Abstract
Background
Increasing numbers of women with breast cancer are electing for contralateral prophylactic mastectomy (CPM) to reduce the risk of developing contralateral breast cancer. The objective of this study was to identify factors that may impact a patient’s decision to undergo CPM.
Methods
We identified 2504 women with stage 0–III unilateral primary breast cancer who underwent breast surgery at our institution from January 2000 to August 2006 from a prospectively maintained database. We performed logistic regression analyses to determine which factors were associated with undergoing CPM.
Results
Of 2504 breast cancer patients, 1223 (48.8%) underwent total mastectomy. Of the 1223 patients who underwent mastectomy, 284 (23.2%) underwent immediate or delayed CPM. There were 33 patients (1.3%) who had genetic testing before the surgery; with the use of testing increasing in the latter years of the study (0.1% in 2000–2002 vs. 2.0% in 2003–2006, P<0.0001). Multivariable analysis revealed several factors that were associated with a patient undergoing CPM: age younger than 50 years, white ethnicity, family history of breast cancer, BRCA1/2 mutation testing, invasive lobular histology, clinical stage and use of reconstruction.
Conclusions
We identified specific patient and tumor characteristics associated with the use of CPM. Although genetic testing is increasing, most women undergoing CPM did not have a known genetic predisposition to breast cancer. Evidence-driven models are needed to better inform women of their absolute risk of contralateral breast cancer as well as their competing risk of recurrence from the primary breast cancer to empower them in their active decision making.
Keywords: contralateral breast cancer, breast cancer prevention, contralateral prophylactic mastectomy, risk-reducing mastectomy
INTRODUCTION
Women diagnosed with breast cancer have a significantly increased lifetime risk of developing contralateral breast cancer (CBC) over the general population 1–4. Gao et al. evaluated the incidence of CBC in 134,501 patients previously diagnosed with breast cancer identified from the Surveillance, Epidemiology, and End Results (SEER) database and found that the actuarial incidence of CBC at 5, 10, 15, and 20 years was 3%, 6.1%, 9.1%, and 12%, respectively, or approximately 0.6% per year 5. Patients with unilateral breast cancer who have germline mutations in BRCA1/2 have a markedly increased risk of developing CBC 6, 7. Specific clinical and pathologic factors that have been associated with an increased risk of developing CBC include age younger than 50 years old, a family history of breast cancer, lobular type histology, multicentric cancer, and previous chest irradiation 4, 8–12. Additional risk factors for CBC that have been identified in other studies include African American ethnicity 5, body mass index > 30 kg/m2 13 medullary carcinoma histology 5, and HER2-positive tumors 13.
To reduce their risk of developing CBC, some breast cancer patients choose to undergo contralateral prophylactic mastectomy (CPM). However, the surgeon’s decision to perform and the patient’s decision to undergo CPM are complex. The decision making process should include assessing the patient’s risk of CBC, which can vary according to age, genetic factors such as BRCA1/2 mutation status, tumor histology and multicentricity. Other issues to consider include available reconstructive options, the ability to achieve symmetry if a unilateral procedure is performed and the projected oncologic outcome from the known ipsilateral breast cancer 14.
In a previous study, we identified the clinicopathologic factors that predicted the presence of an unsuspected CBC at the time of CPM: a 5-year Gail risk ≥ 1.67%, additional ipsilateral moderate- to high-risk pathology, an ipsilateral multicentric tumor, or an ipsilateral tumor of invasive lobular histology 12. These findings may impact the decision-making process regarding CPM in patients with a diagnosis of unilateral breast cancer. The clinicopathologic characteristics associated with a patient’s decision to undergo CPM are less well defined. In the current study, we sought to identify factors that may impact the patients’ decision regarding CPM in women faced with a diagnosis of unilateral breast cancer.
MATERIALS AND METHODS
Patient Selection and Data Collection
We used the prospectively maintained Surgical Breast Oncology Database at The University of Texas M. D. Anderson Cancer Center for patient selection. We retrospectively identified patients with unilateral breast cancer who underwent breast-conserving surgery and/or mastectomy between January 2000 and August 2006. We included patients with unilateral stage 0–III primary breast cancer who had no clinical or radiographic evidence of a contralateral breast malignancy. Patients known to have had bilateral breast cancer prior to CPM were excluded from analysis. Some patients who underwent CPM were included in previous reports from our institution 9, 12. The M. D. Anderson Institutional Review Board approved this study and the need for informed consent was waived.
We reviewed patient charts for demographics, disease, and treatment characteristics, including year of surgery, age at the time of diagnosis, ethnicity, marital status, use of hormone replacement therapy, family history of breast cancer, clinical tumor stage, use of pretreatment magnetic resonance imaging (MRI), type of surgery for primary tumor, performance and timing of CPM, reported reasons for undergoing CPM, surgeon characteristics (gender, age), use and type of reconstructive surgery, genetic testing for BRCA 1/2 mutations and results, histology of the primary tumor (invasive lobular carcinoma versus other histology), estrogen receptor (ER) status, and progesterone receptor (PR) status of the primary tumor.
Breast-conserving surgery or mastectomy was performed on the ipsilateral side with or without lymph node staging as considered appropriate based on diagnostic biopsy findings. In patients who chose to undergo CPM, synchronous or delayed CPM was performed with or without sentinel lymph node biopsy at the discretion of the operating surgeon.
