Abstract
Liquid RNA·DNA hybridization with separated strands of adenovirus type 2 DNA revealed that late nuclear RNA can hybridize to about 85% of the 1-strand and 10-15% of the h-strand, whereas late cytoplasmic RNA hybridizes to 65-70% and 25% of the l- and h-strand, respectively. With separated strands from the six EcoRI fragments of adenovirus type 2 DNA as probes, it was shown that late nuclear RNA hydridizes to 85-90% of the l-strand from all six EcoRI fragments. Since late cytoplasmic RNA hybridizes to 40-50% of the h-strand from both fragments EcoRI-B and EcoRI-C, complementary viral RNA sequences are synthesized during adenovirus infection. Complementarity between nuclear and cytoplasmic RNA could also be demonstrated by showing that late cytoplasmic RNA which had been preincubated with late nuclear RNA hybridized to a smaller fraction of the h-strand of fragment EcoRI-C than without preincubation. Double-stranded RNA which contains sequences that correspond to at least 60% of the viral genome was isolated from infected cells. However, less than 2% of the newly synthesized late RNA became double-stranded after incubation under annealing conditions, which suggests that RNA derived from one of the strands is present at a low concentration. Accordingly, it was shown that nearly all viral cytoplasmic RNA which is synthesized late after infection is derived from the l-strand.
Keywords: nuclear RNA, separated strands, specific DNA fragments
Full text
PDF
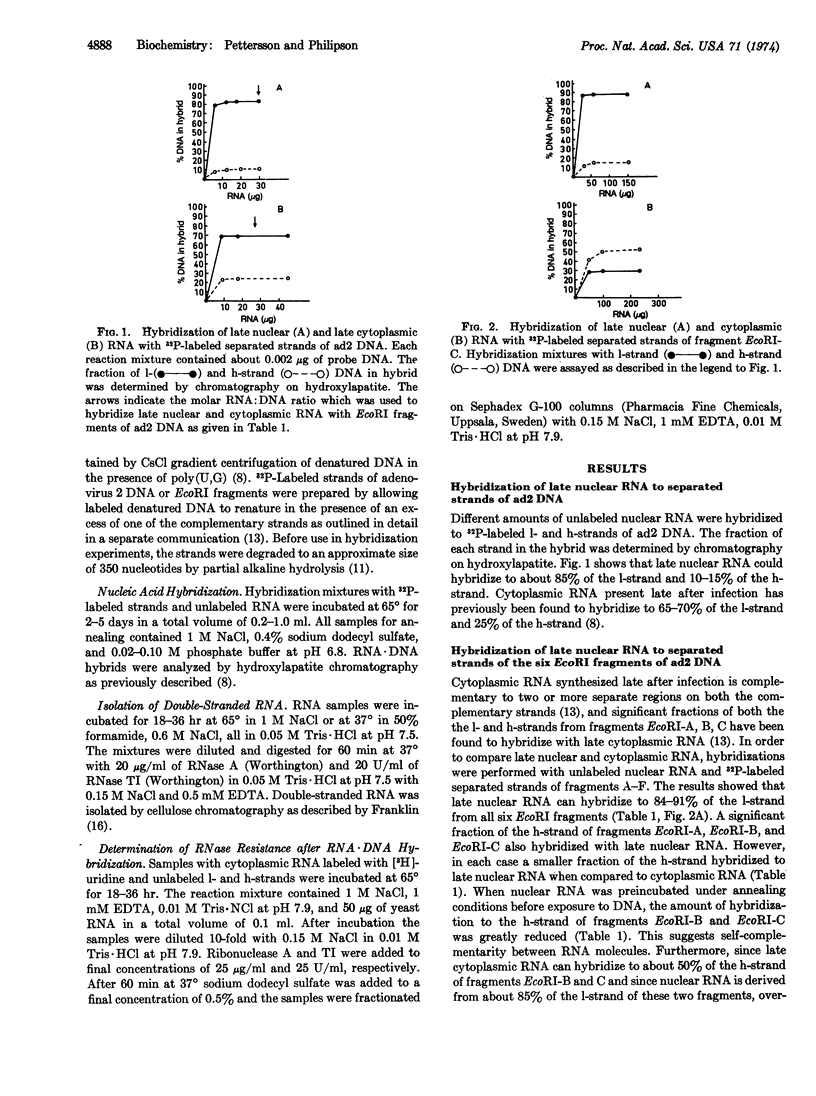
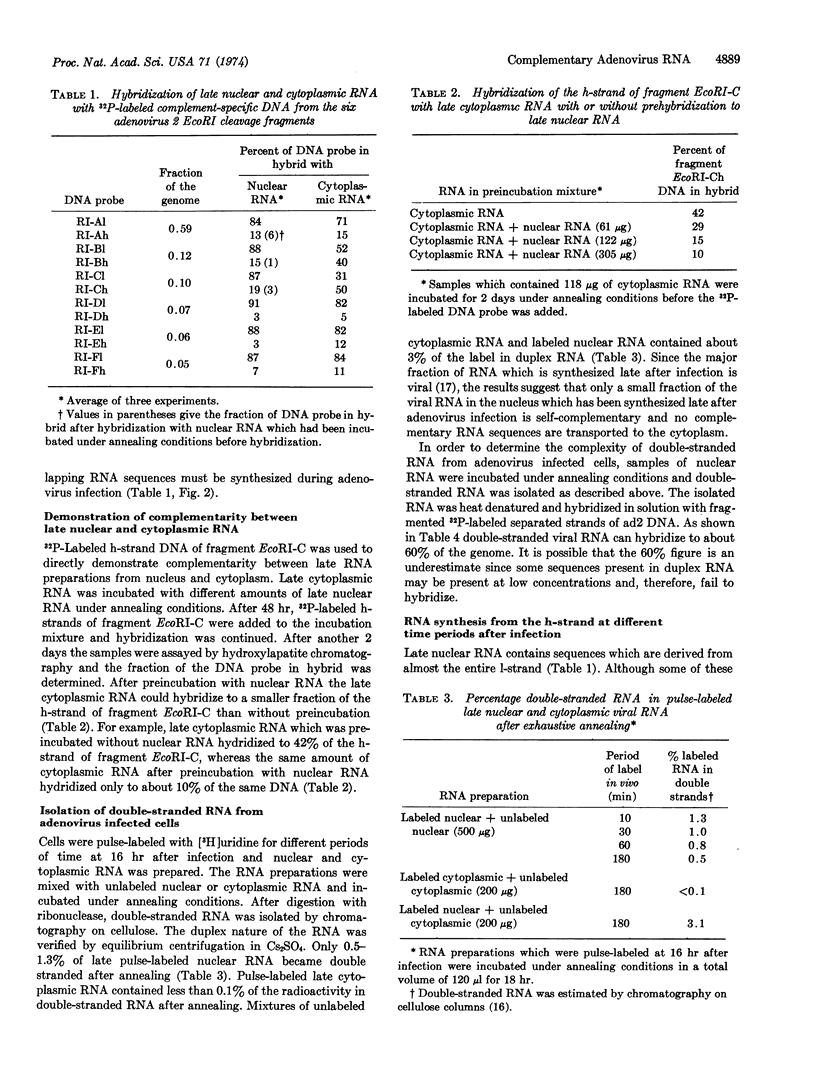


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Attardi G. Symmetrical in vivo transcription of mitochondrial DNA in HeLa cells. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1757–1761. doi: 10.1073/pnas.68.8.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y. Extensive symmetrical transcription of Simian Virus 40 DNA in virus-yielding cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2404–2409. doi: 10.1073/pnas.69.9.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y., Locker H. Symmetrical in vivo transcription of polyoma DNA and the separation of self-complementary viral and cell RNA. Virology. 1973 Aug;54(2):495–505. doi: 10.1016/0042-6822(73)90159-1. [DOI] [PubMed] [Google Scholar]
