Abstract
In this study, stroke patients received constraint-induced movement therapy for 3 weeks. Before and after constraint-induced movement therapy, the flexibility of their upper limbs on the affected side was assessed using the Wolf motor function test, and daily use of their affected limbs was assessed using the movement activities log, and cerebral functional reorganization was assessed by functional magnetic resonance imaging. The Wolf motor function test score and the movement activities log quantity and quality scores were significantly increased, while action performance time in the Wolf motor function test was significantly decreased after constraint-induced movement therapy. By functional magnetic resonance imaging examination, only scattered activation points were visible on the affected side before therapy. In contrast, the volume of the activated area was increased after therapy. The activation volume in the sensorimotor area was significantly different before and after therapy, and the activation area increased and appeared adjusted. In addition to the activated area around the lesions being decreased, there were also some new activated areas, including the supplementary movement area, premotor area and the ipsilateral sensorimotor area. Our findings indicate that constraint-induced movement therapy significantly improves the movement ability and daily use of the affected upper limbs in stroke patients and promotes cerebral functional reorganization.
Keywords: cerebral stroke, constraint-induced movement, functional magnetic resonance imaging, cerebral functional reorganization, rehabilitation, motor function of upper limbs, neural regeneration
Abbreviations
fMRI, functional magnetic resonance imaging; CIMT, constraint-induced movement therapy; WMFT, Wolf motor function test; MAL, movement activities log
INTRODUCTION
Stroke is a leading cause of acquired adult hemiplegia[1]. In general, recovery of upper limb function is difficult in patients with stroke[2]. Constraint-induced movement therapy (CIMT) is an effective rehabilitation treatment for upper extremity impairment in hemiparetic patients after stroke. In this study, we aimed to explore the clinical effects and possible mechanisms underlying CIMT for the rehabilitation of upper limb function in patients with stroke.
In the 1980s, CIMT was used for chronic hemiplegic patients after cerebral stroke, and significant improvements in limb function were observed[3,4,5]. CIMT trains the affected upper extremity in stages while constraining the less affected upper extremity. Previous studies showed that CIMT could significantly improve the flexibility of the upper limb after stroke. Recently, dramatic advances have been made in CIMT. Specifically, CIMT has been successfully used in various neural rehabilitation approaches, and has consequently garnered increasing attention[6,7,8,9,10].
The efficacy of CIMT may be due to its ability to overcome “learned non-use”[11,12,13,14], and the underlying mechanism appears to involve cortical reorganization[15,16]. CIMT studies often examine the interaction between neural plasticity and behavior. Investigating neural plasticity and reorganization of brain function during CIMT may provide a rational basis for the development and implementation of optimal methods for functional recovery. Modern technologies, such as transcranial magnetic stimulation, positron emission tomography, multiple-exposure photography, magnetic encephalography and functional magnetic resonance imaging (fMRI), provide advanced tools for examining functional reorganization after central nervous system injury[17].
In recent years, fMRI has been widely used as an economical, convenient and non-invasive means to examine cerebral function. fMRI, also called blood oxygen level-dependent fMRI, is based on blood oxygen levels[18]. The gradient echo MRI technique enhances contrast further to better reflect blood oxygen levels in the cerebral microvasculature.
CIMT is a relatively new technique for improving upper limb function after stroke, and the mechanisms underlying CIMT-mediated functional recovery remain unclear, particularly as only a few studies have provided an objective assessment. Therefore, in this study, using fMRI, we investigated cerebral functional reorganization to elucidate the mechanisms underlying the recovery of motor function in stroke patients after CIMT. We employed a prospective self-control design and applied quantitative analytical methods for analyzing fMRI data. This study should provide substantial insight into cerebral functional reorganization and further our understanding of the mechanisms underlying the efficacy of CIMT for the treatment of limb impairment following stroke.
RESULTS
Quantitative analysis of participants
A total of 15 stroke patients were enrolled in this study for CIMT treatment, who were hospitalized at the Neurology Department of Beijing Boai Hospital, China Rehabilitation Research Center, between July 2009 and December 2010. Three patients were excluded for failing to undergo full evaluation after treatment. The other 12 stroke patients completed the full treatment and underwent scale measurements, and received fMRI scans before and after CIMT. These twelve stroke patients were included in the final analysis.