Statistical Analysis
For statistical analyses, patients who underwent surgery for breast cancer were separated into two groups—patients who underwent CPM and patients who did not undergo CPM. Clinicopathologic data were tabulated for each of these groups. Student’s t test with appropriate normality checks was used to compare the means of all continuous variables. For univariable comparisons of all categorical variables, Chi squared analysis or Fisher’s exact test (when sample sizes are small) was used. We performed univariable and multivariable analyses, and used a stepwise multiple logistic regression analysis to identify variables that were associated with undergoing CPM in patients who underwent surgery for breast cancer (breast-conserving surgery and/or mastectomy) and separately in those who underwent mastectomy as the primary procedure for their breast cancer. All P values were 2-tailed, and we considered P values ≤ 0.05 to be significant. Stata statistical software (StataSE 10, StataCorp LP, College Station, TX) was used for all statistical analyses.
RESULTS
Patient, Tumor, and Treatment Characteristics
A total of 2504 women with stage 0–III unilateral primary breast cancer who underwent surgical treatment at the University of Texas M. D. Anderson Cancer Center were included in this study. The median age of the patients was 54 years (range, 22–97 years); 1861 (74.3%) were white, and 643 (25.7%) were of other ethnicities (African American, Asian, Hispanic). The clinical stage of the primary tumor at diagnosis was 0, I, II, and III in 16.2%, 37.8%, 32.2%, and 13.8% of the patients, respectively. A total of 304 (12.1%) patients had primary tumors of invasive lobular histology. Of the 2504 patients, 1223 (48.8%) underwent total mastectomy for their known ipsilateral cancer. Of the 284 patients (23.2% of those undergoing ipsilateral mastectomy) who had CPM, 246 underwent CPM at the time of treatment for their ipsilateral breast cancer (immediate) and 38 had CPM at a later time (delayed). Eight patients (2.8%) had an occult malignancy in their CPM specimen, and 50 patients (17.6%) had ADH, ALH, and LCIS histologic findings in the CPM specimen.
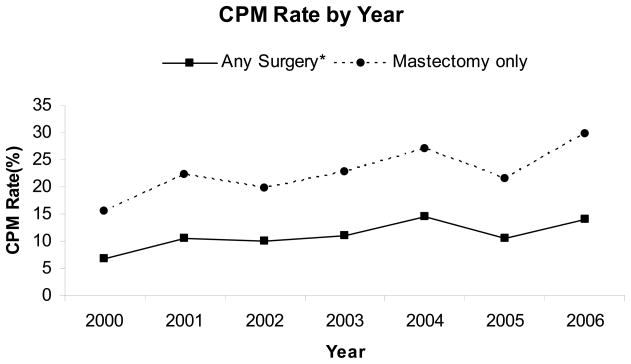
Figure 1 shows the proportion of patients who underwent CPM over time from 2000 to 2006. The CPM rate increased significantly from 6.8% in 2000 to 14.0% in 2006 (a 105% increase, P<0.0001). The CPM rate was also significantly increased when only patients undergoing mastectomy for treatment of their primary tumor were considered. This increased, from 15.5% in 2000 to 29.8% in 2006 (a 92% increase, P<0.0001).
Figure 1.
Patients with unilateral breast cancer who underwent contralateral prophylactic mastectomy.
Abbreviation: CPM, contralateral prophylactic mastectomy.
* Breast-conserving surgery and/or mastectomy for treatment of the patient’s primary tumor.
Factors Associated with CPM
The demographics, tumor, pathologic and treatment characteristics of the patients who did and did not undergo CPM are summarized in Table 1. Univariable analyses revealed that patient age <50, white ethnicity, marital status, family history of breast cancer, use of hormone replacement therapy, undergoing BRCA1/2 genetic testing before surgery, higher clinical tumor stage, multicentric primary tumor, invasive lobular histology, and use of reconstructive surgery were significantly associated with undergoing CPM.
Table 1.
Demographic, tumor, pathologic and treatment characteristics of breast cancer patients who underwent CPM compared with those who did not.