- Aloni Y. Poly A and symmetrical transcription of SV40 DNA. Nat New Biol. 1973 May 2;243(122):2–6. [PubMed] [Google Scholar]
- Brawerman G., Mendecki J., Lee S. Y. A procedure for the isolation of mammalian messenger ribonucleic acid. Biochemistry. 1972 Feb 15;11(4):637–641. doi: 10.1021/bi00754a027. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973 Jun 5;12(12):2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- Landgraf-Leurs M., Green M. DNA strand selection during the transcription of the adenovirus 2 genome in infected and transformed cells. Biochim Biophys Acta. 1973 Jul 27;312(4):667–673. doi: 10.1016/0005-2787(73)90070-1. [DOI] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Identification of double-stranded virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. Biochem Biophys Res Commun. 1972 Oct 6;49(1):39–44. doi: 10.1016/0006-291x(72)90006-x. [DOI] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Synthesis of virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. J Virol. 1971 Aug;8(2):203–214. doi: 10.1128/jvi.8.2.203-214.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Transcription and transport of virus-specific ribonucleic acids in African green monkey kidney cells abortively infected with type 2 adenovirus. J Virol. 1972 Dec;10(6):1109–1117. doi: 10.1128/jvi.10.6.1109-1117.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire P. M., Swart C., Hodge L. D. Adenovirus messenger RNA in mammalian cells: failure of polyribosome association in the absence of nuclear cleavage. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1578–1582. doi: 10.1073/pnas.69.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Tibbetts C., Pettersson U., Johansson K., Philpson L. Relationship of mRNA from productively infected cells to the complementary strands of adenovirus type 2 DNA. J Virol. 1974 Feb;13(2):370–377. doi: 10.1128/jvi.13.2.370-377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Philipson L., Darnell J. E. Processing of adenovirus specific nuclear RNA during virus replication. Virology. 1972 Oct;50(1):27–34. doi: 10.1016/0042-6822(72)90342-x. [DOI] [PubMed] [Google Scholar]



