Abstract
Suspended moxibustion can decrease the expression of prokineticin 1 and its receptor in colonic tissue from rats modeling chronic visceral hyperalgesia. This study aimed to verify if rat spinal cord prokineticin 1 and its receptor contribute to the analgesic effect of suspended moxibustion in a rat model of irritable bowel syndrome where rats display chronic visceral hypersensitivity. Results showed that suspended moxibustion at Tianshu (ST25) point significantly decreased visceral sensitivity to colorectal distention in a chronic visceral hyperalgesia rat model; also protein and mRNA expression of prokineticin 1 and prokineticin receptor 1 in the spinal cord of rats was significantly decreased. Experimental findings indicate that prokineticin 1 and prokineticin receptor 1 are involved in the analgesia using suspended moxibustion in rats with chronic visceral hyperalgesia.
Keywords: suspended moxibustion, chronic visceral hypersensitivity, irritable bowel syndrome, prokineticin 1, prokineticin receptor 1, spinal cord, neural regeneration
Abbreviations
CVH, chronic visceral hypersensitivity; PK1, prokineticin 1; PKR1, prokineticin receptor 1; AWR, abdominal withdrawal reflex
INTRODUCTION
Irritable bowel syndrome is one of the most common intermittent or durative functional gastrointestinal disorders characterized by abdominal discomfort or pain and altered bowel habits[1]. Several studies have suggested that an abnormally enhanced perception of visceral stimulation is an important biological marker of irritable bowel syndrome[2,3,4]. The clinical management of visceral hypersensitivity is typically poor and drugs that are used with some efficacy to treat somatic pain often present unwanted effects on the viscera[1]. Some patients therefore turn to alternative treatment modalities, which many find beneficial[5,6].
Moxibustion involves warm stimulation by moxa combustion at acupoint areas. This form of acupuncture is also a therapy for treating certain disorders in the clinic[7,8,9,10]. Moxibustion includes suspended moxibustion (also named mild moxibustion), scarring moxibustion and herb-partition moxibustion. Previous studies conducted by our research team have indicated that moxibustion has a beneficial effect on irritable bowel syndrome[11]. However, the mechanism of how moxibustion, especially suspended moxibustion, produces its effects has not been clearly elucidated. Visceral sensory nerves have been reported to be closely associated with spinal cord fragments[12,13]. Suspended moxibustion at Tianshu (ST25) has been shown to decrease chronic visceral hypersensitivity (CVH) by increasing the concentrations of dynorphin and endomorphin in the spinal cord of rats used in a model of irritable bowel syndrome induced by mechanical colorectal irritation in the postnatal period[14]. The potential mechanism of spinal cord contribution to the effect of moxibustion therapy merits further study.
Prokineticin 1 (PK1), a new member of the AVIT protein family identified in mammals, was originally thought to regulate intestinal motility, specifically contracting guinea pig longitudinal muscle, fundic muscle and proximal colon. A number of studies indicated that PK1 and its receptor, prokineticin receptor 1 (PKR1) not only participated in regulating multiple gastrointestinal functions, but also mediated the transmission of pain signaling[15,16]. In the colitis rat model mediated by mustard oil, the expression level of PKR1 mRNA obviously increased, indicating that PKR1 was associated with inflammatory pain and was involved in the regulation of acute harmful stimulation and inflammatory pain[17]. Giannini et al[18] found that a PKR antagonist preferentially bound PKR1, and dose-dependently reduced, and eventually abolished, both prokineticin-induced hypernociception and inflammatory hyperalgesia. It is not known whether the analgesic effect of moxibustion is related to spinal cord PK1 and PKR1 of rats with irritable bowel syndrome. Therefore this study aimed to verify whether suspended moxibustion could decrease PK1 and PKR1 expression in the spinal cord of the CVH model rats.
A rat model of CVH was, therefore, established using colorectal distention in the postnatal period[19], and abdominal withdrawal reflex (AWR) scoring was adopted for behavioral assessment in the evaluation of hypersensitivity after moxibustion intervention. We found that suspended moxibustion can relieve CVH and decrease the expression of PK1 and PKR1 in colonic tissue from CVH model rats.