| Characteristics | CPM, (n=284) | No CPM, Any surgery* (n=2220) | P† | No CPM, Mastectomy (n=939) | P† |
|---|---|---|---|---|---|
| Median age (range) year | 49.6 (22–85) | 55.8 (26–97) | <0.0001 | 54.3 (26–88) | <0.0001 |
| Age | <0.0001 | <0.0001 | |||
| <50 year | 152 (53.5) | 711 (32.0) | 345 (36.7) | ||
| >=50 year | 132 (46.5) | 1509 (68.0) | 594 (63.3) | ||
| Ethnicity | <0.0001 | <0.0001 | |||
| White | 244 (85.9) | 1617 (72.8) | 646 (68.8) | ||
| Others | 40 (14.1) | 603 (27.2) | 293 (31.2) | ||
| Marital status | <0.0001 | <0.0001 | |||
| Married | 221 (77.8) | 1466 (66.0) | 611 (65.1) | ||
| Single | 24 (8.5) | 252 (11.4) | 114 (12.1) | ||
| Others | 39 (13.7) | 502 (22.6) | 214 (22.8) | ||
| Family history of breast cancer | <0.0001 | <0.0001 | |||
| Yes | 161 (56.7) | 939 (42.3) | 401 (42.7) | ||
| No | 123 (43.3) | 1281 (57.7) | 538 (57.3) | ||
| Family history of breast cancer | 0.001 | 0.002 | |||
| In a first-degree relative | 75 (26.4) | 399 (18.0) | 169 (18) | ||
| Not in first-degree relatives | 209 (73.6) | 1821 (82.0) | 770 (82.0) | ||
| Hormone replacement therapy | 0.001‡ | 0.1‡ | |||
| Yes | 95 (33.5) | 977 (44.0) | 366 (39.0) | ||
| No | 179 (63.0) | 1192 (53.7) | 547 (58.2) | ||
| Unknown | 10 (3.5) | 51 (2.3) | 26 (2.8) | ||
| BRCA1/2 genetic testing before surgery | <0.0001 | <.0001 | |||
| Yes | 18 (6.3) | 15 (0.7) | 9 (1.0) | ||
| No | 266 (93.7) | 2205 (99.3) | 930 (99.0) | ||
| Breast MRI | <0.0001* | 0.001* | |||
| None or after surgery | 256 (90.1) | 2121 (95.5) | 888 (94.6) | ||
| Primary side | 21 (7.4) | 94 (4.2) | 48 (5.1) | ||
| Contralateral side | 7 (2.5) | 5 (0.3) | 3 (0.3) | ||
| Clinical tumor stage | <0.0001 | 0.003 | |||
| 0 | 39 (13.7) | 367 (16.5) | 142 (15.1) | ||
| I | 81 (28.5) | 865 (39.0) | 238 (25.4) | ||
| II | 122 (43.0) | 684 (30.8) | 330 (35.1) | ||
| III | 42 (14.8) | 304 (13.7) | 229 (24.4) | ||
| Multicentric tumor | <0.0001 | 0.46 | |||
| No | 231 (81.3) | 1981 (89.2) | 745 (79.3) | ||
| Yes | 53 (18.7) | 239 (10.8) | 194 (20.7) | ||
| Histology of primary tumor | <0.0001 | 0.02 | |||
| Invasive lobular | 56 (19.7) | 248 (11.2) | 132 (14.1) | ||
| Others | 228 (80.3) | 1972 (88.8) | 807 (85.9) | ||
| Estrogen receptor status | 0.098‡ | 0.64‡ | |||
| Positive | 184 (64.8) | 1530 (68.9) | 614 (65.4) | ||
| Negative | 88 (31.0) | 582 (26.2) | 274 (29.2) | ||
| Unknown | 12 (4.2) | 108 (4.9) | 51 (5.4) | ||
| Progesterone receptor status | 0.16‡ | 0.80‡ | |||
| Positive | 142 (50) | 1197 (53.9) | 471 (50.2) | ||
| Negative | 129 (45.4) | 906 (40.8) | 413 (44.0) | ||
| Unknown | 13 (4.6) | 117 (5.3) | 55 (5.8) | ||
| Surgery for primary tumor | <0.0001 | ||||
| Breast-conserving surgery | 0 | 1281 (57.7) | |||
| Mastectomy | 284 | 939 (42.3) | |||
| Reconstruction | <.0001 | ||||
| Yes | 184 (64.8) | 348 (37.1) | |||
| No | 100 (35.2) | 591 (62.9) | |||
Abbreviation: CPM, contralateral prophylactic mastectomy.
Note: Data are presented as the number (%) of patients, unless otherwise specified.
Breast-conserving surgery or mastectomy for treatment of the patients’ primary tumor.
Compared to patients who underwent CPM. P-values correspond to t-tests for continuous variables and Chi-square or Fisher’s exact tests for categorical variables
Excluding the unknown category.
MRI was performed only selectively in this series. Patients who underwent CPM were more likely to have undergone an MRI of either breast compared with patients who had a unilateral mastectomy without a CPM (9.9% vs. 5.4%, p<0.0001), however, of the 28 CPM patients who underwent an MRI, 18 only had imaging of the ipsilateral breast, and 7 had imaging of the contralateral breast, and 3 had bilateral imaging. Review of the medical records suggested that none of the 28 CPM patients who had an MRI of either breast decided to pursue a CPM due to MRI findings.
Multivariable logistic regression analysis with backward variable selection (Table 2) revealed that younger patients (< 50 years of age) were significantly more likely to undergo CPM (odds ratio (OR) 1.78, 95% confidence interval (CI): 1.33–2.39): 17.6% of patients < 50 years underwent CPM compared with only 8.0% of women ≥ 50 years. Patients were also significantly more likely to undergo CPM if they were white (OR 2.54, 95% CI: 1.73–3.74), had a family history of breast cancer (OR 1.57, 95% CI: 1.19–2.09), had stage II tumors (OR 2.25, 95% CI: 1.46–3.46) or stage III tumors (OR 2.44, 95%CI: 1.44–4.15), underwent BRCA1/2 genetic testing before surgery(OR 4.51, 95% CI: 1.98–10.25), had reconstructive surgery (OR 8.82, 95% CI: 6.59–11.80), or had invasive lobular histology (OR 1.89, 95% CI: 1.30–2.74). Neither ER/PR status of the primary tumor nor surgeon characteristics (gender, age) was associated with the use of CPM.
