Abstract
Background: Rapid weight gain in infancy is associated with a higher risk of obesity in children and adults. A high relative reinforcing value of food is cross-sectionally related to obesity; lean children find nonfood alternatives more reinforcing than do overweight/obese children. However, to our knowledge, there is no research on how and when food reinforcement develops.
Objective: This study was designed to assess whether the reinforcing value of food and nonfood alternatives could be tested in 9- to 18-mo-old infants and whether the reinforcing value of food and nonfood alternatives is differentially related to infant weight status.
Design: Reinforcing values were assessed by using absolute progressive ratio schedules of reinforcement, with presentation of food and nonfood alternatives counterbalanced in 2 separate studies. Two nonfood reinforcers [Baby Einstein–Baby MacDonald shows (study 1, n = 27) or bubbles (study 2, n = 30)] were tested against the baby’s favorite food. Food reinforcing ratio (FRR) was quantified by measuring the reinforcing value of food (Food Pmax) in proportion to the total reinforcing value of food and a nonfood alternative (DVD Pmax or BUB Pmax).
Results: Greater weight-for-length z score was associated with a greater FRR of a favorite food in study 1 (FRR-DVD) (r = 0.60, P < 0.001) and FRR of a favorite food in study 2 (FRR-BUB) (r = 0.49, P = 0.006), primarily because of the strong association between greater weight-for-length z score and lower DVD Pmax (r = −0.71, P < 0.0001) and BUB Pmax (r = −0.53, P = 0.003). Infant monthly weight gain was positively associated with FRR-DVD (r = 0.57, P = 0.009) and FRR-BUB (r = 0.37, P = 0.047).
Conclusions: Our newly developed paradigm, which tested 2 different nonfood alternatives, demonstrated that lean infants find nonfood alternatives more reinforcing than do overweight/obese infants. This observation suggests that strengthening the alternative reinforcers may have a protective effect against childhood obesity. This research was registered at clinicaltrials.gov as NCT02229552.
Keywords: enriched environment, food reinforcement, infancy, non-food reinforce, obesity
See corresponding editorial on page 421.
INTRODUCTION
Food reinforcement is an important determinant of human food ingestion (1). The reinforcing value of food refers to how hard someone is motivated to work to gain access to food, which can be measured in the laboratory by having individuals perform operant responses for food through the use of a progressive ratio schedule of reinforcement (2). Obese preschoolers (3), children (4), and adults (1, 5) find food more reinforcing than do nonobese populations, and the relative reinforcing value of food is prospectively related to weight gain in children, adolescents, and adults (6, 7).
There is very limited research on the origins of food reinforcement. Mounting evidence suggests that rapid weight gain in the first 2 y of life is associated with a markedly increased risk for obesity later in life (8–10). Studies with infants have shown that sucking pressure and rate predict weight gain (11, 12), and these processes may be related to food reinforcement. The rate of responding for food at 3–5 y of age is related to BMI (3), but research is needed on young children to assess how early the behavioral phenotype of high food reinforcement develops and whether it is related to weight-for-length or weight gain in infants.
In the natural environment, adults and children alike are faced with many choices about how to allocate their time and energy. For infants, eating is often a choice among many other activities, including interacting with parents/siblings and playing with toys. Given this scenario, to determine how motivated an infant is to eat compared with engaging in other activities, we need to compare the reinforcing value of food with nonfood alternatives. Previous research demonstrated that lean children find nonfood alternatives more reinforcing than food, presented in a concurrent schedule, whereas overweight/obese children show the opposite preference (4). This observation might imply that the environment of lean children may have a protective effect against obesity. This implication is supported by the findings of Strauss and Knight (13), who demonstrated that children raised in an enriched home environment where nonfood alternatives (i.e., books, toys) were readily available had a lower risk of becoming obese later in life. In assessing treatment success, the availability of alternative reinforcers and high environment enrichment was associated with greater weight loss among children with a low relative reinforcing value of food (14).
The purpose of our article was to develop a paradigm to study food reinforcement among infants 9–18 mo of age and to study for the first time the relation between weight status and food reinforcement and the reinforcing value of alternatives to food in infants. The food reinforcing ratio (FRR)6 of favorite food to alternatives was assessed by measuring infants’ effort to gain access to food in proportion to the total effort for both food and nonfood alternatives by using developmentally appropriate single schedules. Two studies were conducted. In study 1, Baby Einstein–Baby MacDonald (DVD; Kids II Inc.) was used as a nonfood alternative to represent sedentary activities; in study 2, playing with bubbles (BUB) was used as nonfood alternative to represent more active play. We hypothesized that weight status (birth weight, current weight-for-length, and weight gain since birth) of the infants is positively associated with FRR, and lean infants will find nonfood alternatives, both DVD and BUB, more reinforcing than will overweight/obese infants.