RESULTS
Quantitative analysis of experimental animals
A total of 24 Sprague-Dawley rats were equally and randomly assigned to three groups, namely normal, model (CVH model) and suspended moxibustion (CVH model + suspended moxibustion treatment). Twenty-two rats were included in the final analysis. Two rats were not included. One rat died from narcotic overdose in the normal group and the other, in the model group, died from bowel perforation.
Suspended moxibustion reduced AWR scores in CVH rats
AWR scores obtained on colorectal distention stimulation (2.66, 5.32, 7.98, 10.64 kPa) for the evaluation of visceral hyperalgesia are shown in Figure 1. AWR scores were significantly higher in the model group compared to the normal group (P < 0.01) at differing levels of colorectal distention. AWR was also significantly decreased in the suspended moxibustion group compared with the model group (P < 0.01).
Figure 1.
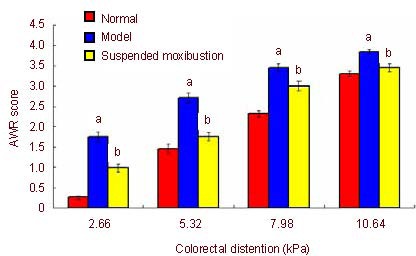
Abdominal withdrawal reflex (AWR) scores following colorectal distention stimulation in chronic visceral hypersensitivity rats.
Data are expressed as mean ± SD (seven rats in the normal and model groups and 8 rats in the suspended moxibustion group). aP < 0.01, vs. normal group; bP < 0.01, vs. model group (analysis of variance). AWR score ranged from 1 to 4, a higher score signifies worse pain.
Suspended moxibustion decreased PK1 and PKR1 expression in the spinal cord of CVH rats
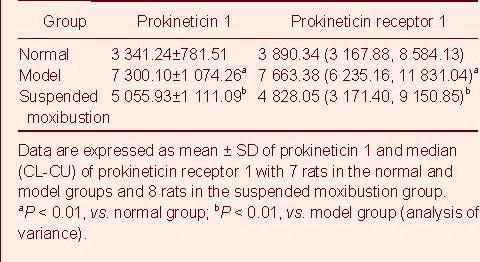
Immunohistochemistry revealed that the expression level of PK1 and PKR1 was significantly higher in the model group than the normal group (P < 0.01). PK1 and PKR1 expression was significantly decreased in the suspended moxibustion group compared with the model group (P < 0.01; Figure 2, Table 1).
Figure 2.
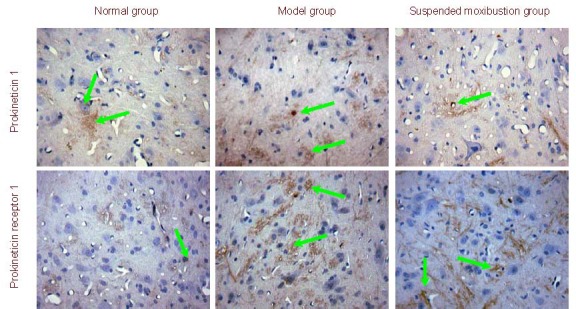
Prokineticin 1 and prokineticin receptor 1 expression in spinal cord from chronic visceral hypersensitivity rats (immunohistochemistry, × 400). Prokineticin 1 and prokineticin receptor 1 positive cells (stained yellow; arrows) were visible in each group. The expression of positive products was increased in the model group showing dark staining, while staining was light and attenuated in the suspended moxibustion and normal groups.
Table 1.
Prokineticin 1 and prokineticin receptor 1 expression (absorbance; immunohistochemistry) in the spinal cord of rats with chronic visceral hypersensitivity
Suspended moxibustion downregulated PK1 and PKR1 mRNA expression in the spinal cord of CVH rats
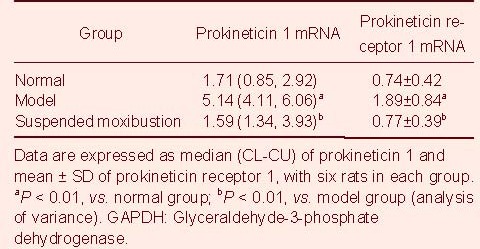
Fluorescent quantitative PCR results showed that the relative expression level of PK1 and PKR1 mRNA was significantly higher in the model group than that of the normal group (P < 0.01). After suspended moxibustion, the relative PK1 and PKR1 mRNA expression level was significantly decreased compared with the model group (P < 0.01; Table 2).