Baseline data of participants
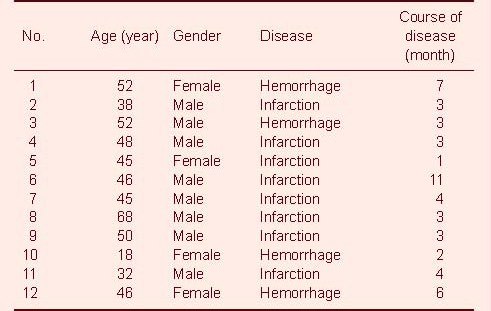
Each of the 12 stroke patients were right-handed and suffered stroke within 180 days from onset, including eight patients with cerebral infarction and four patients with cerebral hemorrhage. There were eight males and four females, with a mean age of 45 ± 12 years (range of 18-68 years), and the mean course of disease was 4.17 ± 2.69 months (Table 1).
Table 1.
Baseline data of participants
Motor function and daily use of affected upper limbs in stroke patients before and after CIMT
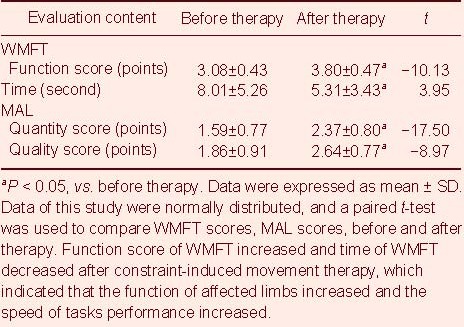
As shown in Table 2, the flexibility of the upper limbs was significantly improved after 3 weeks of CIMT. Using the Wolf motor function test (WMFT), the motor function score was increased while the motor time score was decreased (P < 0.01). Based on the results of the movement activities log (MAL), daily use of affected limbs was significantly improved after 3 weeks of CIMT, both quantitatively and qualitatively (P < 0.01).
Table 2.
Wolf motor function test (WMFT) and movement activities log (MAL) results before and after constraint-induced movement therapy
fMRI results before and after CIMT
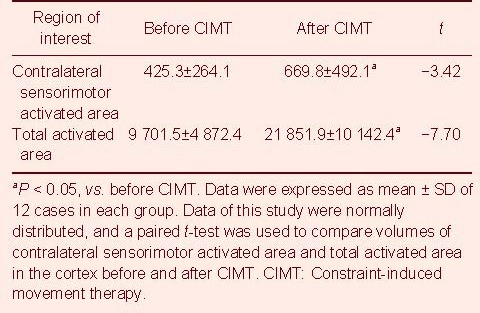
Image processing and statistical analysis was conducted using AFNI software. The volume of the activated area was calculated when the correlation coefficient of the activation signal was 0.8 and the single activated area volume was 70.3 mm3. During finger-tapping movement of the affected hand, only scattered activation points were present on the focal side before CIMT. The volume of the activated area increased after CIMT: The volume of the activated area in the contralateral sensorimotor area (lesion side) was 425.31 ± 264.09 mm3 and 669.77 ± 492.08 mm3 before and after CIMT, respectively, which approached significance (P < 0.01). The total activated area of the brain was 9 701.47 ± 4 872.36 mm3 and 21 851.92 ± 10 142.39 mm3 before and after CIMT, respectively, which approached significance (P < 0.01; Table 3).
Table 3.
Results for the contralateral sensorimotor activated area and total activated area of the cortex before and after CIMT (mm3)
In addition to the volume increase, there were a number of new areas activated after CIMT. Of the twelve patients, 4 cases exhibited activation of the contralateral primary motor area (M1) of the cortex in the hemiplegic side before CIMT, while 7 cases had activation of the contralateral M1 and sensorimotor areas after CIMT. Other common activated areas included the contralateral supplementary movement area, premotor area, top occipital lobe, and ipsilateral M1 and premotor areas (Figures 1, 2). The fMRI results demonstrate that CIMT induced compensatory changes, including functional reorganization of other cortical regions.
Figure 1.
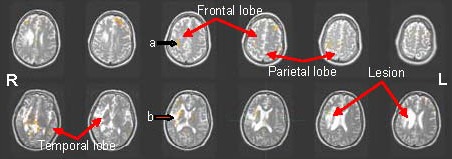
Functional magnetic resonance imaging scan showing the activated brain regions of a patient with left hemiplegia before treatment. R: Right; L: left.