METHODS
Participants
In study 1, we recruited 32 infants aged 9–18 mo and their respective biological mothers by using posted flyers and an existing database. Infants aged 9–18 mo were included in the study because at this age, most infants have the ability to finger-feed themselves due to mastery of the pincer grasp, can express “no” and “all done,” and can sit upright without assistance. To recruit the subject sample, which can be generalizable to the healthy population of infants with no potential developmental delays, we excluded infants from participation if they were born preterm (<37 wk of gestation), had a low birth weight (<2500 g), or had known developmental delay(s); their mother was <18 y old at the time of pregnancy; their mother smoked, drank ≥4 alcoholic drinks on a single occasion or >1 alcoholic drink on a daily basis, and/or used controlled substances while pregnant; or the pregnancy was a high-risk pregnancy. We also excluded infants whose parent(s) would not allow the child to interact with the television screen. Five enrolled infants were excluded from the final analysis due to not playing any of the food/nonfood reinforcement tasks (n = 3) and crying excessively and/or behavioral issues during the task (n = 2). The number of participants who completed the laboratory task and are included in the analysis was 27 infants (15 girls and 12 boys).
In study 2, participants included 37 infants aged 9–18 mo and their respective biological mothers by using posted flyers and an existing database. All other exclusionary criteria were the same as in study 1, except we excluded infants whose parent(s) would not allow the child to play with nonstaining, nontoxic bubbles. Seven enrolled infants were excluded from the final analysis due to not playing any of the food/nonfood reinforcement tasks (n = 3) and crying excessively and/or behavioral issues during the task (n = 4). The number of participants who completed the laboratory task and are included in the analysis was 30 infants (11 girls and 19 boys).
Procedures
For both studies, interested parents were screened via telephone interview, and eligible infants were scheduled for a 1-h laboratory appointment. All visits were scheduled during a time the mother felt the infant would be awake, alert, and willing to do the food/nonfood reinforcement task. Parents were also sent links to study questionnaires to complete before their appointment. Parents were instructed to avoid feeding their child 1 h before the visit and to provide the infant’s favorite solid food for the food portion of the task. On arrival in the laboratory, parents filled out a consent form for their infant’s participation and were briefed on the study protocol.
After the parent signed the consent form, the infant was placed in a high chair or on the parent’s lap, depending on the infant’s comfort level and the parent’s request. The parent was seated next to the infant if the infant was placed in a high chair. Parents were allowed to be present during the task to avoid separation anxiety and anxiety around strangers experienced by infants in this age group (15). Research staff then directed the infant’s attention to the mouse button. To adapt the infants to the task and reduce responding for novelty, we exposed infants to the computer mouse before starting the task. A series of Microsoft PowerPoint slides were presented to make a “cash register” noise when the mouse was pressed, and research staff demonstrated how to press the mouse button to create a “funny noise.” Once the infant appeared to understand how to control the noise with a button press, he or she was allowed 30 s to play with the mouse button. Infants received similar training (up to 5 min in length) for the food and nonfood task right after the “funny noise” period. Throughout the experiment, researchers remained neutral in their instructions to the infant and used only scripted cue phrases. Praise was given after the infant pressed the button during all trials for all reinforcers (e.g., “that’s right,” “good job”). The parent was instructed to use only the scripted phrases or phrases used by the researchers. Finally, research staff measured the height and weight of the parent and infant. Parents were debriefed and received compensation.
Measures
Food/nonfood reinforcement task
Reinforcing values of food and nonfood alternatives (screen time and blowing bubbles) were assessed by using a computerized task (3, 16). Developmentally appropriate modifications were made to the station setup and schedule of the food/nonfood reinforcement task based on a pilot study (n = 13). A touch-sensitive mouse (T620 Wireless Touch Mouse; Logitech) was used as the response manipulandum to easily allow infants to complete the task. The mouse was placed near the edge of the table within reach of the infant and was secured with Velcro strips. The television screen was offset to the left of the infant for study 1. In study 2, the television screen was not present. The researcher blew bubbles while sitting across from the infant. The computer screen, which counted the points earned by the infants, was not visible to the infant. Refer to Supplemental Figure 1 for the laboratory setup.
Progressive fixed-ratio (FR) schedules were used in this study; a reward was presented only after a specified number of responses were given by the infants. The schedules of reinforcement began with one button press (one response, FR1) to earn a reward, increasing linearly in difficulty up to a maximum of FR15 responses (i.e., 1, 1, 2, 2, 3, 3, ..., 15, 15). The number of responses, number of reinforcers earned, and the time spent in each session were recorded.
