Abstract
Our previous research showed that octacosanol exerted its protective effects in 6-hydroxydopamine-induced Parkinsonian rats. The goal of this study was to investigate whether octacosanol would attenuate neurotoxicity in 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP)-treated C57BL/6N mice and its potential mechanism. Behavioral tests, tyrosine hydroxylase immunohistochemistry and western blot were used to investigate the effects of octacosanol in a mouse model of Parkinson's disease. Oral administration of octacosanol (100 mg/kg) significantly improved behavioral impairments in mice treated by MPTP and markedly ameliorated morphological appearances of tyrosine hydroxylase-positive neuronal cells in the substantia nigra. Furthermore, octacosanol blocked MPTP-induced phosphorylation of p38MAPK and JNK, but not ERK1/2. These findings implicated that the protective effects afforded by octacosanol might be mediated by blocking the phosphorylation of p38MAPK and JNK on the signal transduction in vivo. Considering its excellent tolerability, octacosanol might be considered as a candidate agent for clinical application in treating Parkinson's disease.
Keywords: Parkinson's disease, neuroprotecion, mitogen-activated protein kinase, c-Jun N-terminal kinase, p38MAPK, substantia nigra, neural regeneration
Abbreviations
PD, Parkinson's disease; 6-OHDA, 6-hydroxydopamine; ERK1/2, extracellular signal-regulated kinase 1/2; JNK, c-jun N-terminal kinase; MAO-B, monoamine oxidase B; MAPK, mitogen-activated protein kinase; MPP+, 1-methyl-4-phenylpyridium; MPTP, 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine
INTRODUCTION
Parkinson's disease (PD) is a progressive and age-related neurodegenerative disease characterized by degeneration of dopaminergic neurons originating in the substantia nigra pars compacta and projecting to the dorsal striatum[1]. It affects 1.5% of the global population over 65 years of age. To date, the most widely used and effective treatment for PD is currently available dopamine replacement strategies via oral supplementation of dopamine (DA) precursor levodopa, however, long-term treatment with levodopa is often complicated by the development of adverse effects[2]. There have been additional anti-parkinsonian drugs, such as dopamine receptor agonists, but these available therapies could not protect against dopaminergic neurodegeneration. Therefore, it is of utmost importance to develop new drugs that show or halt the rate of progression of PD.
Mitogen-activated protein kinases (MAPKs) play a critical role in promoting survival or inducing cell death. The family of MAPKs comprises of extracellular signal-regulated kinases (ERKs), stress-activated protein kinases (SAPKs), c-Jun N-terminal kinases (JNKs) and p38 kinases. Previous studies demonstrated the selective phosphorylation of p38MAP kinase within the dopamine neurons, whereas JNK activation occurred predominantly in the microglia[3]. p38MAPK activation results in downstream phosphorylation of p53 and increased p53 mediated transcription of Bax and Puma in the ventral midbrain. JNK, a member of the MAPK family, is activated by a variety of stimuli, including neurotoxic insults, environmental stress, and apoptotic agents[4,5]. Activated JNK in turn phosphorylates the substrates, including c-Jun, ELK1, and p53, leading to neuronal death and excitotoxicity[6,7]. Therefore, JNK is an important mediator of signal transduction from the cell surface to the nucleus[8,9].
Octacosanol (CH3[CH2]27OH, molecular weight: 410.77), a low-molecular-weight primary aliphatic alcohol, is the main component of a natural product wax extracted from plants. It has a number of indications for its clinical application, many of which are currently being researched. In particular, the cholesterol-lowering effects, anti-aggregatory properties, and cytoprotective use of octacosanol have been widely investigated[10]. We have recently found that octacosanol exerted its anti-parkinsonism effects in 6-hydroxydopamine (6-OHDA) treated rats and this neuroprotective effect might be associated with its inhibition of proNGF-p75NTR/sortilin mediated cell death[11]. Since 6-OHDA treated rat model and 1-methyl-4-phenyl-1, 2, 3, 6 tetrahydropyridine (MPTP) induced mouse model are both very important and classic animal models for screening drugs to provide the clues for PD treatment, in the present study, we were interested in systematically exploring whether octacosanol would attenuate MPTP induced deficits and its effects on the ERK, JNK and p38MAPKases activities which play a critical role in determining the fate of neural cells in the mouse PD model.