Before treatment, during finger-tapping movement of the affected left hand, only a few scattered brain activation points were visible on the right sensorimotor area and around the right lesion. The lobes are indicated in the figures.
a: Motor area and inner activated area on the right hemisphere. b: Scattered activation points surrounding the right lesion.
Figure 2.
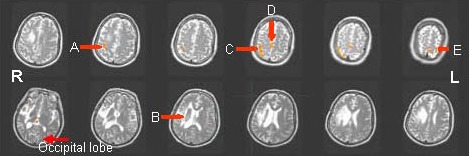
Functional magnetic resonance imaging scan showing the activated brain regions in a patient with left hemiplegia after treatment. R: Right; L: left.
After treatment, more numerous and larger activated regions in the right sensorimotor area, supplementary motor area (middle arrow shows) and the left sensorimotor area (ipsilateral hemiplegia) are observed.
A: Motor area and inner activated area in the right hemisphere. B: Activation points around the right lesion disappear and are adjusted. C: Right sensorimotor area activated after treatment. D: Supplementary motor area activated after treatment. E: Left sensorimotor area (ipsilateral hemiplegia) activated after treatment.
DISCUSSION
Impressive differences were noted after CIMT treatment, irrespective of WMFT scores or the time required to complete the action, which indicates that CIMT can improve the flexibility of the upper limb. Significant improvements of movement quality and speed of movement of the affected limbs after the 3-week CIMT period are based on the principle of centralized repeated enhanced training. As shown by a randomized controlled trial, CIMT was superior to hands training, which is a traditional rehabilitation method[19].
The MAL scale was administered to assess the patients’ subjective impression of movement quantity and quality of the affected limbs within a specific period. Importantly, MAL shifts the emphasis from laboratory conditions to daily living. As shown by the MAL results, CIMT significantly reversed the habit of not using the affected hands, and significantly improved the quantity and quality of affected upper limb movement in daily life. Thus, progress made in the laboratory was successfully transferred to the household setting, which is a CIMT objective. As shown by other studies[3,4], this progress can be maintained until the follow-up period, and can persist at least one year after treatment.
The findings of this study show that CIMT not only improves motor function after damage, but also promotes brain reorganization, as shown by the fMRI results. Cortical functional reorganization was displayed on fMRI as expansion of the original volume of activation, emergence of new regions of activation and re-adjustment of the original pattern of activation.
First, fMRI imaging in this study showed decreased activation of the contralateral hemisphere, which controls the affected limb, in most patients before treatment, with only a few scattered regions activated during finger-tapping movement. A possible cause may be the blocking of efferent pathways. The area of the contralateral hemisphere activated prior to treatment was expanded after treatment. Specifically, the volume of the contralateral sensorimotor area increased significantly compared with the volume before treatment (r = 0.8 and minimum activation area = 141 mm3 [2 pixels] calculated by AFNI software). The blockade of efferent sensorimotor pathways, reduced motor cortex excitability, as well as the diminished cortical area controlling the affected limb, may, at least in part, have been due to limited use of the hemiplegic limb, or it may have been caused by the brain damage itself[20,21]. Through repeated intensive training and feedback from the affected limbs, neuronal excitability, metabolism, blood flow and motor cortical oxygen content increased. In the fMRI scans, an expanded active area and cortical functional compensation was visible in the corresponding brain areas, as well as in nearby areas[20].
Second, the volume of the contralateral sensorimotor area, reflected in both mean and maximum volume, was significantly enhanced after CIMT. In 10 out of 12 subjects, the mean volume of the activated contralateral sensorimotor area (669.77 mm3) was greater than that of other activated areas, suggesting that the sensorimotor area on the lesion side continued to exert primary control of the affected upper limb. Kim et al[21] demonstrated that the recovery of motor function at the subacute stage is mainly due to activation of the contralateral sensorimotor area, while at the chronic stage, recovery mainly involves the sensorimotor region on the side of the lesion. Their results were slightly different from our findings, which may be due to the different intervention techniques used. The centralized enhanced training in CIMT, which promotes excitability of the cerebral cortex, likely improves excitability of the sensorimotor area on the lesion side.