When food was earned, the researcher placed a piece of favorite solid food in front of the infant. When screen time was earned in study 1, researchers allowed the infant to watch the DVD for approximately 10 s. When bubbles were earned in study 2, researchers blew bubbles toward the infant and engaged him or her in playing with the bubbles (i.e., popping or catching) for approximately 10 s. Instead of using a concurrent schedule to assess relative reinforcing value, we modified the task to independently assess the reinforcing value of favorite food and the alternative (DVD or bubbles) separately. This modification was necessary due to some challenges we observed (i.e., infants failed to manipulate one of 2 buttons, pressing 2 buttons at the same time) with this age group of infants in a pilot study. In both studies, infants worked for access to these 2 rewards sequentially, with the order of the reward counterbalanced between participants. The infant continued to play the task until he or she gave signs of wanting to stop (e.g., crying, signing “all done,” saying “all done,” head turning away). In between tasks, parents were instructed to play with the infant in a separate play area for 1.5 min (no food, drinks, or toys were used).
The infant’s favorite food was used. In piloting the task (n = 13), we initially attempted to standardize the foods to Cheerios (General Mills) and puffs, but some infants had not experienced these foods, did not like these foods, and would not work for these foods. Given that the study was designed to assess the reinforcing value of food, we had mothers provide their infant’s favorite solid food for the food portion of the task. This approach also allowed us to avoid food allergies and to ensure that the food used in the food/nonfood reinforcement task was not a novel food to the infants. Parents brought in a wide range of foods, including Cheerios, bananas, avocados, and hot dogs. If necessary, food was cut into small pieces (approximately 1 × 1 × 1 cm) to standardize reward size. The total amount of food consumed by the infants was measured. Total energy consumption during the task and energy density of the food were than analyzed by using Nutritionist Pro (version 5.2.0; Axxya Systems LLC). Energy density of the favorite solid food was categorized as very low to medium energy density (<4 kcal/g) and high energy density (>4 kcal/g) (17).
Anthropometrics
The parent’s height was measured to the nearest 0.01 cm by using a calibrated stadiometer (SECA). The parent’s weight was measured to the nearest 0.1 kg with a calibrated digital weight scale (Tanita). The parent’s BMI (kg/m2) was calculated. The infant’s length was measured by using an infantometer with infants placed in a supine position (SECA). The infant’s weight was measured to the nearest 0.001 kg with a calibrated scale (SECA). The infant’s weight-for-length z score, weight-for-age z score, and length-for-age z score were calculated by using the WHO infant growth chart.
Questionnaires: demographics and baby eating behavior
Socioeconomic status and demographics were assessed by using a standardized questionnaire. Data were collected on age, race, ethnicity, household income, educational attainment, employment, and marital status. The Baby Eating Behaviour Questionnaire, an adapted version of the Child Eating Behaviour Questionnaire, is a parent-report psychometric measure of infant appetite (18). It encompasses 4 appetitive measurement scales: enjoyment of food (Cronbach’s α = 0.67), food responsiveness (Cronbach’s α = 0.85), slowness in eating (Cronbach’s α = 0.63), and satiety responsiveness (Cronbach’s α = 0.67). All questions in each subscale were scored on a 5-point Likert scale as never (1), rarely (2), sometimes (3), often (4), or always (5), and mean scores for each subscale were then calculated (range: 1–5). In addition to these 4 subscales, a general appetite item was also included in this questionnaire: how would you rate your child’s appetite? This item was scored on a 5-point Likert scale as poor (1), okay (2), good (3), very good (4), or excellent (5).
Laboratory environment
The laboratory room used for this experiment was designed for eating experiments. The room is equipped with negative pressure inside the laboratory rooms, HEPA filtration, and noise-dampening dry wall. The study was approved by the State University of New York at Buffalo Social and Behavioral Sciences Institutional Review Board.
Data analysis
Reinforcing values of food and nonfood alternatives (DVD or bubbles) were determined by using the maximum schedule achieved for the favorite food (Food Pmax) and nonfood alternative (DVD Pmax or BUB Pmax). The food reinforcing ratio of the favorite food in study 1 (FRR-DVD) and in study 2 (FRR-BUB) was determined by calculating the proportion of the food responses in comparison to the nonfood alternative [e.g., Food Pmax ÷ (Food Pmax + DVD Pmax)]. Infant monthly weight gain was calculated by subtracting laboratory-measured current weight from birth weight and then dividing by the age of the infant in months. Using the weight-for-length percentile, we classified infant obesity status as lean or overweight/obese (≥85th percentile).
Descriptive demographic data (means ± SDs) are presented in Table 1. Individual linear regression models were used to estimate the associations of FRR-DVD, FRR-BUB, Food Pmax, DVD Pmax, and BUB Pmax with continuous variables (weight-for-length z score, birth weight, 5 appetitive scales from the Baby Eating Behaviour Questionnaire, energy consumption, and energy density) and categorical variables (order, sex, and infant obesity status) separately. A linear regression model was used to estimate the associations of FRR-DVD, FRR-BUB, Food Pmax, DVD Pmax, and BUB Pmax with infant monthly weight gain by controlling for birth weight. All data analyses were conducted with JMP, version 7 (SAS Institute Inc.).