RESULTS
Effects of octacosanol on rotarod test and spontaneous movement
Rotarod test could reveal motor coordination ability of mice[12] and spontaneous movement test is a behavioral index of Parkinsonian hypokinesia[13]. To test that whether octacosanol would attenuate MPTP induced behavioral deficits in mice, rotarod test (F(4,70) = 4.219, P = 0.004) and spontaneous movement test (F(4,70) = 3.506, P = 0.011) were adopted in the present study. As shown in Figure 1, mice treated with MPTP showed a clear reduction in the duration of rotations compared with sham control group (P = 0.005). Octacosanol treatment (25 mg/kg, 50 mg/kg and 100 mg/kg) increased stay duration of rotations (P = 0.049, P = 0.015, P = 0.025), and ameliorated motor coordination ability impairment due to MPTP. Octacosanol treatment for 2 weeks attenuated MPTP-induced impairment of the counts of spontaneous movement. As shown in Figure 2, 2 weeks of treatment with octacosanol (100 mg/kg) effectively increased the number of spontaneous movement (P = 0.046) and improved MPTP-induced hypokinesia while lesioned animal group exhibited a significant decrease in spontaneous movement compared with sham control group (P = 0.008).
Figure 1.

Effects of 2-week treatment with octacosanol at 25 (Ocs-L), 50 (Ocs-M), 100 mg/kg (Ocs-H) on rotarod test in 1-methyl-4-phenyl-1, 2, 3, 6 tetrahydropyridine (MPTP)-induced motor coordination ability impairment 2 weeks after MPTP injection. n = 15 mice in each group.
Data are expressed as mean ± SD. aP < 0.01, vs. sham control group; bP < 0.05, vs. model group.
Figure 2.
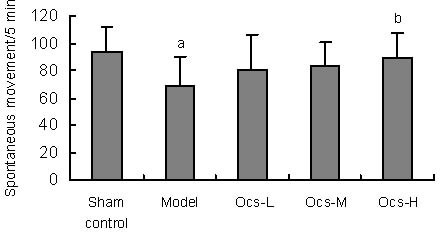
Effects of 2-week treatment with octacosanol at 25 (Ocs-L), 50 (Ocs-M), 100 mg/kg (Ocs-H) on spontaneous movement in 2 weeks after 1-methyl-4- phenyl-1, 2, 3, 6 tetrahydropyridine injection. n = 15 mice in each group.
Data are expressed as mean ± SD. aP < 0.01, vs. sham control group; bP < 0.05, vs. model group.
Octacosanol increased the numbers of tyrosine hydroxylase (TH)-positive dopamine neurons in the substantia nigra
TH immunohistochemical staining (F(4,25) = 18.973, P < 0.001) was performed to assess the neurotoxicity of MPTP and protection conferred by octacosanol. As shown in Figures 3A, B, it was obvious that MPTP significantly impaired TH-positive neurons in the substantia nigra in MPTP-treated animals as compared to that in the sham control group (P < 0.001) and the mice treated with octacosanol (100 mg/kg, octacosanol- high group) for 14 days markedly reversed the MPTP- induced changes in the substantia nigra (P < 0.001).
Figure 3.

Effects of 2-week treatment with octacosanol at 25 (Ocs-L), 50 (Ocs-M), 100 mg/kg (Ocs-H) on a loss of dopamine neurons induced by 1-methyl-4-phenyl-1, 2, 3, 6 tetrahydropyridine.
(A) Representative photomicrographs of tyrosine hydroxylase (TH)-positive neurons immunohistochemistry in substantia nigra (An inset of TH-positive neurons was also included).
(B) Quantitative analysis of TH positive cells in substantia nigra.
Scale bar = 200 μm. n = 6 mice in every group. Data were represented as mean ± SD. aP < 0.001, vs. sham control group; bP < 0.001, vs. model group.
Octacosanol inhibited MPTP-induced increase in p38 MAPK and JNK phosphorylation in the substantia nigra
To further explore the mechanisms underlying the protective effects of octacosanol on MPTP-induced toxicity, levels of p38MAPK (F(4,10) = 40.080, P < 0.001) and JNK (F(4,10) = 191.115, P < 0.001) phosphorylation were analyzed. It was well known that apoptosis which played a key role in the neurodegenerative processes in PD[14,15] was involved in several signaling pathways. Among them, p38MAPK and JNK signaling cascades were the key mediators in MPTP-induced neuronal apoptosis. As shown in Figures 4A, B, western blot analysis revealed that a robust increase was detected in the substantia nigra of p38MAPK phosphorylation in MPTP-treated group (P < 0.001), meanwhile, we also found MPTP significantly increased JNK phosphorylation in the same region (Figures 5A, B; P < 0.001).
