Abstract
Chitosan, collagen I and gelatin were mixed in appropriate quantities to develop a new nerve repair material, with good arrangement and structure, as well as even aperture size. The composite material was sterilized by 60Co irradiation for 24 hours prior to implantation in the right thigh of rats following sciatic nerve damage. Results showed that the material was nontoxic to the kidneys and the liver, and did not induce an inflammatory response in the muscles. The composite material enhanced the recovery of sciatic nerve damage in rats. These experimental findings indicate that the composite material offers good biocompatibility and has a positive effect on injured nerve rehabilitation.
Keywords: chitosan, collagen I, gelatin, biomaterials, biocompatibility, nerve repair, neural regeneration
INTRODUCTION
Nerve damage is commonplace in clinics due to accidents and injuries[1]. However, mature nerve cells have a limited capacity for regeneration. Injured nerves will trigger various functional disturbances to the body if they are not repaired in a timely manner. An ideal nerve repair material should be biocompatible, biodegradable and enhance nerve function rehabilitation.
Chitosan is a polysaccharide with great biocompatibility and biodegradability that facilitates tissue regeneration; thus, it has been widely used for nerve repair. However, chitosan has a ’crispy’ texture that makes it difficult to suture to the host tissues, and the absorption of chitosan is slow, which affects its efficacy as a treatment strategy[2,3,4,5,6,7,8,9,10,11,12]. Gelatin, on the other hand, is a constituent of bones, tendons, and connective tissues of animals, and is widely used in clinics for skin, cartilage, bone and nerve tissue repair[13]. Gelatin is biodegradable; however, it is a brittle substance, absorbs water well and expands. The combination of gelatin and chitosan can compensate for the deficiencies in each of these two compounds. Type I collagen is the most abundant of all the collagen proteins that undergoes natural degradation in the body. Collagen has been widely used for drug delivery systems, particularly in burn repair, as it provides a supporting structure for cells. Collagen may enhance the affinity of chitosan to nerve cells[14]. Published papers have used combinations of chitosan and gelatin, chitosan and collagen, or gelatin and collagen for nerve or tissue repair[15,16,17].
However, there is currently no research using the combination of chitosan, gelatin and collagen I in nerve repair. Thus, the aim of this study is to explore the biocompatibility and nerve repair potential of a chitosan/gelatin/collagen I biomaterial.
RESULTS
Quantitative analysis of experimental animals
A total of 156 rats were included in this study: 96 rats were used to determine the biocompatibility of the composite, and 60 rats were examined for nerve repair. For each experiment, the rats were equally divided into three groups: control (uninjured), injury (injured only) and materials (injured and treated with material placement). Overall, 156 rats were involved in the results analysis.
Ultrastructure of chitosan-collagen I-gelatin composite
The structure of the nerve repair material was cylindrical and uniform, with good flexibility and strong elasticity. The microstructure of the transverse, longitudinal and diagonal sections of the sterilized material under a scanning electronic microscope showed that the outer structure of the material was fully enclosed. The inner structure showed a uniform aperture, with parallel microtubules travelling uniformly. The structure was not affected by pore size, and the trabecular wall of the pores had good continuity, smooth surfaces, and no folds; the outer surface was imbricated. The longitudinal orientation of the microtubules was independent of each other and in a closed state. There were no exchanges for the bridge connecting pipes and the overall structure was irregular, with an inconsistent diameter. The transverse sections of the material were predominantly circular, with a regular shape and uniform diameter (Figure 1). The internal structure of the material was also regular, and the ideal microstructure was conducive to the regeneration of fiber-oriented growth. The basic direction of the microtubules was parallel and independent, with a uniform diameter. These structures helped the anatomical and functional reconstruction of the nerve after injury.
Figure 1.
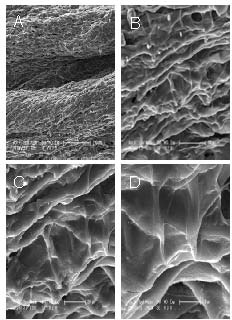
Surface sections of the repair materials under electron microscopy.