Table 2.
Prokineticin 1 and prokineticin receptor 1 mRNA expression (ratio to GAPDH; fluorescent+quantitative PCR assay) in the spinal cord of rats with chronic visceral hypersensitivity
DISCUSSION
Studies have shown that 90.7% of irritable bowel syndrome patients presented with abdominal pain when the pressure of colorectal distention reached 7.98 kPa[2]. The severity of irritable bowel syndrome symptoms was closely related to threshold, with no obvious differences among the subtypes of irritable bowel disease[20]. In the present study, under different colonic distension pressures (2.66, 5.32, 7.98, 10.64 kPa), AWR scores markedly increased in the model group compared with those in the normal group. After being treated by suspended moxibustion, the abnormally increased AWR scores in irritable bowel syndrome rats obviously decreased, which indicated that suspended moxibustion at Tianshu may have an analgesic effect in CVH rats, which is in accordance with our previous studies[14,21].
It is known that the spinal cord is important for transmitting sensation and motor neuron impulses. Nerve impulses transmit into the dorsal root ganglion, which in turn transmits to the brain through the spinal cord. Neurophysiology of somatesthesia and visceral sensation state that dorsal horn neurons interact with the peripheral tissues or descending system of the brain stem when the dorsal horn neurons are over-excited. This mechanism plays a crucial role in the occurrence and development of gastrointestinal chronic hyperalgesia in humans[22]. Research studying the harmful exion reflex in irritable bowel syndrome patients has demonstrated that hyper-excitability existed in the spinal cord of this population[23].
PK1 and PKR1 are new members of a peptide family newly identified in mammals, which have been proven to be closely related with transmission of pain signals[15,16]. PK receptors include two subtypes in mammals, PKR1 and PKR2. PKR1 expression in the central nervous system is eight times higher than PKR2. This peptide is widely expressed in the brain, spinal cord, dorsal root ganglion and enteric plexus[16,24,25]. PKR1 signaling was reported to be a requirement associated with activation and sensitization of primary afferent fibers[15]. Blockade of PKR1 may present a novel strategy which can diminish the activation and sensitization of primary afferent nociceptors. In fact, rats lacking the PKR1 gene developed deficient responses to noxious heat, capsaicin and protons compared with wild-type littermates[16].
In the present study, PK1 and PKR1 showed low expression in neurons of the spinal cord in the normal group. Expression sporadically appeared in large, medium, and small neurons, vascular endothelial cell and interstitial substances. PK1 and PKR1 mRNA expression was also low. Expression of PK1 and PKR1 was obviously increased in the model group. The relative expression of PK1 and PKR1 mRNA in the spinal cord was also increased. These findings demonstrate that the increased expression of PK1 and PKR1 in the spinal cord may be related to abdominal pain in a rat model of irritable bowel syndrome. In the suspended moxibustion group, there was a significant decrease in the positive expression of PK1 and PKR1. PK1 and PKR1 mRNA expression was also decreased. These observations indicated that suspended moxibustion may diminish the abnormally increased expression of PK1/PKR1 and their mRNA in the spinal cord of irritable bowel syndrome rats.
In conclusion, suspended moxibustion at Tianshu increased pain thresholds in a rat model of irritable bowel syndrome and decreased the expression of PK1 and PKR1 in the spinal cord. We suggest that suspended moxibustion exerts its effect on this model by reducing the abnormal increased transcription of the PK1/PKR1 gene and protein expression in the spinal cord.
MATERIALS AND METHODS
Design
A randomized, controlled, animal study.
Time and setting
This experiment was performed at the Open Animal Laboratory, Shanghai Medical College, Fudan University, from October to December in 2009.