Figure 4.
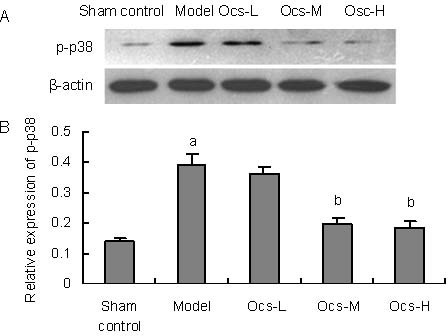
Inhibitory effects of octacosanol treatment on 1-methyl-4-phenyl-1, 2, 3, 6 tetrahydropyridine-induced phospho-p38 MAPK activation in treatment with 20, 50, 100 mg/kg octacosanol in substantia nigra.
(A) Western blotting for p-p38 MAPK in substantia nigra in every group.
(B) Image analysis of the relative phosphorylation level of p38MAPK.
Relative absorbance was normalized to β-actin. Each value represents mean ± SD, n = 6 mice in every group. aP < 0.001, vs. sham control group; bP < 0.001, vs. model group.
Figure 5.
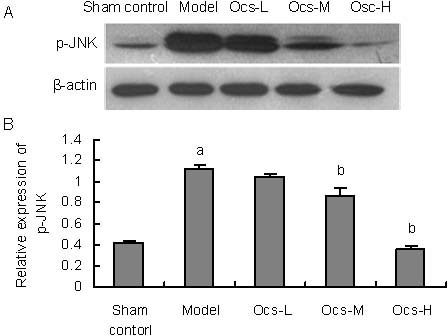
Octacosanol treatment blocked 1-methyl-4-phenyl-1, 2, 3, 6 tetrahydropyridine-induced phospho-Thr183/Tyr185 JNK activation in treatment with 25, 50, 100 mg/kg octacosanol in substantia nigra.
(A) Western blotting for p-JNK in substantia nigra in each group.
(B) Image analysis of the relative phosphorylation level of JNK.
Relative absorbance was normalized to β-actin. Each value represents mean ± SD. n = 6 mice in every group. aP < 0.001, vs. sham control group; bP < 0.001, vs. model group.
Both 50 and 100 mg/kg treatment with octacosanol could effectively inhibit MPTP-induced phosphorylation of p38MAPK and JNK in the substantia nigra in a dose dependent manner (Figures 4, 5, P < 0.001).
Octacosanol did not alter MPTP-induced decrease in Erk1/2 phosphorylation in the substantia nigra
Among MAPKs, JNK and p38MAPK participated in stress responses and often triggered apoptosis, while Erk1/2 signaling regulated cell proliferation, differentiation and survival[16]. In Figures 6A, B, western blot analysis demonstrated that the relative phosphorylation level of Erk1/2 (F(4,10) = 23.409, P < 0.001) in the substantia nigra were significantly decreased by MPTP treatment as compared to sham control group (P < 0.001). Although the relative expression of p-Erk1/2 in octacosanol treated animals tended to increase, however, there was no significant difference between MPTP alone and octacosanol treatment groups.
Figure 6.
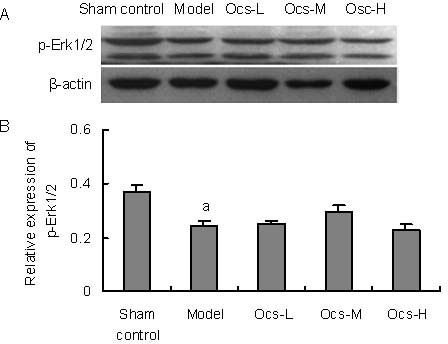
Treatment with 25, 50, 100 mg/kg octacosanol (Ocs-L, -M, -H) had no effects on 1-methyl-4-phenyl-1, 2, 3, 6 tetrahydropyridine-induced decreased Erk1/2 phosphorylation in substantia nigra.
(A) Western blotting for phospho-Erk1/2 in substantia nigra in every group.
(B) Image analysis of the relative phosphorylation level of Erk1/2. Relative absorbance was normalized to β-actin.
Each value represents mean ± SD. n = 6 mice in every group. aP < 0.001, vs. sham control group.
DISCUSSION
Our findings have demonstrated that oral administration of octacosanol, especially at the dose of 100 mg/kg, significantly improved MPTP-induced behavioral impairments and decrease in TH-positive neurons in the substantia nigra, suggesting protective effects of octacosanol against neurotoxicity. This neuroprotective effect might be mediated by blocking the phosphorylation of p38MAPK and JNK on the signal transduction pathway.