(A) Longitudinal section of the material showed an irregular shape and an inconsistent diameter (× 150).
(B) Transverse section of the material showed that the internal structure was regular (× 601).
(C) Transverse section of the material showed the trabecular walls of the pores had good continuity (× 1 201).
(D) Transverse section of the material showed the surface was smooth, with no folds (× 2 403).
The biocompatibility of chitosan/collagen I/gelatin composite
After surgery, the activity level for the rats in the injury and material groups reduced significantly. The foot attached to the operated limb was lame, yet there was no notable inflammatory reaction or self-mutilation. On day 2 after surgery, the operated muscle cicatrized well. At days 3, 7, 14, 21 after surgery, we noted no obvious changes to the embedded material.
Gross and histological changes of liver, kidney and muscles
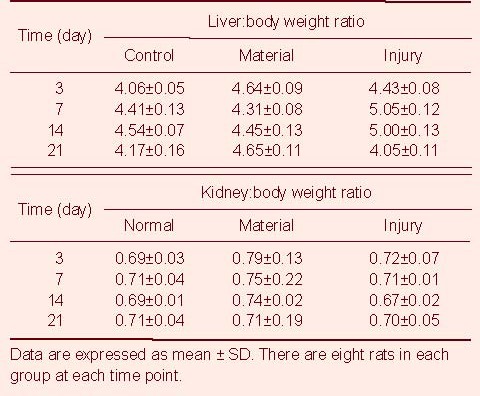
Under a light microscope, the structures of the liver and the kidneys in the rats from the three groups were similar, and no abnormal changes were found. In the left quadriceps femoris muscles of rats in the materials group, numerous inflammatory cells had infiltrated around the material on days 3 and 7. Furthermore, the material had been subjected to phagocytosis, degraded and a foreign granuloma had formed. An inflammatory response was also seen in the rats of the injury group. On days 14 and 21, the inflammatory response in the thigh muscles of rats from the material group had disappeared, but the foreign granuloma was still visible. For rats in the injury group, the inflammation had also disappeared, similar to that seen in the rats in the control group (Figure 2). There was no significant difference in the organ:body weight ratio in rats among these three groups (P > 0.05; Table 1).
Figure 2.
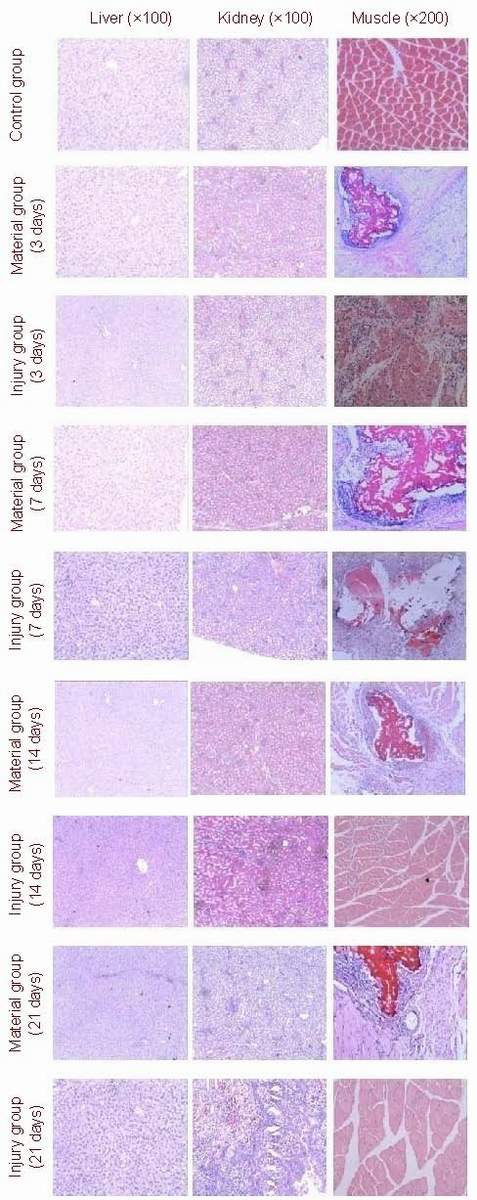
Hematoxylin-eosin stained sections. Morphology changes in the liver, kidneys and muscles were determined using light microscopy.