TABLE 1.
Child, maternal, and household characteristics of participants
| Study 1 (n = 27) |
Study 2 (n = 30) |
|||||
| Variable | Mean ± SD | n (%) | Range | Mean ± SD | n (%) | Range |
| Child | ||||||
| Sex | ||||||
| Male | 12 (44.4) | 19 (63.3) | ||||
| Female | 15 (55.6) | 11 (36.7) | ||||
| Age, mo | 13.2 ± 3.2 | 9.0–18.6 | 13.1 ± 2.6 | 8.9–17.8 | ||
| Race | ||||||
| Caucasian | 21 (77.7) | 25 (89.3) | ||||
| Minority | 6 (22.2) | 3 (10.7) | ||||
| Birth weight, kg | 3.53 ± 0.44 | 2.78–4.54 | 3.47 ± 0.47 | 2.52–4.25 | ||
| Weight-for-length z score1 | 0.50 ± 1.00 | −1.50 to 2.55 | 0.68 ± 0.97 | −1.03 to 2.65 | ||
| Weight-for-age z score1 | 0.33 ± 0.92 | −1.51 to 1.99 | 0.33 ± 1.04 | −1.96 to 2.14 | ||
| Length-for-age z score1 | 0.02 ± 0.95 | −2.12 to 2.19 | −0.28 ± 1.25 | −2.91 to 3.03 | ||
| BMI percentile1 | 64.7 ± 28.5 | 6.8–99.5 | 69.0 ± 27.4 | 15.4–99.6 | ||
| ≥85th percentile | 9 (33.3) | 13 (43.3) | ||||
| Breastfeeding duration | ||||||
| <6 mo | 10 (37.0) | 10 (33.3) | ||||
| ≥6 mo | 17 (63.0) | 20 (66.7) | ||||
| First introduction to solids | ||||||
| <4 mo | 0 (0) | 0 (0) | ||||
| 4–5 mo | 12 (44.4) | 12 (40.0) | ||||
| ≥6 mo | 15 (55.6) | 18 (60.0) | ||||
| Mother | ||||||
| Age, y | 29.5 ± 4.0 | 21.0–40.0 | 31.3 ± 4.4 | 21.8–41.5 | ||
| Race | ||||||
| Caucasian | 24 (88.8) | 26 (92.9) | ||||
| Minority | 3 (11.1) | 2 (7.1) | ||||
| Educational level | ||||||
| Some college or lower | 4 (14.8) | 7 (23.3) | ||||
| College graduate | 12 (44.4) | 8 (26.7) | ||||
| Postgraduate | 11 (40.7) | 15 (50.0) | ||||
| Parity | ||||||
| Nulliparous2 | 11 (41.8) | 14 (46.7) | ||||
| Parous ≥12 | 16 (59.3) | 16 (53.3) | ||||
| Current BMI, kg/m2 | 25.1 ± 4.8 | 18.6–38.0 | 27.7 ± 8.1 | 18.7–59.9 | ||
| Normal weight | 15 (55.6) | 14 (48.3) | ||||
| Overweight | 8 (29.6) | 7 (24.1) | ||||
| Obese | 4 (14.8) | 8 (27.6) | ||||
| Household | ||||||
| Total income | ||||||
| <$25,000 | 3 (11.0) | 4 (13.3) | ||||
| $25,000 to $49,999 | 3 (11.0) | 7 (23.3) | ||||
| $50,000 to $74,999 | 12 (44.4) | 4 (13.3) | ||||
| ≥$75,000 | 5 (19.5) | 12 (40.0) | ||||
| No response | 4 (15.5) | 3 (10.0) | ||||
Calculated by using the WHO growth chart.
Nulliparous, first-time pregnant women; parous ≥1, women with at least one pregnancy.
RESULTS
Study 1
Characteristics of infants and their mothers are presented in Table 1. Mothers of the infants were predominately highly educated (≥college graduate = 85.1%) and Caucasian (88.8%). There were 18 different types of food provided by the mothers. The energy density of the food ranged between 0.32 and 5.12 kcal/g. For their favorite solid food, 74.1% of the infants (n = 20) had a very low- to medium-energy density food (<4 kcal/g; i.e., grapes, puffs, and peas), and 25.9% of the infants (n = 7) had a high-energy density food (>4 kcal/g; i.e., animal crackers, cheese-flavored crackers, and goldfish crackers). No order effects (food or DVD first) were observed for FRR-DVD (P = 0.21); however, boys had higher FRR-DVD than did girls (P = 0.003).