Third, the emergence of compensatory activation was observed. Most of the newly active areas were located in the premotor area of the lesion side (7/12), supplementary motor area (8/12), primary somatosensory area, the rear of the parietal cortex, around the lesion and ipsilateral sensorimotor area (6/12) after CIMT. The common areas of activation and compensation were in the premotor area of the lesion side, supplementary motor area and the ipsilateral sensorimotor area, which were the main regions undergoing compensatory changes after CIMT. Research has shown that the supplementary motor area and the premotor area participate in the planning and execution of various activities[22]. Through repeated intensive training and feedback from the affected limb, CIMT may improve the excitability of cortical neurons which control the affected hand. CIMT may also induce the formation of new circuits and promote compensatory activation (as revealed by fMRI).
We found that in most patients with hemiplegia, both the contralateral and ipsilateral sensorimotor areas were activated. This demonstrates that after CIMT, motor cortical representation of the ipsilateral upper extremity, originally inhibited, was activated, thereby compensating for the damage caused to the efferent pathways originally controlling movement. Indeed, previous research has shown that an important mechanism of motor functional recovery after cerebral injury involves activation of the sensorimotor cortex ipsilateral to the affected upper limb[22].
Fourthly, readjustment of the original activation area was observed. The emergence of new compensatory activation after CIMT was found by fMRI examination, which was also observed in previous research[17]. Readjustment of the original activation pattern was found. In three subjects, scattered activation points around foci visible before CIMT disappeared after CIMT. Compensatory activation occurred in the supplementary movement area, premotor area and ipsilateral sensorimotor area of the affected upper extremity. While the mechanism of readjustment of the activation area is unknown[22], it may involve redistribution of blood flow, enhanced metabolism in the compensatory areas, as well as decreased metabolism around foci.
In summary, we studied the efficacy of CIMT for upper limb motor dysfunction, as well as the associated brain functional reorganization. This preliminary study revealed that CIMT could significantly improve motor function and daily use of the upper limbs, and promote compensatory cortical functional reorganization, which is the neurophysiological basis for motor function improvement in patients with cerebral injury.
The limitations of our study include small sample size and the fact that patients were in the early phase of recovery after stroke. Despite these shortcomings, we were able to observe significant cortical functional changes induced by CIMT. Consequently, we consider our results even more remarkable and clinically relevant.
SUBJECTS AND METHODS
Design
A self-control study.
Time and setting
This study was performed at the Department of Neurology, Beijing Boai Hospital, China Rehabilitation Research Center in China between July 2008 and December 2010.
Subjects
Diagnostic criteria
All stroke patients were diagnosed according to the diagnostic criteria of Cerebrovascular Disease published by the Chinese Neurosurgery Association, Chinese Thoracic Surgery Association in 1996. Diagnostic procedures upon study entry included medical history, general physical examination, neurological examination, CT or MRI scans. Subarachnoid hemorrhage and transient ischemic attack were not included.
A total of 12 right-handed stroke patients were enrolled, who were hospitalized at the Neural Rehabilitation Department of China Rehabilitation Research Center between July 2009 and December 2010.
Inclusion criteria
Patients, aged 18-80 years, suffering from subacute stroke were included in the study if the following criteria were met: (1) improvement of the affected arm—both interphalangeal and metacarpophalangeal joints can be extended by at least 10°, and the wrist joint can be extended by at least 20°. (2) no severe balance or walking disorder. (3) being able to care for oneself independently (e.g., meals, bathroom use and walking). (4) no clear sign of dementia (MMSE > 22 points), mental disorder or aphasia, which may hinder the patient's ability to undergo examination or therapy. (5) lack of excessive pain, spasticity, ataxia or frailty, as determined by clinical judgment, and (6) the absence of severe end-stage or uncontrolled medical conditions. In addition, all participants were required to desire better recovery and have access to adequate family support. All participants were informed of the program and associated risks before and after the experiment, and signed informed consent was obtained[23].
Exclusion criteria
Participants were excluded if they had any of the following: evidence of significant medical disease (e.g., cancer, cardiovascular disease, diabetes or hepatic, renal, cardiac or pulmonary disorders), on blood pressure lowering medication or the presence of uncontrolled hypertension, visual or hearing disabilities, or other relevant health conditions that could affect cognition or hinder the administration of neuropsychological tests.