Table 2.
Multivariable analysis of the factors associated with undergoing contralateral prophylactic mastectomy.
| Variable | Any surgery* | Mastectomy only | ||||
|---|---|---|---|---|---|---|
| OR | 95 % CI | P | OR | 95 % CI | P | |
| Age | ||||||
| ≥50 (referent) | - | - | - | - | - | - |
| <50 | 1.78 | 1.33–2.39 | 0.001 | 1.84 | 1.35–2.50 | <0.0001 |
| Race | ||||||
| Others (referent) | - | - | - | - | - | - |
| White | 2.54 | 1.73–3.74 | <0.0001 | 2.63 | 1.78–3.88 | <0.0001 |
| Marital status | ||||||
| Single (referent) | - | - | - | - | - | - |
| Married | 1.62 | 0.99–2.64 | 0.055 | 1.66 | 1.002–2.73 | 0.049 |
| Other | 1.16 | 0.64–2.09 | 0.62 | 1.16 | 0.63–2.11 | 0.63 |
| Family history of breast cancer | ||||||
| No (referent) | - | - | - | - | - | - |
| Yes | 1.57 | 1.19–2.09 | 0.001 | 1.58 | 1.17–2.11 | 0.002 |
| BRCA1/2 genetic testing | ||||||
| No test before surgery (referent) | - | - | - | - | - | - |
| Test before surgery | 4.51 | 1.98–10.25 | <0.0001 | 5.16 | 2.15–12.35 | <0.0001 |
| Clinical tumor stage | ||||||
| 0 (referent) | - | - | - | - | - | - |
| I | 1.07 | 0.68–1.67 | 0.83 | 1.37 | 0.85–2.19 | 0.195 |
| II | 2.25 | 1.46–3.46 | <0.0001 | 1.82 | 1.11–2.79 | 0.011 |
| III | 2.44 | 1.44–4.15 | 0.001 | 1.15 | 0.85–2.19 | 0.622 |
| Invasive lobular histology | ||||||
| No (referent) | - | - | - | - | - | - |
| Yes | 1.89 | 1.30–2.74 | 0.001 | 1.58 | 1.07–2.31 | 0.019 |
| Reconstruction | ||||||
| No (referent) | - | - | - | - | - | - |
| Yes | 8.82 | 6.59–11.80 | <0.0001 | 2.72 | 1.97–3.76 | <0.0001 |
Abbreviation: CI, confidence interval; OR, Odds Ratio
Breast-conserving surgery and/or mastectomy for treatment of the patient’s primary tumor.
Multivariable analysis was done with logistic regression with backward variable selection
When only patients who underwent total mastectomy for treatment of their primary tumor were included in the analysis, univariable analysis (Table 1) revealed that patient age, ethnicity, marital status, family history of breast cancer, undergoing BRCA1/2 genetic testing before surgery, higher clinical tumor stage, invasive lobular histology, and use of reconstructive surgery were significantly associated with undergoing CPM. Multivariable logistic regression analysis (Table 2) revealed that younger patients (< 50 years of age) were significantly more likely to undergo CPM (OR 1.72, 95% CI: 1.26–2.25): 30.6% of patients < 50 years of age underwent CPM compared with only 18.2% of women ≥ 50 years of age. Patients were also significantly more likely to undergo CPM if they were white (OR 2.51, 95% CI: 1.70–3.71), had a family history of breast cancer (OR 1.45, 95% CI: 1.08–1.94), had stage II tumors (OR 1.76, 95% CI: 1.11–2.79), underwent BRCA1/2 genetic testing (OR 3.92, 95% CI: 2.30–6.67), underwent reconstructive surgery (OR 2.70, 95% CI: 1.95–3.74), or had invasive lobular histology (OR 1.63, 95% CI: 1.11–2.39).
Factors Associated with Timing of CPM
The demographics, tumor, and treatment characteristics of the patients who underwent immediate CPM as compared with delayed CPM are summarized in Table 3. Patients with younger age, other ethnicity, single status, who had BRCA1/2 genetic testing before surgery, higher clinical tumor stage, and use of reconstructive surgery were more likely to undergo delayed CPM instead of immediate CPM.
Table 3.
Demographic, tumor, pathologic and treatment characteristics of breast cancer patients who underwent immediate CPM as compared with delayed CPM.
| Characteristics | Immediate CPM N=246 |
Delayed CPM N=38 |
P value |
|---|---|---|---|
| Median age (range) year | 49 (22–85) | 43.5 (29–68) | 0.005 |
| Age | 0.055* | ||
| <50 year | 120 (48.8) | 12 (31.6) | |
| >=50 year | 126 (51.2) | 26 (68.4) | |
| Ethnicity | 0.01* | ||
| White | 217 (88.2) | 27 (71.1) | |
| Others | 29 (11.8) | 11 (28.9) | |
| Marital status | 0.03 | ||
| Married | 197 (80.1) | 24 (63.2) | |
| Single | 17 (6.9) | 7 (18.4) | |
| Others | 32 (13.0) | 7 (18.4) | |
| Clinical tumor stage | 0.012* | ||
| 0 | 37 (15.0) | 2 (5.2) | |
| I | 76 (30.9) | 5 (13.2) | |
| II | 100 (40.8) | 22 (57.9) | |
| III | 33 (13.5) | 9 (23.7) | |
| Family history of breast cancer | 0.6 | ||
| Yes | 141 (57.3) | 20 (52.6) | |
| No | 105 (42.7) | 18 (47.4) | |
| BRCA1/2 genetic testing before surgery | <0.0001 | ||
| Yes | 9 (3.7) | 9 (23.7) | |
| No | 237 (96.3) | 29 (76.3) | |
| Reconstruction | 0.027* | ||
| Yes | 153 (62.0) | 31 (81.6) | |
| No | 93 (37.8) | 7 (18.4) | |
Abbreviation: CPM, contralateral prophylactic mastectomy.