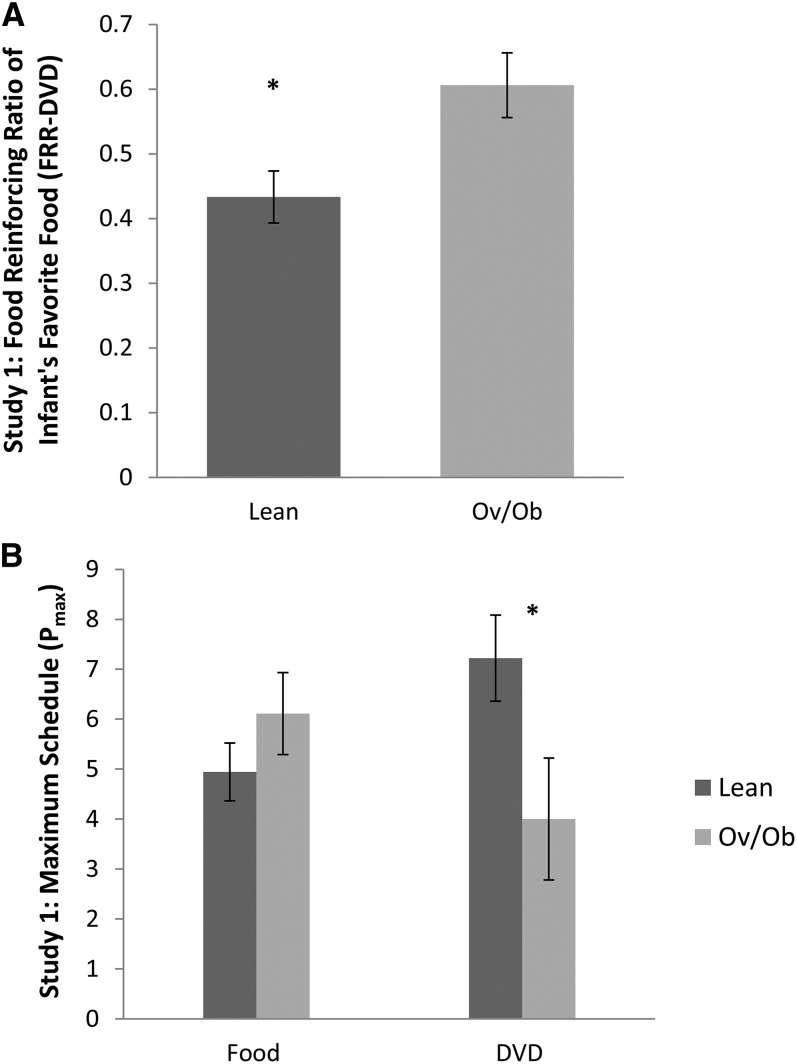
Greater weight-for-length z score was associated with greater FRR-DVD (r = 0.60, P < 0.001). There was no association between Food Pmax and weight-for-length z score (r = 0.02, P = 0.93). Greater DVD Pmax was associated with a lower weight-for-length z score (r = −0.71, P < 0.0001). Figure 1 illustrates the aforementioned results by using obesity status of the infants. Consistent with the absence of a relation between Food Pmax and weight-for-length z score, total energy consumed (P = 0.69) and energy density (P = 0.26) of the food were not associated with weight-for-length z score. Energy density of the food (P = 0.36) was not associated with Food Pmax. We did not observe significant associations between birth weight and FRR (P = 0.13), Food Pmax (P = 0.51), and DVD Pmax (P = 0.25). However, infant monthly weight gain was positively associated with FRR-DVD (r = 0.57, P = 0.009), controlling for birth weight. There was no association between infant monthly weight gain and Food Pmax (r = 0.20, P = 0.45), but greater infant monthly weight gain was associated with lower DVD Pmax (r = −0.56, P = 0.006).
FIGURE 1.
Infant obesity status in relation to food/nonfood reinforcement in study 1. Infants aged 9–18 mo (n = 27) performed the developmentally appropriate food/nonfood reinforcement task. There were 18 lean and 9 Ov/Ob infants. Reinforcing values of food and nonfood alternatives (DVD) were determined by using the maximum schedule achieved for Food Pmax and DVD Pmax. FRR-DVD was determined by calculating the proportion of food responses among all responses [Food Pmax ÷ (Food Pmax + DVD Pmax)]. The linear regression model shows that Ov/Ob infants had significantly higher FRR-DVD (mean ± SEM; lean: 0.43 ± 0.04; Ov/Ob: 0.61 ± 0.05; P = 0.009) (A). There was no difference between lean and Ov/Ob infants in Food Pmax (mean ± SEM; lean: 4.94 ± 0.58; Ov/Ob: 6.11 ± 0.82; P = 0.25) but a greater difference in DVD Pmax (lean: 7.22 ± 0.86; Ov/Ob: 4.00 ± 1.22; P = 0.04) (B). DVD, Baby Einstein–Baby MacDonald shows (Kids II Inc.); DVD Pmax, reinforcing value of nonfood alternative; Food Pmax, reinforcing value of food; FRR-DVD, food reinforcing ratio of favorite food in study 1; Ov/Ob, overweight and obese; Pmax, reinforcing value.