Materials
A total of 24 neonatal male Sprague-Dawley rats (5 days old) were obtained from the Department of Experimental Animal Science of Shanghai Medical College, Fudan University, China (license No. SCXK (Hu) 2009-0019). Eight rats were housed with a nursing adult female rat in a cage at room temperature (22 ± 2°C) and controlled humidity (60 ± 10%) in a 12-hour day-light cycle. Studies were performed in strict accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[26].
Methods
Establishment of the CVH rat model
Neonatal rats in the model and suspended moxibustion groups received colorectal distention on day 8 after birth to assess CVH by AWR. The experimental rat model of CVH was established as previously described[19]. Neonatal rats in the normal group were treated by grabbing and massaging the perineum, while the other rats received colorectal distention stimulation. Vaseline was smeared on the surface of the balloon (Shanghai Tianfeng plastic Co., Ltd., Shanghai, China), then slowly inserted into the anus following the physiological curve of the colorectum (2 cm in depth). The balloon was then inflated with 0.5 mL of air for 1 minute and then deflated, whereupon the balloon was slowly withdrawn. Distention was repeated twice daily, following a 30-minute interval, for a period of 14 days in total.
Suspended moxibustion treatment
After distention was finished, rats were kept until they reached adulthood (42 days after birth). Suspended moxibustion was performed using smoldered moxibustion sticks (Nanyang Hanyi Moxa Co., Ltd., Nanyang, Henan province, China; 0.5 cm in diameter and 0.3 cm high), 2 cm above the Tianshu (located on a horizontal line 5 cun above the symphysis pubis and 2 cun laterally to the midline) for 10 minutes, once daily, 7 times in total[21]. Rats in the normal group were used as controls and those in the model group were used for comparison with the suspended moxibustion group.
AWR scores
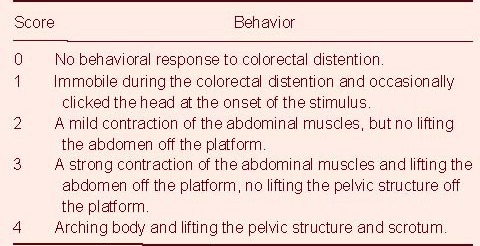
After seven treatments, AWR was assessed within 90 minutes after colorectal distention based on semi-quantitative analysis. Prior to colorectal distention, the rats were gently touched around the anus to activate defecation. When the balloon was inserted into the descending colon, colorectal distention was produced by rapidly inflating the balloon at strengths of 2.66, 5.32, 7.98, 10.64 kPa for a period of 20 seconds. Each score was tested five times, and each rat was tested by two different people, who were not involved in this research project. There were 4-minute intervals between the two tests, to allow the rats to adapt. The scoring criteria of AWR were obtained from the method used by AL-chaer et al[19] (Table 3).
Table 3.
Abdominal withdrawal reflex scoring criteria
Preparation of spinal cord specimens
Animals were sacrificed after AWR assessment by intraperitoneal anesthesia using 3% pentobarbital sodium (0.1 mL/100 g). Spinal cord (T6-L5) segments of rats from every group were carefully isolated. Half segments were fixed with paraformaldehyde, while half parts from 6 rats in every group were immediately frozen in a -80°C refrigerator.
Immunohistochemistry for the expression of PK1 and PKR1
Paraffin sections of spinal cord were routinely deparaffinized in water. After hotfixing the antigen under room temperature using 3% H2O2, slices were immerged into citrate buffer solution (pH 6.0), then diluted primary antibody in 5% bovine serum albumin was added dropwise to the sections, incubated at room temperature for 20 minutes (mouse anti-rat PK1 monoclonal antibody 1:400, rabbit anti-rat PKR1 monoclonal antibody 1:100; Abcam Co., Cambridge, UK), then preserved overnight. After incubation for 2 hours at 37°C, corresponding secondary antibody was added to the sections (PK1: biotin goat anti-mouse IgG; PKR1: biotin goat anti-rabbit IgG; 1:100; Wuhan Boster Bio-Engineering Co., Ltd., Wuhan, China), and incubated at 37°C for 20 minutes. Staining was then developed using DAB after incubation with streptavidin-biotin complex (Wuhan Boster Bio-Engineering Co., Ltd.) for 20 minutes at 37°C, counterstained with hematoxylin for 1 minute, and observed under the light microscope (H2; Olympus, Tokyo, Japan).