Octacosanol has many potential indications for treating diseases as a health agent. It is the predominant component of policosanol, which is a commercial mixture of very long-chain alcohols isolated from sugarcane, composed primarily of octacosanol (60%), triacontanol (13%), and hexacosanol (6%). Many studies have shown that octacosanol was very effective in lowering LDL and increasing HDL. Octacosanol also offers cytoprotective effects. For example, octacosanol attenuated disrupted hepatic reactive oxygen species metabolism associated with acute liver injury progression in CCl4-intoxicated rats[17]. Additionally, policosanol, in which octacosanol represents its main component, showed a protective effect on the myocardial necrosis induced by isoprenaline in a rat experimental model[18]. Results of another study showed an anti-ischemic effect of policosanol administered after induction of cerebral ischemia in two different experimental models in Mongolian gerbils, suggesting a possible therapeutic effect in cerebral vascular disorders[19]. It should be noted that in our rotarod test, the low dose of octacosanol seemed to provide reversal of MPTP-induced behavioral deficits, but no improvement in the biochemical parameters such as western blot. We have speculated this disconnect result was probably due to the athletic performance of octacosanol which was summarized by Taylor et al[10]. Since oxidative stress may be attributed as a causative factor in the pathogenesis of PD, some studies found that octacosanol could preserve the free radical scavenging capability in rat striatums[11], attenuate disrupted hepatic reactive oxygen species metabolism of rats[17], and reduce the potential of lipoprotein to undergo lipid peroxidaiton in rat plasma[20]. Thus, octacosanol shows its anti-parkinsonism effects in MPTP treated mice probably through improvement of the oxidative-stress microenvirmonent in nigrostriatal systems. In addition, a recent study reported that long-chain fatty alcohols from pomace olive oil, in which octacosanol was the third main component, had inhibited the release of different proinflammatory mediators including eicosanoids, cytokines and nitric oxide in vitro[21], suggesting that octacosanol might also have a protective effect on some mediators involved in the inflammatory process during PD pathogenesis.
MAPKs control many cellular events, including differentiation, proliferation, and apoptosis[22], and to date at least three major MAPK subfamilies, ERKs, p38MAPKs and JNKs, have been described. Though there have been reports in which it was suggested that they might be implicated in apoptotic and/or necrotic pathways activated in certain neurodegenerative conditions, ERKs have primarily been hypothesized to play a pivotal role in cell growth and differentiation in different PD models in vivo and in vitro. For example, ERK1/2 had appeared to mediate the neuroprotective effects induced by glial cell derived neurotrophic factor in cultured striatal neurons or a MPTP mouse model[23,24]. The second family of MAPKs is p38MAPKs. Karunakaran et al[3] have reported that activation of p38MAPK was potentially an important contributor to the disease process, leading to the degeneration of dopaminergic neurons of the substantia nigra pars compacta in PD. Now there is also substantial body of evidence that JNKs are activated in the models of PD. Saporito et al[25] have shown that MPTP activated JNK within 4 hours of a single MPTP treated administration, and Xia et al[26] confirmed this in multiple dosing regimens showing that JNK was active at 6 hours after the second and third injections of MPTP and was still evident after the fifth injection. Another research showed that the activation of JNK was observed in MPTP-induced animal models of PD, and the inactivation of JNK by CEP-1347/KT-7515, an inhibitor of JNK activation, was neuroprotective[25]. Consistent with previous reports, we also observed the elevation of p38 MAPK and JNK as well as the reduction of ERK in the MPTP treated mice. After octacosanol treatment, the phosphorylation levels of p38MAPK and JNK were inhibited. However, octacosanol treatment showed no effect on the phosphorylation of ERK, indicating that octacosanol exerted different influences on the MAPKs family.