Table 2 provides the relationships between the different measures of food or nonfood reinforcement and the 5 appetitive scales measured with the Baby Eating Behaviour Questionnaire. In separate models, we observed significant positive associations between food responsiveness and general appetite and infant’s FRR-DVD (r = 0.44, P = 0.02; r = 0.41, P = 0.03, respectively). Satiety, enjoyment of food, and slowness in eating were not associated with FRR-DVD (r = −0.34, P = 0.08; r = −0.05, P = 0.79; and r = −0.11, P = 0.59, respectively).
TABLE 2.
Correlations of infant appetitive traits and food reinforcing ratio of favorite food1
| Study 1 |
Study 2 |
|||||
| Appetitive traits | FRR-DVD | Food Pmax | DVD Pmax | FRR-BUB | Food Pmax | BUB Pmax |
| Food responsiveness | 0.442 | 0.008 | −0.34 | 0.22 | 0.02 | −0.19 |
| General appetite | 0.412 | 0.04 | −0.30 | 0.392 | −0.05 | −0.472 |
| Satiety responsiveness | −0.34 | −0.07 | 0.31 | −0.06 | 0.04 | 0.15 |
| Enjoyment of eating | −0.05 | −0.16 | −0.15 | −0.05 | 0.00 | −0.01 |
| Slowness in eating | −0.11 | 0.05 | 0.16 | −0.25 | 0.02 | 0.25 |
BUB Pmax, reinforcing value of nonfood alternative (Bubbles); DVD Pmax, reinforcing value of nonfood alternative (DVD); FRR-BUB, food reinforcing ratio of favorite food in study 2; FRR-DVD, food reinforcing ratio of favorite food in study 1.
Significant at P < 0.05.
Study 2
Characteristics of infants and their mothers are presented in Table 1. Mothers of the infants were predominately educated (≥college graduate = 76.7%) and Caucasian (92.9%). There were 18 different types of food provided by the mothers. The energy density of the food ranged between 0.32 and 5.0 kcal/g. For their favorite solid food, 63.3% of the infants (n = 19) had a very low- to medium-energy density food (<4 kcal/g), and 36.6% of the infants (n = 11) had a high-energy density food (>4 kcal/g). No order (food or bubbles first) and sex effects were observed for FRR-BUB (order, P = 0.13; sex, P = 0.17).
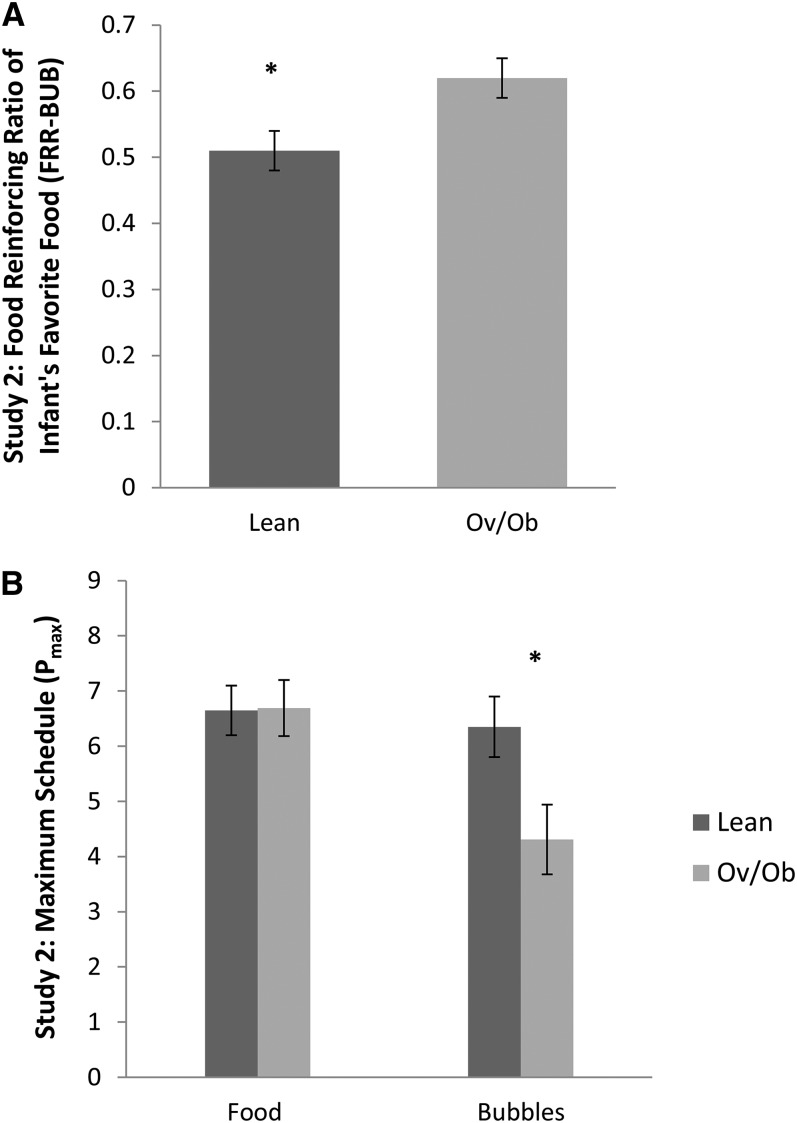
Greater weight-for-length z score was associated with greater FRR-BUB (r = 0.49, P = 0.006). There was no association between Food Pmax and weight-for-length z score (r = −0.08, P = 0.69). Greater BUB Pmax was associated with lower weight-for-length z score (r = −0.53, P = 0.003). Figure 2 illustrates the aforementioned results by using obesity status of the infants. Consistent with study 1, total energy consumption and energy density of the food were not associated with weight-for-length z score (P = 0.18 and P = 0.57, respectively), and energy density of the food was not associated with Food Pmax (P = 0.49). Similarly, we did not observe significant associations between birth weight and FRR-BUB (P = 0.85), Food Pmax (P = 0.80), and BUB Pmax (P = 0.84). However, infant monthly weight gain was positively associated with FRR-BUB (r = 0.37, P = 0.047), controlling for birth weight. There was no association between infant monthly weight gain and Food Pmax (r = 0.15, P = 0.46) or BUB Pmax (r = −0.26, P = 0.18).
FIGURE 2.