All samples were analyzed using the Motic Med 6.0 image analysis system (Motic Group Co., Ltd., Xiamen, China). Brown and dark brown granulation was observed with a background of purple blue. The integrated absorbance of PK and PKR1 under three fields was averaged.
Fluorescent quantitative PCR assay for PK1 and PKR1 mRNA expression
Total RNA was extracted according to the instructions provided by Trizol. Reverse transcribing cDNA: 5 × reverse transcription buffer solution 4 μL, oligo(dT) 0.5 μL, dNTPs 0.5 μL, reverse transcriptase MMLV 1 μL, DECP-treated water 10 μL, and RNA template 4 μL were maintained at 37°C for 1 hour, heated to 95°C for 5 minutes to deactivate MMLV. Reverse transcription- PCR: PCR augmentation was conducted using prepared cDNA, 5× PCR buffer 10 μL, forward primer (Da’an Bio-Technology Co,. Ltd., Qingdao, China) 0.5 μL, the reverse primer 0.5 μL, dNTPs 0.5 μL, TaqMan fluorescent probe (Da’an Bio-Technology Co,. Ltd.) 0.5 μL, Taq enzyme 1 μL, ddH2O 32 μL, and cDNA template 5 μL. The solution was then subjected to 40 cycles at 50°C for 2 minutes, 95°C for 5 minutes, 95°C for 15 seconds, 60°C for 45 seconds. The sequence of the PK1 probe (150 bp) was 5’-CCG CTC TGT GGA CCG CCT CAA GGA-3’; forward primer, 5’-GGA TGT TGG CGA TGG ATT CTG-3’; reverse primer, 5’-CTC GTA GCA CCA GCA TCA TTG-3’. The sequence of the PKR1 probe (149 bp) was 5’-TCT CTA TGT CTC CAC CAA TGC-3’; forward primer, 5’-AGG CCA GTG GCT GTT TGG CA-3’; reverse primer, 5’-GGC CAC GTC CTG TGC ACC TC-3’. The mRNA level was analyzed using ABI Prism 7500 SDS Software (ABI Co., Foster City, CA, USA). The relative expression value of mRNA = 2-ΔCT × 100%; in which ΔCT = CT value of the target gene – CT value of GAPDH.
Statistical analysis
Data were expressed as mean ± SD for normally distributed continuous variables and Median (CL-CU) for non-normal variables. Statistical analyses were performed using SPSS 10.0 (SPSS, Chicago, IL, USA). Differences in mean were compared by one-way analysis of variance. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This work was supported by the National Basic Research Program of China (973 Program) (No. 2009CB522900), Nanjing University of Chinese Medicine, Key Laboratory of Acupuncture Combined with Medication (No. KJA200914) and the National Natural Science Foundation of China (No. 30973783).
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Review Committees of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine affiliated to Shanghai University of Traditional Chinese Medicine in China.