P-values correspond to t-tests for continuous variables and Chi-square or Fisher’s exact tests for categorical variables
Patients’ Decision-Making Regarding the Use of CPM
The patients’ reasons for undergoing CPM (as attributed by the surgical team and prospectively collected in the database) are summarized in Table 4. Most patients (87%) chose to undergo CPM because of a family history of breast cancer, a psychological fear of developing another breast cancer, or perceived difficulty in surveillance for CBC because of clinically and mammographically dense breast tissue or diffuse microcalcifications in the contralateral breast.
Table 4.
Factors influencing a patient’s decision to undergo contralateral prophylactic mastectomy (as documented in the patients’ records)
| Factors | Number of patients* (%) |
|---|---|
| Family history of breast cancer | 85 (30) |
| Difficult surveillance | 91 (32) |
| Psychological fear | 135 (48) |
| Family history of other cancer | 66 (23) |
| Multicentric/multifocal primary breast cancer | 50 (18) |
| Reconstructive issue | 30 (11) |
| Unknown | 17 (6.0) |
Some factors were listed more than once in an individual patient’s medical records
Genetic Testing for Germline Mutations in BRCA1/2
Table 5 summarizes the impact of genetic testing on the decision to undergo CPM. Thirty-three patients had genetic testing prior to surgery. Eight of these patients had a deleterious BRCA1/2 mutation identified and then proceeded to undergo CPM. Interestingly, 10 patients who underwent genetic testing and were found not to carry a deleterious BRCA1/2 mutation still chose to undergo CPM.
Table 5.
Incidence and results of genetic testing in patients who did and did not undergo CPM
| Characteristics | Number of patients (%)
|
P* | |
|---|---|---|---|
| No CPM | CPM | ||
| Genetic testing before surgery(n=33) | (n=15) | (n=18) | 0.003 |
| BRCA1/2 mutation | 0 | 8 | |
| No BRCA1/2 mutation | 15 | 10 | |
Abbreviation: CPM, contralateral prophylactic mastectomy.
Fisher’s exact tests
DISCUSSION
Over the past decade, increasing numbers of women with breast cancer have been electing to undergo CPM to reduce the risk of contralateral breast cancer 15. In this study, we sought to identify factors that were associated with the decision to undergo CPM. Of 2504 women with stage 0–III unilateral breast cancer who underwent breast-conserving surgery or mastectomy for their primary tumor at our institution, we found that 11.3% underwent CPM. Several patient and tumor characteristics were associated with patients undergoing CPM including patient age, ethnicity, family history of breast cancer, undergoing BRCA1/2 genetic testing, higher clinical tumor stage, invasive lobular histology, and use of reconstructive surgery.
We found that the proportion of patients who chose to undergo CPM at our institution doubled between 2000 and 2006. Similarly, Tuttle et al. found that the rate of CPM in patients with stage I–III unilateral breast cancer increased by 150% from 1998 to 2003 16 and that the rate of CPM in patients with ductal carcinoma in situ increased by 148% in the United States from 1998 to 2005 17. There are many factors that may have contributed to the increased use of CPM over this timeframe, including an increased patient interest in cancer prevention, the availability of genetic counseling and BRCA 1/2 testing, and the increased utilization of skin-sparing mastectomy and availability of breast reconstruction.
MRI is increasingly used in the preoperative assessment of both the ipsilateral and contralateral breasts of women with newly diagnosed breast cancer18, 19. Katipamula and colleagues recently reported that among women treated for early-stage breast cancer at the Mayo Clinic between 1997 and 2006, 52% of those who had a breast MRI underwent mastectomy compared with 38% of women who did not receive an MRI20. Sorbero and colleagues reported that women who underwent MRI were nearly twice as likely to have CPM21. In this study, the overall use of MRI is low, with MRI being used selectively. Although more of the patients that underwent CPM had a MRI, only 3.5% underwent a contralateral MRI, and a retrospective review of the medical records of the CPM patients who had an MRI of either breast suggested that the MRI results did not lead to the decision for CPM in our patients. Thus the trend of increasing CPMs in our institution appears to not be driven by the use of MRI. However, in our institution, more recently, the contralateral breast is also imaged when an ipsilateral MRI is ordered. How this change in practice will affect CPM rates will need to be followed.
