [DOI] [PubMed] [Google Scholar]
- 27.Bisgard GE, Forster HV, Klein JP. Recovery of peripheral chemoreceptor function after denervation in ponies. J Appl Physiol. 1980;49:964–970. doi: 10.1152/jappl.1980.49.6.964. [DOI] [PubMed] [Google Scholar]
- 28.Serra A, Brozoski D, Hodges M, et al. Effects of carotid and aortic chemoreceptor denervation in newborn piglets. J Appl Physiol. 2002;92:893–900. doi: 10.1152/japplphysiol.00819.2001. [DOI] [PubMed] [Google Scholar]
- 29.Serra A, Brozoski D, Simeon T, et al. Serotonin and serotonin receptor expression in the aorta of carotid intact and denervated newborns. Respir Physiol Neurobiol. 2002;132:253–264. doi: 10.1016/s1569-9048(02)00119-2. [DOI] [PubMed] [Google Scholar]
- 30.Hodges MR, Opansky C, Qian B, et al. Carotid body denervation alters ventilatory responses to ibotenic acid injections or focal acidosis in the medullary raphe. J Appl Physiol. 2005;98:1234–1242. doi: 10.1152/japplphysiol.01011.2004. [DOI] [PubMed] [Google Scholar]
- 31.Liu Q, Wong-Riley MT. Postnatal changes in cytochrome oxidase expressions in brain stem nuclei of rats: implications for sensitive periods. J Appl Physiol. 2003;95:2285–2291. doi: 10.1152/japplphysiol.00638.2003. [DOI] [PubMed] [Google Scholar]
- 32.Hoop B, Masjedi MR, Shih VE, et al. Brain glutamate metabolism during hypoxia and peripheral chemodenervation. J Appl Physiol. 1990;69:147–154. doi: 10.1152/jappl.1990.69.1.147. [DOI] [PubMed] [Google Scholar]
- 33.Roux JC, Peyronnet J, Pascual O, et al. Ventilatory and central neurochemical reorganisation of O 2 chemoreflex after carotid sinus nerve transection in rat. J Physiol. 2000;522(Pt 3):493–501. doi: 10.1111/j.1469-7793.2000.t01-4-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roux JC, Pequignot JM, Dumas S, et al. O2-sensing after carotid chemodenervation: hypoxic ventilatory responsiveness and upregulation of tyrosine hydroxylase mRNA in brainstem catecholaminergic cells. Eur J Neurosci. 2000;12:3181–3190. doi: 10.1046/j.1460-9568.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- 35.Mulkey DK, Stornetta RL, Weston MC, et al. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 36.Takakura AC, Moreira TS, Colombari E, et al. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thor KB, Helke CJ. Serotonin and substance P colocalization in medullary projections to the nucleus tractus solitarius: dual-colour immunohistochemistry combined with retrograde tracing. J Chem Neuroanat. 1989;2:139–148. [PubMed] [Google Scholar]
- 38.Jean A. The nucleus tractus solitarius: neuroanatomic, neurochemical and functional aspects. Arch Int Physiol Biochim Biophys. 1991;99:A3–52. doi: 10.3109/13813459109145916. [DOI] [PubMed] [Google Scholar]
- 39.Nuding SC, Segers LS, Shannon R, et al. Central and peripheral chemoreceptors evoke distinct responses in simultaneously recorded neurons of the raphe- pontomedullary respiratory network. Philos Trans R Soc Lond B Biol Sci. 2009;364:2501–2516. doi: 10.1098/rstb.2009.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan LG, Forster HV, Ohtake PJ, et al. Effect of carotid chemoreceptor denervation on breathing during ventrolateral medullary cooling in goats. J Appl Physiol. 1995;79:1120–1128. doi: 10.1152/jappl.1995.79.4.1120. [DOI] [PubMed] [Google Scholar]