It has been well shown that, proNGF, an unprocessed precursor form of neurotrophin family members (pro-neurotrophins), can act through a co-receptor system of p75NTR and sortilin to mediate cell apoptosis while NGF induces neuronal survival with TrkA and low-affinity binding p75NTR[27,28,29]. Based on this mechanism, we have recently demonstrated that octacosanol showed its anti-parkinsonism effects in 6-OHDA induced parkinsonian rats and these protective effects might be associated with its inhibition of proNGF-p75NTR/sortilin mediated cell death[11]. A recent study has found that accumulation of proNGF induced by oxidative stress could activate a signaling pathway, RhoA/p38MAPK to mediate neurovascular injury in samples from human patients and retinal Müller glial culture cells[30]. Besides, proNGF was proved to be produced by microglia via the p38MAPK-mediated pathway to induced oligodendrocytes death after spinal cord injury[31]. Therefore, p38MAPK has played an important role in production of proNGF and subsequent proNGF mediated neural injury. In our study, we have speculated that by inhibition of the phosphorylation of p38MAPK, octacosanol might also probably affect the expression of proNGF or its activity so that octacosanol could exert neuroprotective effects in the MPTP-treated mouse model. Moreover, our previous study has found that octacosanol attenuated neuronal apoptosis especially via inhibition of a key proapoptotic signalling pathway mediated by JNK[11]. And it has been well demonstrated that p75NTR-dependent apoptosis was associated with an increase in JNK activity[32,33,34] and JNK-dependent mitochondrial pathway including cytosolic accumulation of cytochrome c and caspase activation in vitro or in vivo[35,36]. Consistent with our results in 6-OHDA treated rats, we found that octacosanol might exert its neuroprotection in MPTP-treated mice probably through inhibition of JNK activation. Thus, under the background that p75NTR-induced JNK activation was a consistent feature of p75NTR-responsive cell types[37], we have made a hypothesis that p75NTR, the upstream regulation factors of JNK, might be in part involved in the protective effects afforded by octacosanol in MPTP-treated mouse model of this study. However, at present, we cannot elucidate the exact mechanism underlying how octacosanol might interfere with MAPKs signaling, but we thought that it was not directly related to the chemical structure of octacosanol. And more studies should be done in order to validate this speculation.
In conclusion, our present data confirm the protective anti-parkinsonian effect of octacosanol in MPTP-treated mouse model of Parkinson's disease. To our knowledge, this series of studies have been the first basic research on the neuroprotective effects of octacosanol in PD. Together with the evidence of protective effects of octacosanol on 6-OHDA-induced parkinsonism in rats, our studies strongly support the protective potential of octacosanol in its anti-parkinsonian effects in animal models. Furthermore, because of the non-toxicity in animal studies[38] and its excellent tolerability in clinical trials[39], it would be feasible for the healthy general population to use octacosanol without major side effects. Thus, taking its excellent tolerability and non-toxicity together, octacosanol deserves to be further explored on its anti-parkinsonian effects and mechanism in PD animal models. If further studies confirm its effects, octacosanol may be considered as a candidate agent for clinical application in PD treatment.
MATERIALS AND METHODS
Materials
Reagents
Octacosanol (CH3[CH2]27OH) was synthesized by Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, with purity more than 99%. Rabbit polyclonal antibodies for phospho-Thr183/Tyr185 JNK, phospho-Erk1/2, phospho-p38 were purchased from Cell Signaling Technology (Beverly, MA, USA). A rabbit antibody for β-actin was purchased from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). MPTP was purchased from Sigma Chemical (St. Louis, MO, USA); secondary goat anti-rabbit IgG, SP immunohistochemical staining kit and 3,3’-diaminobenzidine were purchased from Zhongshan Biotechnology Co (Beijing, China). Whole Protein Extracion Kit was purchased from Applygen Technologies Inc (Beijing, China).
Animals
Seventy-five adult male C57BL/6N mice (Weitonglihua Experimental Animal Central, Beijing, China) weighing 23-25 g were used. Mice were maintained in a constant temperature (22 ± 1°C) and humidity (60 ± 10%) environment under a 12-hour light/dark cycle. Food and water were available ad libitum. Animal treatment and maintenance were carried out in accordance the guidelines established by the National Institutes of Health for the care and use of laboratory animals and were approved by the Animal Care Committee of the Peking Union Medical College and Chinese Academy of Medical Sciences. Adequate measures were taken to minimize pain or discomfort.
Mice were divided randomly into five groups with 15 in each group: sham control (saline only), model (MPTP, i.p., 15 mg/kg for 4 times, with 2 hours interval), octacosanol-low (Ocs-L, MPTP + 25 mg/kg octacosanol), octacosanol-medium (Ocs-M, MPTP + 50 mg/kg octacosanol), and octacosanol-high (Ocs-H, MPTP + 100 mg/kg octacosanol) groups. The next day after injection of MPTP, mice in the three dose groups were intragastricly administered octacosanol (dissolved in saline containing 0.5% carboxymethylcellulose sodium) once per day for subsequent 14 days. Sham control and treatment groups received the same volume of saline (10 mL/kg body weight) with 0.5% carboxymethylcellulose sodium as that of the octacosanol.