There were no changes in liver and kidneys in rats from the three groups. In the thigh muscles, numerous inflammatory cells had infiltrated after surgery in the materials and injury groups. By days 14 and 21, the inflammation had disappeared, but foreign granuloma could still be observed in the materials group.
Table 1.
Organ:body weight ratio (%) changes in rats among three groups
Changes of blood routine and biochemical indicators in rats
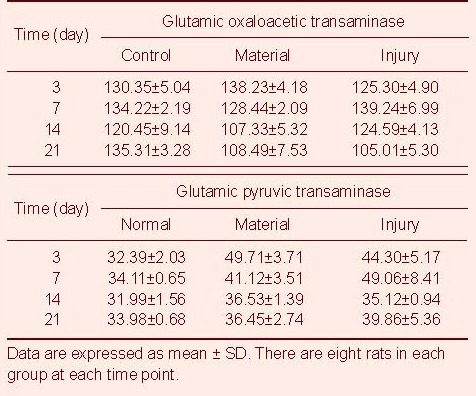
There were no significant differences in the peripheral hemoglobin and the level of blood urine nitrogen and urine creatine from rats among the three groups (P > 0.05). On days 3 and 7 after injury, the number of peripheral white blood cells in rats from the material and injury groups was significantly higher than that from the control group (P < 0.05). By day 14, the number of peripheral white blood cells decreased to normal levels. The changes in glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, lactate dehydrogenase and creatine kinase levels from day 3 to day 21 are shown in Tables 2–5.
Table 2.
Changes in hemoglobin (g/L) and white blood cell numbers (× 103/mL) in rats among groups
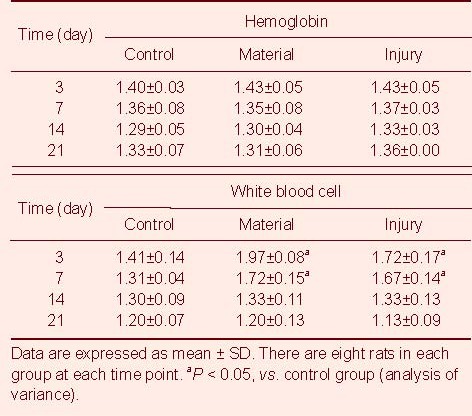
Table 5.
Changes of lactate dehydrogenase (U/L) and creatine kinase (U/L) in rats from three groups
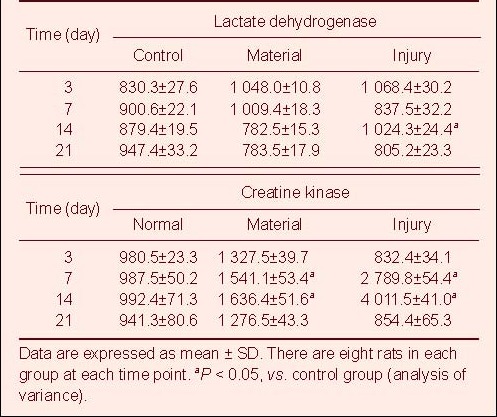
Table 3.
Changes in blood urea nitrogen (mM) and creatinine (μM) in rats among three groups
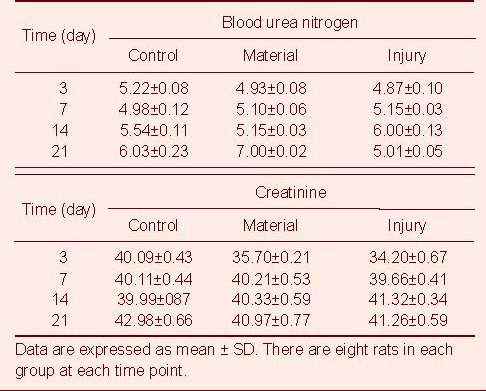
Table 4.