At our institution, several patient variables were associated with the decision to undergo CPM. For example, in all patients who underwent surgery for breast cancer and in those who underwent mastectomy for treatment of their primary tumor, Caucasian race was associated with significantly higher CPM rates. Ethnicity was also found to be an important predictor of CPM in studies examining the SEER database by Tuttle et al. 16, 17. Race and ethnicity are known to effect the delivery of definitive local therapy 22, choice of breast-conserving surgery versus mastectomy 23, and utilization of immediate breast reconstruction in patients undergoing mastectomy 24. In our study, Caucasian race was a predictor for patients undergoing CPM independent of tumor stage and use of reconstruction. Further study is warranted to evaluate both the patient and heath care provider variables that may contribute to this ethnic disparity.
Age younger than 50 years and invasive lobular histology were both significantly associated with patients undergoing CPM in our study. Using data from the SEER registry and the Connecticut Tumor Registry, Tuttle et al. 16 and Polednak 25 also reported that young age and lobular histology were associated with higher CPM rates. The increased utilization of CPM in younger patients is not surprising, as patients who are younger in age at breast cancer onset are more likely to have a hereditary breast cancer. In addition, among BRCA1/2 mutation carriers, younger age at diagnosis of the first breast cancer predicts a higher risk of CBC 26. Furthermore, even among women without a recognized genetic predisposition to breast cancer, younger women would still be expected to derive more benefit from CPM owing to their longer life expectancy and subsequently higher expected lifetime risk of developing another primary breast cancer.
The increased utilization of CPM that we and others found in patients with invasive lobular carcinoma is of particular interest. Although patients with invasive lobular carcinoma have been reported to be at a significantly increased risk of CBC 4, 9–12, many of these studies were performed more than twenty years ago when there was still controversy regarding the diagnoses of lobular carcinoma in situ and invasive lobular histology. Others, such as our recent study, have determined predictors of incidental CBC on CPM specimens 12, and found that ipsilateral lobular histology increased the likelihood of identifying a CBC on CPM. However, these studies do not truly answer the question of whether patients with invasive lobular carcinoma undergoing current standard-of-care treatments for unilateral cancer are more likely to develop a clinically significant CBC in the future. In fact, Gao et al. 5 did not observe a higher rate of CBC in patients with invasive lobular cancer compared with invasive ductal cancers. Similarly, in our previous work, we have found no difference in the CBC rates of patients undergoing breast conservation for invasive lobular carcinoma compared with those with ductal carcinoma. 27 As most lobular carcinomas are ER positive, it is likely that even if there is a propensity for CBC development, it may be overcome by the chemopreventive effect of current adjuvant endocrine therapy regimens.
When considering CPM, it is important to weigh the absolute risk of CBC against the risk of the proposed surgery, patient comorbidities, and competing risk of recurrence from the primary tumor. The absolute risk of CBC varies not only with the factors discussed above but also with the treatment of the primary tumor. An overview of randomized polychemotherapy trials demonstrated that adjuvant chemotherapy was associated with a 20% reduction in CBC risk and that the use of tamoxifen was associated with a 47% risk reduction 28. In considering the risk of the proposed surgery, one must also consider how the surgery will effect adjuvant therapy; even minor wound complications may potentially delay recommended chemotherapy or radiation therapy for the primary cancer after surgery. Indeed, in a previous study, we found that bilateral mastectomy for unilateral cancer conferred a higher risk of complications than unilateral mastectomy, with 8.4% of the complications occurring on the primary side, 6.3% occurring on the contralateral side, and 1.7% occurring bilaterally; thus, CPM does increase the complexity of the surgical procedure and may increase the complications incurred 1.
Breast cancer patients with deleterious BRCA1/2 mutations have a significantly increased risk of developing CBC 6, 7. Thus, it is important to pursue genetic risk stratification for patients with a family history of breast or ovarian cancer or those who are at a young age at breast cancer onset. We found that genetic testing for the BRCA1/2 mutation has increased in recent years at our institution. This may reflect increased awareness and acceptance of genetic testing by patients and physicians, or it may reflect a more recent restructuring of the medical cancer genetics services at our institution. In our study, genetic testing for BRCA1/2 mutations was associated with patients choosing to undergo CPM. Interestingly, several patients who underwent testing and were negative for any deleterious mutations in the BRCA genes still chose to undergo CPM. In fact, patients who had pursued genetic testing were more likely to undergo CPM regardless of results of BRCA testing.
Although there is evidence that CPM does in fact decrease CBC risk 2, 29–31, the impact of CPM on survival has been more controversial. If there is indeed a survival benefit from CPM, it could be hypothesized that the benefit would be greatest in patients with early-stage cancer, who have the lowest risk of death from the primary cancer. Shrag et al. reported that in BRCA1/2 mutation carriers, patients with early-stage node-negative breast cancer would be expected to have the greatest gains in life expectancy after CPM 32. Interestingly, among all patients who underwent surgery for breast cancer in our study, patients who had higher-stage disease (stage II or III) were more likely to choose to undergo CPM; however, this was no longer true when only patients who underwent mastectomy for treatment of their primary tumor were considered. Whether our findings reflect the greater psychologic fear of patients who have a more aggressive primary tumor at diagnosis or whether it reflects that patients with larger tumors that necessitate mastectomy are more likely to elect a contralateral procedure remains unclear. However, it emphasizes that we need better tools to inform women of their absolute risk of CBC as well as the competing risk of recurrence from their primary breast cancer to assist them in their decision-making process.