(Edited by Wei JZ, Yang LP/Yang Y/Wang L)
REFERENCES
- 1.Robinson DR, Gebhart GF. Inside information: the unique features of visceral sensation. Mol Interv. 2008;8(5):242–253. doi: 10.1124/mi.8.5.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouin M, Plourde V, Boivin M, et al. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122(7):1771–1777. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- 3.Mertz H, Naliboff B, Munakata J, et al. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109(1):40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 4.Naliboff BD, Munakata J, Fullerton S, et al. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41(4):505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in the irritable bowel syndrome. Arch Intern Med. 2003;163(3):265–274. doi: 10.1001/archinte.163.3.265. [DOI] [PubMed] [Google Scholar]
- 6.Giese LA. A study of alternative health care use for gastrointestinal disorders. Gastroenterol Nurs. 2000;23(1):19–27. doi: 10.1097/00001610-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Lim B, Manheimer E, Lao L, et al. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD005111.pub2. CD005111. [DOI] [PubMed] [Google Scholar]
- 8.Joos S, Wildau N, Kohnen R, et al. Acupuncture and moxibustion in the treatment of ulcerative colitis: a randomized controlled study. Scand J Gastroenterol. 2006;41(9):1056–1063. doi: 10.1080/00365520600580688. [DOI] [PubMed] [Google Scholar]
- 9.Joos S, Brinkhaus B, Maluche C, et al. Acupuncture and moxibustion in the treatment of active Crohn's disease: a randomized controlled study. Digestion. 2004;69(3):131–139. doi: 10.1159/000078151. [DOI] [PubMed] [Google Scholar]
- 10.Ouyang H, Chen JD. Review article: therapeutic roles of acupuncture in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20(8):831–841. doi: 10.1111/j.1365-2036.2004.02196.x. [DOI] [PubMed] [Google Scholar]
- 11.Wu HG, Shi Y, Zhang W, et al. Advances and thinking about prevention and treatment of inflammatory bowel diseases by acupuncture and moxibustion. Zhongguo Zhenjiu. 2006;26(6):454–458. [PubMed] [Google Scholar]
- 12.Hwang SL, Lin CL, Lieu AS, et al. Punctate midline myelotomy for intractable visceral pain caused by hepatobiliary or pancreatic cancer. J Pain Symptom Manage. 2004;27(1):79–84. doi: 10.1016/j.jpainsymman.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Honda CN. Visceral and somatic afferent convergence onto neurons near the central canal in the sacral spinal cord of the cat. J Neurophysiol. 1985;53(4):1059–1078. doi: 10.1152/jn.1985.53.4.1059. [DOI] [PubMed] [Google Scholar]
- 14.Liu HR, Qi L, Wu LY, et al. Effects of moxibustion on dynorphin and endomorphin in rats with chronic visceral hyperalgesia. World J Gastroenterol. 2010;16(32):4079–4083. doi: 10.3748/wjg.v16.i32.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negri L, Lattanzi R, Giannini E, et al. Modulators of pain: Bv8 and Prokineticins. Curr Neuropharmacol. 2006;4(3):207–215. doi: 10.2174/157015906778019518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negri L, Lattanzi R, Giannini E, et al. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: focus on interaction between PKR1 and thecapsaicin receptor TRPV1 in pain behavior. J Neurosci. 2006;26(25):6716–6727. doi: 10.1523/JNEUROSCI.5403-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimball ES, Prouty SP, Pavlick KP, et al. Stimulation of neuronal receptors, neuropeptides and cytokines during experimental oil of mustard colitis. Neurogastroenterol Motil. 2007;19(5):390–400. doi: 10.1111/j.1365-2982.2007.00939.x. [DOI] [PubMed] [Google Scholar]
- 18.Giannini E, Lattanzi R, Nicotra A, et al. The chemokine Bv8/prokineticin 2 is up-regulated in inflammatory granulocytes and modulates inflammatory pain. Proc Natl Acad Sci U S A. 2009;106(34):14646–14651. doi: 10.1073/pnas.0903720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AL-chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119(5):1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 20.Kanazawa M, Palsson OS, Thiwan SI, et al. Contributions of pain sensitivity and colonic motility to IBS symptom severity and predominant bowel habits. Am J Gastroenterol. 2008;103(10):2550–2561. doi: 10.1111/j.1572-0241.2008.02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou EH, Liu HR, Wu HG, et al. Suspended moxibustion relieves chronic visceral hyperalgesia via serotonin pathway in the colon. Neurosci Lett. 2009;451(2):144–147. doi: 10.1016/j.neulet.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 22.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107(1):271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 23.Coffin B, Bouhassira D, Sabate JM, et al. Alteration of the spinal modulation of nociceptive processing in patients with irritable bowel syndrome. Gut. 2004;53(10):1465–1470. doi: 10.1136/gut.2003.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soga T, Matsumoto S, Oda T, et al. Molecular cloning and characterization of prokineticin receptors. Biochimicaet Biophysica Acta. 2002;1579(2-3):173–179. doi: 10.1016/s0167-4781(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 25.Negri L, Lattanzi R, Giannini E, et al. Nociceptive sensitization by the secretory protein Bv8. Br J Pharmacol. 2002;137(8):1147–1154. doi: 10.1038/sj.bjp.0704995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]