Methods
Rotarod test
For rotarod test, each animal was placed on a 3-cm diameter, 10-cm long wooden rod, rotating with a constant speed at 16 r/m to measure fore- and hindlimb motor dysfunction. A trial was terminated when the mouse fell from the rotarod and the total time before the mice fell off the rod was automatically calculated. The maximum time per trial is 5 minutes. And the total time on the rotarod was used for analysis. Three pretrainings were performed before the behavioral testing and then the fourth trial was regarded as the probe test.
Spontaneous locomotor test
To evaluate the effects of octacosanol on behavioral impairments induced by MPTP, spontaneous locomotor test and rotarod test were performed just after the last administration of octacosanol. Locomotor activity was measured in a circular open field arena (diameter 50 cm, height 40 cm) made of white plastic in a dimly illuminated room. Each mouse was put into the spontaneous movement monitor and allowed to freely explore the open field for 2 minutes. Then every movement of the mice would be acquired by the infrared ray detector and counted by a computer for the consecutive 5 minutes.
Brain tissue preparation
Animals (n = 7 in each group) for western blot analysis were decapitated, and the brains were immediately taken out and rinsed in ice-cold isotonic physiologic saline. Tissues of the substantia nigra were isolated and kept frozen in liquid nitrogen before analysis. For immunohistochemistry, the remaining animals were anesthetized via pentobarbital sodium overdose and perfused transaortically first with 0.1 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde (pH 7.4). The brains were removed and postfixed in the same 4% paraformaldehyde solution at 4 °C, after which they were equilibrated in 0.1 M PBS containing 15%, 20% and 30% sucrose at 4°C, respectively. Coronal sections (35 μm) were cut using a cryostat.
TH immunohistochemistry
Free-floating brain slices (35 μm) were treated for TH immunohistochemistry. Sections were washed in PBS to remove cryopreservative and subsequently incubated for 10 minutes at room temperature in 3% H2O2 solution to reduce endogenous peroxidase activity, and then washed in PBS. The samples were placed in goat serum for 1 hour at room temperature (SP immunohistochemical staining kit). Overnight incubation at 4°C was performed with rabbit polyclonal anti-TH antibody. After incubation, slices were washed with PBS and incubated with biotinylated goat anti-rabbit IgG for 1 hour at room temperature, again washed, and then incubated in avidin-biotin horseradish peroxidase macromolecular complex for 1 hour at room temperature. To develop color, slices were incubated briefly in 3,3’-diaminobenzidine substrate kit. After a final set of washes in PBS, the slices were mounted on slides, dehydrated, cleared, and coverslipped with mounting medium.
Quantitative morphology
All the histological quantification was performed blindly and automatically, similar as the previous literature described[40]. Neuroanatomical sites were identified using the Paxinos and Franklin atlas[41]. The observed anteroposterior (AP) localizations from bregma of the analyzed areas were from AP: –3.11 mm to AP:–3.64 mm in substantia nigra. Neurons which were positively stained exhibited labeled soma and dendrites. The number of tyrosine hydroxylase positive neurons in the substantia nigra was counted by using a microscope (Olympus BX53) equipped with a 40 x objective and connected to an image analysis system (Image-Pro Plus, Media Cybernetics, Inc.) equipped for stereological application. Results are expressed as the mean density of cells (number of positive neurons/mm2 of the structure). Every section (35 μm) from each of the 6 animals was included for stereological assessment, resulting in 15 sections per animal. And we calculated the mean value of the 15 sections per animal for the analysis. The density of positive cells in histochemistrical analysis was calculated with Image-Pro Plus image analysis software.
Western blot analysis
Whole protein lysates was preparation by using a Protein Extraction Kit (Applygen Technologies Inc., Beijing, China) according to the manufacturer's instructions. After protein content was estimated by using Bradford's method, proteins were separated by a sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene difluoride membrane. The membrane was blocked with 5 % skim milk in Tris buffer saline. The membrane was then incubated at 4°C overnight with primary antibodies. After washing, the membranes were incubated with a horseradish peroxidase conjugated secondary antibody (goat anti-rabbit IgG, 1:10 000, Zhongshan Biotechnology Co., Beijing, China) for 1 hour at room temperature. The antibody-reactive bands were visualized on X-ray film using superECL plus detection reagent (Applygen Technologies Inc.,). All western blot experiments were done at least in triplicate from different samples. The band intensity in western blot analysis was calculated by AlphaEase®FC Software.
Statistical analysis
Data were analyzed using SPSS 13.0 software and presented as mean ± SD. Differences between mean values were evaluated by analysis for statistical significance by one-way analysis of variance, followed by Tukey's honestly significant difference multiple comparison. Statistical significance was set at P < 0.05.