Although absolute indications for CPM have not been established, the Society of Surgical Oncology has published criteria for considering the use of CPM, listing reconstructive issues, such as symmetry and/or balance as a consideration 33. Also, in a study of breast cancer patients from the National Cancer Institute-funded Cancer Research Network between 1979 and 1999, Geiger et al. reported that patients who underwent CPM were more likely to undergo breast reconstruction than patients who did not undergo CPM 34. In our study, use of reconstructive surgery was the strongest factor associated with patients undergoing CPM on multivariable analysis. Some patients may pursue CPM for reconstructive issues, such as the desire to achieve symmetry (especially a concern with implant reconstructions) or the ability to undergo autologous abdominal tissue-based reconstruction only once. Alternatively, there may be other variables driving the decision to pursue reconstruction and/or CPM.
Our study had several possible limitations, including those inherent to any single-institution, retrospective study. One limitation is that we did not evaluate patient satisfaction with and psychosocial outcomes after CPM. Given the retrospective nature of the study, we were also unable to discern the role of the patient’s choice versus the surgeon’s influence on the decision to pursue CPM. Furthermore, this study reflects the patterns of care at a major cancer center. The CPM rates may have been influenced by an increased number of informed patients seeking out certain treatments, the ready availability of genetic counseling, and the patients’ desire and ability to undergo immediate reconstruction. Regardless, results of this study may be valuable to other institutions with respect to their own recommendations on the use of CPM in patients with unilateral breast cancer. Our study opens this subject up to further critical analysis and debate among patients and clinicians who treat women with breast cancer.
In conclusion, we found that various patient and tumor characteristics were associated with the decision to undergo CPM. Evidence-driven models are needed to better inform women of their absolute risk of CBC and the competing risk of recurrence from their primary breast cancer in order to empower these women during the active decision-making process. Genetic counseling and selective use of genetic testing may also guide rational decision making.
Footnotes
Presented in part at the 2009 Annual Meeting of the American Society of Clinical Oncology, May 29–June 2, 2009, Orlando, FL
References
- 1.Goldflam K, Hunt KK, Gershenwald JE, et al. Contralateral prophylactic mastectomy. Predictors of significant histologic findings. Cancer. 2004;101(9):1977–1986. doi: 10.1002/cncr.20617. [DOI] [PubMed] [Google Scholar]
- 2.Herrinton LJ, Barlow WE, Yu O, et al. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a cancer research network project. J Clin Oncol. 2005;23(19):4275–4286. doi: 10.1200/JCO.2005.10.080. [DOI] [PubMed] [Google Scholar]
- 3.Kollias J, Ellis IO, Elston CW, Blamey RW. Clinical and histological predictors of contralateral breast cancer. Eur J Surg Oncol. 1999;25(6):584–589. doi: 10.1053/ejso.1999.0711. [DOI] [PubMed] [Google Scholar]
- 4.Robbins GF, Berg JW. Bilateral Primary Breast Cancer; a Prospective Clinicopathological Study. Cancer. 1964;17:1501–1527. doi: 10.1002/1097-0142(196412)17:12<1501::aid-cncr2820171202>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 5.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003 Jul 15;56(4):1038–1045. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 6.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62(3):676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhoog LC, Brekelmans CT, Seynaeve C, et al. Survival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1. Lancet. 1998 Jan 31;351(9099):316–321. doi: 10.1016/s0140-6736(97)07065-7. [DOI] [PubMed] [Google Scholar]
- 8.Adami HO, Bergstrom R, Hansen J. Age at first primary as a determinant of the incidence of bilateral breast cancer. Cumulative and relative risks in a population-based case-control study. Cancer. 1985;55(3):643–647. doi: 10.1002/1097-0142(19850201)55:3<643::aid-cncr2820550328>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Boughey JC, Khakpour N, Meric-Bernstam F, et al. Selective use of sentinel lymph node surgery during prophylactic mastectomy. Cancer. 2006 Oct 1;107(7):1440–1447. doi: 10.1002/cncr.22176. [DOI] [PubMed] [Google Scholar]
- 10.Erdreich LS, Asal NR, Hoge AF. Morphologic types of breast cancer: age, bilaterality, and family history. South Med J. 1980;73(1):28–32. doi: 10.1097/00007611-198001000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Gogas J, Markopoulos C, Skandalakis P, Gogas H. Bilateral breast cancer. Am Surg. 1993;59(11):733–735. [PubMed] [Google Scholar]