Infant obesity status in relation to food/nonfood reinforcement in study 2. Infants aged 9–18 mo (n = 30) performed the developmentally appropriate food/nonfood reinforcement task. There were 17 lean and 13 Ov/Ob infants. Reinforcing values of food and nonfood alternatives (bubbles) were determined by using the maximum schedule achieved for Food Pmax and BUB Pmax. FRR-BUB was determined by calculating the proportion of food responses among all responses [Food Pmax ÷ (Food Pmax + BUB Pmax)]. The linear regression model shows that Ov/Ob infants had significantly higher FRR-BUB (mean ± SEM; lean: 0.51 ± 0.03; Ov/Ob: 0.62 ± 0.03; P = 0.01) (A). There was no difference between lean and Ov/Ob infants in Food Pmax (mean ± SEM; lean: 6.65 ± 0.45; Ov/Ob: 6.69 ± 0.51; P = 0.95) but a greater difference in BUB Pmax (lean: 6.35 ± 0.55; Ov/Ob: 4.31 ± 0.63; P = 0.02) (B). BUB Pmax, reinforcing value of nonfood alternative (bubbles); Food Pmax, reinforcing value of food; FRR-BUB, food reinforcing ratio of favorite food in study 2; Ov/Ob, overweight and obese; Pmax, reinforcing value.
In terms of the 5 appetitive scales for study 2, consistent with study 1, we observed a significant positive association between general appetite and infant’s FRR-BUB (r = 0.39, P = 0.03) but not satiety, enjoyment of food, and slowness in eating (r = −0.06, P = 0.75; r = −0.05, P = 0.78; and r = −0.25, P = 0.19, respectively). In this study, however, we did not observe a positive association between food responsiveness and infant’s FRR-BUB (r = 0.22, P = 0.24).
DISCUSSION
After adapting a developmentally appropriate food/nonfood reinforcement task for infants aged 9–18 mo, we demonstrated for the first time, in 2 separate studies by using 2 different types of alternatives, that food/nonfood reinforcement can be observed in infants. Weight-for-length z score of the infants and rapid weight gain, but not birth weight, were strongly associated with FRR in both studies. Maternal report of infant’s food responsiveness and appetite was positively associated with FRR of the infants.
Previous infant research has not directly measured food reinforcement but measured infant sucking for milk. Agras and colleagues (11) showed that infant sucking pressure measured in the laboratory at 2 and 4 wk of age is positively associated with BMI at ages 1, 2, and 3 y. Stunkard and colleagues (12) also demonstrated that the total number of sucks measured during a test meal at age 3 mo was associated with body weight at 1 y of age. Sucking harder or faster may be related to the reinforcing value of food, suggesting this behavioral phenotype may be observed at a very early age. Research is needed to verify these observations by using a sucking apparatus that can vary the amount of work needed to gain access to the milk.
Eating is often a choice among many other available activities. Interestingly, we observed no relation between the absolute reinforcing value of food and weight status of the infants, but we observed low motivation to work for access to a nonfood alternative among overweight/obese infants. Even though these results may be due, in part, to the type of alternative reinforcer we studied, our results are consistent with previous work in 8- to 12-y-old children; lean children were more motivated to work for the nonfood alternative than were overweight/obese children (4). Being able to identify nonfood behaviors that can help overweight and obese children to shift their effort away from eating is an imperative strategy for obesity prevention.