Acknowledgments
The authors would like to extend their appreciation to Professor Haibo Zhu for offering octacosanol.
Footnotes
Funding: This work was supported by the grants from National Basic Research Program of China (973 Program), No. 2007B507400, 2010CB934002, 2011CB504101, 2011CBA00408; the National Natural Science Foundation of China, No. 81050025; and the grant from the Ministry of Science and Technology of China Eleventh 5-year Plan-Technical Platform for Drug Development, No. 2009ZX09303-8.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Ethnics Committee of the Peking Union Medical College and Chinese Academy of Medical Sciences.
(Edited by Eriksen J/Zhao LJ/Song LP)
REFERENCES
- 1.Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988;334(6180):345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- 2.Lewitt PA. Levodopa for the treatment of Parkinson's disease. N Engl J Med. 2008;359(23):2468–2476. doi: 10.1056/NEJMct0800326. [DOI] [PubMed] [Google Scholar]
- 3.Karunakaran S, Saeed U, Mishra M, et al. Selective activation of p38 mitogen-activated protein kinase in dopaminergic neurons of substantia nigra leads to nuclear translocation of p53 in 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine-treated mice. J Neurosci. 2008;28(47):12500–12509. doi: 10.1523/JNEUROSCI.4511-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarzschild MA, Cole RL, Hyman SE. Glutamate, but not dopamine, stimulates stress-activated protein kinase and AP-1-mediated transcription in striatal neurons. J Neurosci. 1997;17(10):3455–3466. doi: 10.1523/JNEUROSCI.17-10-03455.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22(6):631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Borsello T, Clarke PG, Hirt L, et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9(9):1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- 7.Repici M, Borsello T. JNK pathway as therapeutic target to prevent degeneration in the central nervous system. Adv Exp Med Biol. 2006;588:145–155. doi: 10.1007/978-0-387-34817-9_13. [DOI] [PubMed] [Google Scholar]
- 8.Brint EK, Fitzgerald KA, Smith P, et al. Characterization of signaling pathways activated by the interleukin 1 (IL-1) receptor homologue T1/ST2. A role for Jun N-terminal kinase in IL-4 induction. J Biol Chem. 2002;277(51):49205–49211. doi: 10.1074/jbc.M209685200. [DOI] [PubMed] [Google Scholar]
- 9.Waetzig V, Herdegen T. The concerted signaling of ERK1/2 and JNKs is essential for PC12 cell neuritogenesis and converges at the level of target proteins. Mol Cell Neurosci. 2003;24(1):238–249. doi: 10.1016/s1044-7431(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 10.Taylor JC, Rapport L, Lockwood GB. Octacosanol in human health. Nutrition. 2003;19(2):192–195. doi: 10.1016/s0899-9007(02)00869-9. [DOI] [PubMed] [Google Scholar]
- 11.Wang T, Liu YY, Wang X, et al. Protective effects of octacosanol on 6-hydroxydopamine-induced Parkinsonism in rats via regulation of ProNGF and NGF signaling. Acta Pharmacol Sin. 2010;31(7):765–774. doi: 10.1038/aps.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong XY, Cai Z, Pan L, et al. Transplantation of human amniotic cells exerts neuroprotection in MPTP-induced Parkinson disease mice. Brain Res. 2008;1205:108–115. doi: 10.1016/j.brainres.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 13.Cass WA, Peters LE, Smith MP. Reductions in spontaneous locomotor activity in aged male, but not female, rats in a model of early Parkinson's disease. Brain Res. 2005;1-2(1034):153–161. doi: 10.1016/j.brainres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1(2):120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 15.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407(6805):802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 16.Xia Z, Dickens M, Raingeaud J, et al. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270(5240):1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 17.Ohta Y, Ohashi K, Matsura T, et al. Octacosanol attenuates disrupted hepatic reactive oxygen species metabolism associated with acute liver injury progression in rats intoxicated with carbon tetrachloride. J Clin Biochem Nutr. 2008;42(2):118–125. doi: 10.3164/jcbn.2008017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noa M, Herrera M, Magraner J, et al. Effect of policosanol on isoprenaline-induced myocardial necrosis in rats. J Pharm Pharmacol. 1994;46(4):282–285. doi: 10.1111/j.2042-7158.1994.tb03794.x. [DOI] [PubMed] [Google Scholar]