Methods
Modified CIMT program
Subjects were treated with the modified CIMT program, which was established by Taub et al[5,11]. In brief, the use of healthy hands and upper limbs were restrained through hand orthosis at rest position and the use of a suspender, respectively. These mandatory devices were required to be used 8 hours per day for 3 weeks, and could be removed when the activities impaired balance, safety, bathing, self-relieving or sleep. In contrast, the affected upper limb was intensively trained using the shaping technique. Intensive training was required for 5 hours per day, 5 days a week, for 3 weeks.
WMFT and MAL before and after CIMT
WMFT and MAL were performed by a researcher using the blinded method, to evaluate the flexibility of upper limbs and the use of upper limbs in daily life, respectively.
WMFT is a graded neurological scale for assessing the function and dexterity of the affected upper limb in patients with moderate to severe upper extremity motor deficits. It comprises 15 items, each of which tests single- or multiple-joint motions and functional tasks[24]. The WMFT function score is a mean score of a total of fifteen tasks performed with the affected arm, ranging from 0 to 5: 0, no movement; 5, normal; 3, subject finishing the task, but action is abnormal. The WMFT time is the mean time (in seconds) required to complete all fifteen tasks. A shorter time indicates more rapid completion of the task.
The MAL scale is a structured questionnaire which involves 30 activities in daily life. The MAL score ranges from 0 to 5: 0, unable to perform the activity quantitatively or qualitatively using the affected arm; 5, movement of affected limb quantitatively and qualitatively normal (i.e., similar to that prior to onset of stroke); 3, activity half completed, quantitatively and qualitatively (i.e., subject completes the task, but action abnormal).
The final measurements and the fMRI scan were performed after completion of the CIMT.
fMRI scans before and after CIMT
The fMRI sessions were conducted before and after intervention to assess recovery of brain function. All images were acquired on a GE Signa 1.5 Tesla magnetic resonance imaging scanner (Fairfield, Connecticut, USA) using Block-fMRI. Image parameters for the fMRI sequence were: echo time = 40 ms, repetition time = 3 000 ms, 64 × 64 image matrix, slice thickness = 5 mm, field of view = 24 mm × 24 mm. Pointwise landmark setting was done. Each measurement was performed with constant parameters by a neuroradiologist who was blinded to clinical information. Participants performed finger flexion/extension of the affected hand or unaffected hand at 2 Hz with six 30-second rest periods, six 24-second preparation periods and six 30-second movement periods. Slices were oriented horizontally, and they covered the cerebral hemispheres and variable portions of the superior part of the cerebellum due to variations in brain size. Image processing and analysis were performed using AFNI software, and images were reconstructed automatically. AFNI software for Linux gcc32 was downloaded from the AFNI/NIfTI Server at the NIMH (Bethesda, MD, USA).
Statistical analysis
All data were expressed as mean ± SD. All statistical analyses were performed using SPSS 10.0 software for Windows (SPSS, Chicago, IL, USA). Baseline data in this study were normally distributed, as assessed by testing, and paired t-test analysis was used to compare WMFT scores, MAL scores, activation volume, stimulation intensity and total activation volume in the sensorimotor area before and after therapy. A P value < 0.05 indicated significance.
Acknowledgments
We would like to thank the patients for their participation and Dr. Xuchu Weng and his graduate students from the Institute of Psychology, Chinese Academy of Sciences for their technical support and helpful discussions.
Footnotes
Funding: This project was funded by Capital Medical Development Foundation, No. 2009-2098.
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Ethics Committee of China Rehabilitation Research Center in China.