- 12.Yi M, Meric-Bernstam F, Middleton LP, et al. Predictors of contralateral breast cancer in patients with unilateral breast cancer undergoing contralateral prophylactic mastectomy. Cancer. 2009 Mar 1;115(5):962–971. doi: 10.1002/cncr.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li CI, Malone KE, Porter PL, Daling JR. Epidemiologic and molecular risk factors for contralateral breast cancer among young women. Br J Cancer. 2003 Aug 4;89(3):513–518. doi: 10.1038/sj.bjc.6601042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery LL, Tran KN, Heelan MC, et al. Issues of regret in women with contralateral prophylactic mastectomies. Ann Surg Oncol. 1999;6(6):546–552. doi: 10.1007/s10434-999-0542-1. [DOI] [PubMed] [Google Scholar]
- 15.Tuttle T, Habermann E, Abraham A, Emory T, Virnig B. Contralateral prophylactic mastectomy for patients with unilateral breast cancer. Expert Rev Anticancer Ther. 2007 Aug;7(8):1117–1122. doi: 10.1586/14737140.7.8.1117. [DOI] [PubMed] [Google Scholar]
- 16.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007 Nov 20;25(33):5203–5209. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 17.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009 Mar 20;27(9):1362–1367. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 18.Morris EA, Liberman L, Ballon DJ, et al. MRI of occult breast carcinoma in a high-risk population. AJR Am J Roentgenol. 2003 Sep;181(3):619–626. doi: 10.2214/ajr.181.3.1810619. [DOI] [PubMed] [Google Scholar]
- 19.Smith RA. The evolving role of MRI in the detection and evaluation of breast cancer. N Engl J Med. 2007 Mar 29;356(13):1362–1364. doi: 10.1056/NEJMe078006. [DOI] [PubMed] [Google Scholar]
- 20.Katipamula R, Degnim AC, Hoskin T, et al. Trends in Mastectomy Rates at the Mayo Clinic Rochester: Effect of Surgical Year and Preoperative Magnetic Resonance Imaging. J Clin Oncol. 2009 Jul 27; doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorbero ME, Dick AW, Beckjord EB, Ahrendt G. Diagnostic breast magnetic resonance imaging and contralateral prophylactic mastectomy. Ann Surg Oncol. 2009 Jun;16(6):1597–1605. doi: 10.1245/s10434-009-0362-3. [DOI] [PubMed] [Google Scholar]
- 22.Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009 Feb 10;27(5):713–719. doi: 10.1200/JCO.2008.17.9234. [DOI] [PubMed] [Google Scholar]
- 23.Hiotis K, Ye W, Sposto R, Skinner KA. Predictors of breast conservation therapy: size is not all that matters. Cancer. 2005 Mar 1;103(5):892–899. doi: 10.1002/cncr.20853. [DOI] [PubMed] [Google Scholar]
- 24.Tseng JF, Kronowitz SJ, Sun CC, et al. The effect of ethnicity on immediate reconstruction rates after mastectomy for breast cancer. Cancer. 2004 Oct 1;101(7):1514–1523. doi: 10.1002/cncr.20529. [DOI] [PubMed] [Google Scholar]
- 25.Polednak AP. Frequency of prophylactic contralateral mastectomy among breast cancer patients. J Am Coll Surg. 2001 Jun;192(6):804–805. doi: 10.1016/s1072-7515(01)00912-7. [DOI] [PubMed] [Google Scholar]
- 26.Verhoog LC, Brekelmans CT, Seynaeve C, Meijers-Heijboer EJ, Klijn JG. Contralateral breast cancer risk is influenced by the age at onset in BRCA1-associated breast cancer. Br J Cancer. 2000 Aug;83(3):384–386. doi: 10.1054/bjoc.2000.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vo TN, Meric-Bernstam F, Yi M, et al. Outcomes of breast-conservation therapy for invasive lobular carcinoma are equivalent to those for invasive ductal carcinoma. Am J Surg. 2006 Oct;192(4):552–555. doi: 10.1016/j.amjsurg.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998 Sep 19;352(9132):930–942. [PubMed] [Google Scholar]
- 29.McDonnell SK, Schaid DJ, Myers JL, et al. Efficacy of contralateral prophylactic mastectomy in women with a personal and family history of breast cancer. J Clin Oncol. 2001;19(19):3938–3943. doi: 10.1200/JCO.2001.19.19.3938. [DOI] [PubMed] [Google Scholar]
- 30.Peralta EA, Ellenhorn JD, Wagman LD, Dagis A, Andersen JS, Chu DZ. Contralateral prophylactic mastectomy improves the outcome of selected patients undergoing mastectomy for breast cancer. Am J Surg. 2000;180(6):439–445. doi: 10.1016/s0002-9610(00)00505-5. [DOI] [PubMed] [Google Scholar]
- 31.van Sprundel TC, Schmidt MK, Rookus MA, et al. Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. Br J Cancer. 2005;93(3):287–292. doi: 10.1038/sj.bjc.6602703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrag D, Kuntz KM, Garber JE, Weeks JC. Benefit of prophylactic mastectomy for women with BRCA1 or BRCA2 mutations. Jama. 2000 Jun 21;283(23):3070–3072. [PubMed] [Google Scholar]
- 33.Giuliano AE, Boolbol S, Degnim A, Kuerer H, Leitch AM, Morrow M. Society of Surgical Oncology: position statement on prophylactic mastectomy. Approved by the Society of Surgical Oncology Executive Council, March 2007. Ann Surg Oncol. 2007;14(9):2425–2427. doi: 10.1245/s10434-007-9447-z. [DOI] [PubMed] [Google Scholar]
- 34.Geiger AM, West CN, Nekhlyudov L, et al. Contentment with quality of life among breast cancer survivors with and without contralateral prophylactic mastectomy. J Clin Oncol. 2006 Mar 20;24(9):1350–1356. doi: 10.1200/JCO.2005.01.9901. [DOI] [PubMed] [Google Scholar]