- 19.Molina V, Arruzazabala ML, Carbajal D, et al. Effect of policosanol on cerebral ischemia in Mongolian gerbils. Braz J Med Biol Res. 1999;32(10):1269–1276. doi: 10.1590/s0100-879x1999001000014. [DOI] [PubMed] [Google Scholar]
- 20.Menendez R, Fraga V, Amor AM, et al. Oral administration of policosanol inhibits in vitro copper ion-induced rat lipoprotein peroxidation. Physiol Behav. 1999;67(1):1–7. doi: 10.1016/s0031-9384(99)00004-9. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Arche A, Marquez-Martin A, de la Puerta Vazquez R, et al. Long-chain fatty alcohols from pomace olive oil modulate the release of proinflammatory mediators. J Nutr Biochem. 2009;20(3):155–162. doi: 10.1016/j.jnutbio.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Martinez JM, Perez-Navarro E, Gavalda N, et al. Glial cell line-derived neurotrophic factor promotes the arborization of cultured striatal neurons through the p42/p44 mitogen-activated protein kinase pathway. J Neurosci Res. 2006;83(1):68–79. doi: 10.1002/jnr.20713. [DOI] [PubMed] [Google Scholar]
- 24.Lindgren N, Leak RK, Carlson KM, et al. Activation of the extracellular signal-regulated kinases 1 and 2 by glial cell line-derived neurotrophic factor and its relation to neuroprotection in a mouse model of Parkinson's disease. J Neurosci Res. 2008;86(9):2039–2049. doi: 10.1002/jnr.21641. [DOI] [PubMed] [Google Scholar]
- 25.Saporito MS, Brown EM, Miller MS, et al. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons In vivo. J Pharmacol Exp Ther. 1999;288(2):421–427. [PubMed] [Google Scholar]
- 26.Xia XG, Harding T, Weller M, et al. Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson's disease. PNAS. 2001;98(18):10433–10438. doi: 10.1073/pnas.181182298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee R, Kermani P, Teng KK, et al. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 28.Nykjaer A, Lee R, Teng KK, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427(6977):843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 29.Harrington AW, Leiner B, Blechschmitt C, et al. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. PNAS. 2004;101(16):6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali TK, Al-Gayyar MM, Matragoon S, et al. Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia. 2011;54(3):657–668. doi: 10.1007/s00125-010-1935-1. [DOI] [PubMed] [Google Scholar]
- 31.Yune TY, Lee JY, Jung GY, et al. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. 2007;27(29):7751–7761. doi: 10.1523/JNEUROSCI.1661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, et al. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383(6602):716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- 33.Yoon SO, Casaccia-Bonnefil P, Carter B, et al. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18(9):3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roux PP, Bhakar AL, Kennedy TE, et al. The p75 neurotrophin receptor activates Akt (protein kinase B) through a phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem. 2001;276(25):23097–23104. doi: 10.1074/jbc.M011520200. [DOI] [PubMed] [Google Scholar]
- 35.Tournier C, Hess P, Yang DD, et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288(5467):870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 36.Salehi AH, Xanthoudakis S, Barker PA. NRAGE, a p75 neurotrophin receptor-interacting protein, induces caspase activation and cell death through a JNK-dependent mitochondrial pathway. J Biol Chem. 2002;277(50):48043–48050. doi: 10.1074/jbc.M205324200. [DOI] [PubMed] [Google Scholar]
- 37.Bhakar AL, Howell JL, Paul CE, et al. Apoptosis induced by p75NTR overexpression requires Jun kinase- dependent phosphorylation of Bad. J Neurosci. 2003;23(36):11373–11381. doi: 10.1523/JNEUROSCI.23-36-11373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aleman CL, Mas R, Hernandez C, et al. A 12-month study of policosanol oral toxicity in Sprague Dawley rats. Toxicol Lett. 1994;70(1):77–87. doi: 10.1016/0378-4274(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez L, Mas R, Illnait J, et al. Policosanol: results of a postmarketing surveillance study of 27,879 patients. Curr Ther Res Clin Exp. 1998;59(10):717–722. [Google Scholar]
- 40.Gomes MZ, Raisman-Vozari R, Del Bel EA. A nitric oxide synthase inhibitor decreases 6-hydroxydopamine effects on tyrosine hydroxylase and neuronal nitric oxide synthase in the rat nigrostriatal pathway. Brain Res. 2008;1203:160–169. doi: 10.1016/j.brainres.2008.01.088. [DOI] [PubMed] [Google Scholar]
- 41.Paxinos G, Franklin K. 2nd ed. San Diego: Academic Press; 2001. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]