Changes of glutamic oxaloacetic transaminase (μM) and glutamic pyruvic transaminase (μM) in blood of rats among the three groups
Chitosan/collagen I/gelatin composite promotes the functional rehabilitation of sciatic nerve
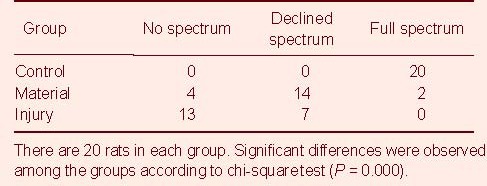
On day 45 after surgery, a muscular electromyogram showed that the injured nerve in the material group recovered from the lesion quicker than the nerves in the injury group (P < 0.01; Table 6).
Table 6.
Muscular electromyography in sciatic nerve 45 days after the surgery
DISCUSSION
Nerve injury is very common clinically. When nerve injury is severe and the nerve function is lost, the patient often exhibits a series of functional disturbances in other parts of the body, which is sometimes puzzling for clinicians. As such, nerve repair has attracted a lot of attention within the medical profession. Various tissue engineering methods have been devised to repair and regenerate the impaired nerve. The key to successful tissue engineering is the use of a material that mimics the capabilities of the original tissue and is highly biodegradable so that the host tissue can eventually replace the substitute material. Chitosan, type I collagen and gelatin are all natural extracellular matrix constituents and are consequently considered as highly suitable substitute material for nerve repair. Many studies have shown that the application of a tissue engineered material can promote the repair and regeneration of injured nerves[18,19]. In this study, a compound nerve repair material was made from chitosan, type I collagen and gelatin. This novel, nerve repair material is cylindrical, with a uniform appearance, good flexibility and strong elasticity. The internal structure of the material was regular, and the ideal microstructure was conducive to the regeneration of fiber-oriented growth. In addition, the basic direction of the independent microtubules was parallel, with a uniform diameter. These features can help the anatomical and function reconstruction of the nerve after injury.
In this study, the material was implanted into the thigh muscles of Wistar rats to observe the biocompatibility of the material. Our results showed that, after surgery, the rats from the injury and material groups had a significant loss of function and were unable to use the injured limb. At 21 days after surgery, we observed no difference between the three groups. The wound had completely healed, and the interface between the material and the surrounding musculature could not be identified. On days 3 and 7, numerous inflammatory cells were observed surrounding the material in the muscles of rats from the material group, with obvious phagocytosis, material degradation, and the formation of a foreign granuloma. An inflammatory response was also seen in rats from the injury group. On days 14 and 21, this inflammation in both the material and injury groups was no longer apparent, but foreign granuloma could still be observed in the materials group. There was no significant difference in the organ:body weight ratio in rats among these three groups. Light microscopy results showed normal liver and kidney structure in rats from all the three groups. The material did not produce toxic effects on the kidneys or liver, as there was no significant differences in the levels of blood urine nitrogen and urine creatine in rats among the control, material and injury groups. Compared with the normal group, there were also no significant differences in the glutamic oxalacetic transaminase, glutamic pyruvic transminase, lactate dehydrogenase and creatine kinase in the blood serum at 21 days after surgery.
For nerve function repair experiments, the sciatic nerve was injured by hemostat. Our composite material enhanced the healing of the injured nerve, as compared with the control and injured groups that did not receive the material. This study shows that our composite material is biocompatible and biodegradable, and possesses the capacity to repair damaged nerves in vivo. Therefore, our composite nerve repair material may offer a useful material for nerve repair strategies.
MATERIALS AND METHODS
Design
A contrast observation regarding nerve tissue engineering.
Time and setting
Experiments were performed at Functional Laboratory, Norman Bethune College of Medicine, Jilin University in May 2010.