(Edited by Chen LJ, Wen CS/Yang Y/Wang L)
REFERENCES
- 1.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8(8):741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 2.Brunner IC, Skouen JS, Strand LI. Recovery of upper extremity motor function post stroke with regard to eligibility for constraint-induced movement therapy. Top Stroke Rehabil. 2011;18(3):248–257. doi: 10.1310/tsr1803-248. [DOI] [PubMed] [Google Scholar]
- 3.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke. JAMA. 2006;296(17):2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 4.Taub E, Uswatte G, King DK, et al. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37(4):1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- 5.Page SJ, Levine P, Leonard A, et al. Modified constraint- induced therapy in chronic stroke: Results of a single-blinded randomized controlled trial. Phys Ther. 2008;88(3):333–340. doi: 10.2522/ptj.20060029. [DOI] [PubMed] [Google Scholar]
- 6.Shi YX, Tian JH, Yang KH, et al. Modified constraint-induced movement therapy versus traditional rehabilitation in patients with upper-extremity dysfunction after stroke: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2011;92(6):972–982. doi: 10.1016/j.apmr.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 7.Sheng B, Lin M. A longitudinal study of functional magnetic resonance imaging in upper-limb hemiplegia after stroke treated with constraint-induced movement therapy. Brain Inj. 2009;23(1):65–70. doi: 10.1080/02699050802635299. [DOI] [PubMed] [Google Scholar]
- 8.Wu CY, Hsieh YW, Lin KC, et al. Brain reorganization after bilateral arm training and distributed constraint-induced therapy in stroke patients: a preliminary functional magnetic resonance imaging study. Chang Gung Med J. 2011;33(6):628–637. [PubMed] [Google Scholar]
- 9.Wang Q, Zhao JL, Zhu QX, et al. Comparison of conventional therapy, intensive therapy and modified constraint-induced movement therapy to improve upper extremity function after stroke. J Rehabil Med. 2011;43(7):619–625. doi: 10.2340/16501977-0819. [DOI] [PubMed] [Google Scholar]
- 10.Massie C, Malcolm MP, Greene D, et al. The effects of constraint-induced therapy on kinematic outcomes and compensatory movement patterns: an exploratory study. Arch Phys Med Rehabil. 2009;90(4):571–579. doi: 10.1016/j.apmr.2008.09.574. [DOI] [PubMed] [Google Scholar]
- 11.Taub E, Burgio L, Miller NE, et al. An operant approach to rehabilitation medicine:overcoming learned non-use by shaping. J Exp Anal Behav. 1994;61(2):281–293. doi: 10.1901/jeab.1994.61-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf SL, Lecraw DE, Barton LA, et al. Forced use of hemiplegic upper extremities to reverse the effect of learned non-use among chronic stroke and head-injured patients. Exp Neurol. 1989;104(2):125–132. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 13.Taub E. Somatosensory deafferentation research with monkeys: implications for rehabilitation medicine. In: Ince LP, editor. Behavioral Psychology in Rehabilitation Medicine: Clinical Applications. Baltimore: Williams and Wilkins; 1980. [Google Scholar]
- 14.Sterr A, Freivogel S, Schmalohr D. Neurobehavioral aspects of recovery: assessment of the learned non-use phenomenon in hemiparetic adolescents. Arch Phys Med Rehabil. 2002;83(12):1726–1731. doi: 10.1053/apmr.2002.35660. [DOI] [PubMed] [Google Scholar]
- 15.Liepert J, Miltner WH, Bauder H, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250(1):5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- 16.Liepert J, Bauder H, Wolfgang HR, et al. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31(6):1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 17.Lin KC, Chung HY, Wu CY, et al. Constraint-induced therapy versus control intervention in patients with stroke: a functional magnetic resonance imaging study. Am J Phys Med Rehabil. 2010;89(3):177–185. doi: 10.1097/PHM.0b013e3181cf1c78. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van der lee JH, Wagenaar RC, Lankhorst GJ, et al. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke. 1999;30(11):2369–2375. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- 20.Ward NS, Brown MM, Thompson AJ, et al. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126(11):2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YH, You SH, Kwon YH, et al. Longitudinal fMRI study for locomotor recovery in patients with stroke. Neurology. 2006;67(2):330–333. doi: 10.1212/01.wnl.0000225178.85833.0d. [DOI] [PubMed] [Google Scholar]
- 22.Ward NS, Brown MM, Thompson AJ, et al. Neural correlates of outcome after stroke:a cross-sectional fMRI study. Brain. 2003;126(Pt 6):1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.State Council of the People's Republic of China. Administrative Regulations on Medical Institution. 1994-09-01 [Google Scholar]
- 24.Hosomi M, Koyama T, Takebayashi T, et al. A modified method for constraint-induced movement therapy: a supervised self-training protocol. J Stroke Cerebrovasc Dis. 2011 doi: 10.1016/j.jstrokecerebrovasdis.2011.04.004. [DOI] [PubMed] [Google Scholar]