Materials
A total of 156 Wistar rats, aged 6-8 weeks, equal split in gender and weighing 200-220 g, were purchased from Animal Center of Jilin University, China (license No. SCXK (Ji) 2003-0007). The experiments conducted in the study are in compliance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[20].
Methods
Preparation of novel nerve repairing material and ultrastructural observation
Two-hundred milligrams of collagen I (Sigma, St. Louis, MO, USA) was dissolved in 10 mL of 1% acetic acid solution and stirred at 1 000 r/min at 4°C for 6 hours, to obtain a final concentration of 2% gel mixture. Chitosan (1 g; Sigma) was dissolved in 100 mL of 1% acetic acid solution, stirred at 1 000 r/min at 4°C for 6 hours, to obtain a final concentration of 1% “sticky solution”. The type I collagen and chitosan solutions were merged and then combined with 3.33 g gelatin (Sigma), stirring at 1 000 r/min at 4°C overnight. The obtained solution was vacuum-filtered with a 200-mesh gauze, and any precipitate was removed. The mixture was injected into a 12 cm silica gel tube, with an internal diameter of 3 mm. Both ends of the tube were sealed and quickly frozen in liquid nitrogen, then cut into 4 cm-long segments, freeze-dried and sterilized under 60Co irradiation for 24 hours. Scanning electronic microscopy (Hitachi, Tokyo, Japan) was used to observe the cross and longitudinal sectional structure of the material.
Biocompatibility test of novel nerve repairing material
The rats from both the material group and injury group were anesthetized with an intraperitoneal injection of 10% chloral hydrate. The hair on the right thigh was removed, and the skin sterilized by 75% alcohol. An incision was then made in the left quadriceps femoris muscles. For rats in the material group, the sterilized nerve repair materials were cut into 2 cm pieces and placed into the muscles in the same direction as the muscle fibers. Rats in the injury group did not receive the nerve repair materials. All rats were sutured with operating thread. Normal rats were set up as a control group. The rats in all three groups were fed the same conditions ad libitum, and subjected to a controlled temperature and a 12-hour light-dark cycle. Before the rats were sacrificed, the body weights were taken. On days 3, 7, 14 and 21 after surgery, eight rats from each group were over-anaesthetized and decapitated to collect the blood sample. The number of red blood cells and white blood cells, the level of blood urine nitrogen, urine creatinine, blood hemoglobin, glutamic oxalacetic transaminase, glutamic pyruvic transminase, lactate dehydrogenase and creatine kinase were determined in the serum of rats from the three groups. The serum was tested using a biochemical meter (Shanghai Analytical Instruments Co., Ltd., China). The liver, kidneys and muscles in the right thigh were removed, and the weight of these organs determined before fixation in 10% paraformaldehyde solution for pathological examination.
Nerve repairing measurement
Rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate. The hair on the right thigh was removed and the skin sterilized with 75% alcohol. The sciatic nerve in the right thigh was dissected from the surrounding tissues. For rats in the material group, the sciatic nerve was injured using a fine hemostat for 30 seconds and then packed with the repair material. The muscles around the nerve and material were threaded using biodegradable thread. For rats in the injury group, the sciatic nerve was injured using a hemostat, but no repair material was used. On day 45 after surgery, muscular electromyography (Atlantis Shanghai Medical Electronic Instrument Co., Ltd., China) was performed on the sciatic nerve.
Statistical analysis
Data from the experiments are expressed as mean ± SD. Distribution of the data was verified to be normal using tests of normality by SPSS 13.0 (SPSS, Chicago, IL, USA). Statistical differences of biocompatibility parameters were analyzed by analysis of variance. Nerve repair was analyzed by chi-square test. A P < 0.05 was considered as a statistically significant difference.
Footnotes
Funding: This study was supported by the Department of Science and Technology of Jilin Province, China, No. 20070417.
Conflicts of interest: None declared.
Ethical approval: The experimental protocol was approved by the Animal Ethical Board of School of Public Health, Jilin University in China.
(Edited by Yin SM, Yang JP/Yang Y/Song LP)
REFERENCES
- 1.Schlosshauer B, Dreesmann L, Schaller HE, et al. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery. 2006;59(4):740–748. doi: 10.1227/01.NEU.0000235197.36789.42. [DOI] [PubMed] [Google Scholar]
- 2.Chung YC, Su YP, Chen CC, et al. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol Sin. 2004;25(7):932–936. [PubMed] [Google Scholar]
- 3.Zhang Y, Zhang M. Cell growth and function on calcium phosphate reinforced chitosan scaffolds. J Mater Sci Mater Med. 2004;15(3):255–260. doi: 10.1023/b:jmsm.0000015485.94665.25. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Ding S, Zhou C. Preparation and biological evaluation of PLA/chitosan composite materials. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2003;20(3):398–400. [PubMed] [Google Scholar]
- 5.Nugraha E, Copinet A, Tighzert L, et al. Mechanical and barrier properties of biodegradable films made from chitosan and poly(lactic acid) blends. J Polym Environ. 2004;12:1–6. [Google Scholar]
- 6.Peesan M, Supaphol P, Rujiravanit R. Electrospinning of hexanoyl chitosan/polylactide blends. J Bimater Sci Polym Ed. 2006;17(5):547–565. doi: 10.1163/156856206776986251. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Dong L, Cheung MK. Preparation and characterization of biodegradable poly(l-lactide)/chitosan blends. Eur Polym J. 2005;41:958–966. [Google Scholar]
- 8.Wan Y, Wu H, Yu A, et al. Biodegradable polylactide/chitosan blend membranes. Biomacromolecules. 2006;7(4):1362–1372. doi: 10.1021/bm0600825. [DOI] [PubMed] [Google Scholar]
- 9.Wu L, Zhang J, Jing D, et al. “Wet-state” mechanical properties of three-dimensional polyester porous scaffolds. J Biomed Mater Res A. 2006;76(2):264–271. doi: 10.1002/jbm.a.30544. [DOI] [PubMed] [Google Scholar]
- 10.Wan Y, Fang Y, Wu H, et al. Porous polylactide/chitosan scaffolds for tissue engineering. J Biomed Mater Res A. 2007;80(4):776–789. doi: 10.1002/jbm.a.31025. [DOI] [PubMed] [Google Scholar]
- 11.Leffler CC, Müller BW. Influence of the acid type on the physical and drug liberation properties of chitosan-gelatin sponges. Int J Pharm. 2000;194(2):229–237. doi: 10.1016/s0378-5173(99)00383-x. [DOI] [PubMed] [Google Scholar]
- 12.Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24(13):2339–2349. doi: 10.1016/s0142-9612(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 13.Young S, Wong M, Tabata Y, et al. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109(1-3):256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Cheng M, Deng J, Gong Y, et al. Chitosan and collagen compound as nerve repairing material. Ninth National Biology and Biophysics Symposium Abstract Collection. 2002:416. [Google Scholar]
- 15.Cheng M, Deng J, Yang F, et al. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials. 2003;24(17):2871–2880. doi: 10.1016/s0142-9612(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Stegemann JP. Thermogelling chitosan and collagen composite hydrogels initiated with beta-glycerophosphate for bone tissue engineering. Biomaterials. 2010;31(14):3976–3985. doi: 10.1016/j.biomaterials.2010.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Ma YS, Mu CZ, et al. Preparation and biological characteristics of collagen-chitosan composite scaffold: Proportion of chitosan and collagen. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2010;14(29):5367–5370. [Google Scholar]
- 18.Liang W, Luo ZJ, Wang SS, et al. A tissue engineering biomaterial used to repair injured nerve and its biocompatibility. Zhonghua Chuangshang Guke Zazhi. 2004;6(9):1037–1041. [Google Scholar]
- 19.Bahr M, Bonhoeffer F. Perspectives on axonal regeneration in the mammalian CNS. Trends Neurosci. 1994;17(11):473–479. doi: 10.1016/0166-2236(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 20.The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]