Availability of alternative reinforcers is hypothetically related to environmental enrichment, and access to alternative reinforcers can make choice of not eating or eating easier. For example, Strauss and Knight (13) showed that enriched cognitive stimulation in environments of infant to 8-y-old children was associated with a lower risk of developing obesity. Similarly, access to alternative reinforcers negatively predicted the development of smoking among adults aged 18–22 y (19), and experimental evidence supports that the use of alternative reinforcers reduces substance use (20–22). Grimm and colleagues (23) demonstrated that rats reared in a socially enriched environment responded less to the sucrose-paired cue compared with rats housed singly without novel objects. Research has shown that when enriching the environment with a running wheel concurrent with access to cocaine, infusions of cocaine decreased by 21.9% in males and 70.6% in females compared with the cocaine-only condition (20). We previously have shown that an intervention providing nonfood alternatives to 8- to 12-y-old children increased their time spent on those activities and reduced their episodes of eating but did not result in lower energy intake and weight status (24). It is possible that the number, intensity, or duration of the approaches used to enhance the nonfood alternatives in this study was not enough to modify energy balance (25). Results from the present study suggest that the ratio of food reinforcement to alternative reinforcers may be established during infancy; thus, providing an enriched environment by parents at an early age might be useful to alter child choice of food and weight gain.
Furthermore, our study showed that infants with high FRR also had high food responsiveness and general appetite. It has been well established that appetitive traits, specifically responsiveness to external food cues and internal satiety cues, are associated with weight status in children and adults (26–28). Longitudinal studies have shown that higher appetite is prospectively associated with faster growth (11, 29). Van Jaarsveld et al. (30) showed that among discordant twins, the sibling with a heartier appetite measured at 3 mo was significantly heavier by 15 mo compared with the other sibling. Maternal report of infant food enjoyment was not related to infant FRR, consistent with previous research showing food reinforcement was a better predictor of child energy intake than food liking (4). Research has implicated both liking and motivation to obtain food as independent pathways for eating, which are regulated by different neurobiological systems (31, 32).
Understanding the progression of choice of food compared with other alternatives from infancy into childhood and adulthood may be important to preventing obesity. Because this is the first observation of the differential patterns of responding for food compared with alternative reinforcers in infants, these results need to be replicated and extended to other types of food and nonfood alternatives. In addition, a different method, such as eye tracking, typically used in infant developmental research to measure attention (33), could be used to examine infants’ differential attention between 2 objects when food and nonfood alternatives are presented in a concurrent fashion. Infant favorite foods were used as food reinforcers, which led to variation in the types of foods studied. However, infant’s food response was not influenced by the energy density of the food, because infants worked equally for access to high or low energy-dense foods. Food reinforcement is typically measured by using the food that participants like and enjoy eating (4), and using the infant’s favorite food facilitates the study of food/nonfood reinforcement. We studied 2 types of nonfood alternative reinforcers (watching a DVD or playing with bubbles), and the reinforcing value of other types of nonfood alternatives may be different. This is an important point because the food reinforcement ratio may depend on what is chosen as the alternative to food.
Although the results are interesting, there are important limitations to the study. Our sample population consisted of highly educated, middle-class families, and infants recruited in the studies were of mothers who did not drink, smoke, or use drugs during pregnancy. Food and nonfood reinforcement needs to be measured in infants of low socioeconomic backgrounds or infants of mothers who smoke, given their increased risk of becoming obese (34, 35) and the potential that alterations in food or nonfood reinforcement may be a risk factor for obesity. In addition, it is possible that the reinforcers used in these 2 studies might not have the same rewarding value (i.e., 10 s of watching a DVD vs. 10 s of playing with bubbles). Although having different magnitude reinforcers may alter responding for the food or nonfood alternative, shifting the food/nonfood reinforcement ratio, it is expected the pattern of individual differences in responding to the food or nonfood alternatives would still be observed. In the past, the reinforcing value of food compared with the nonfood alternative was measured by using concurrent schedules, which provides an individual a choice between 2 reinforcers presented simultaneously. However, to facilitate the infants learning the methods, we focused on separately measuring the absolute reinforcing value of the alternatives and used the ratio of food to nonfood reinforcement derived from the 2 absolute schedules. Future research is needed to develop ways of assessing relative reinforcing value in infants by using a concurrent schedule.
In summary, the results from our studies extend our knowledge of the relation between weight status and food/nonfood reinforcement in infants. Arguably, obesity occurs because there is a lack of pleasurable alternatives in one’s environment, thereby increasing the reinforcing value of eating (14). Infancy is a unique period for cellular differentiation and development, which makes it a vulnerable period for the development of obesity. By understanding the origins of food reinforcement, future interventions can be implemented at a young age to help overweight and obese populations alter their reinforcing value of food. Novel obesity prevention strategies, beginning as early as infancy, may prevent negative health consequences faced by obese children and the trajectory toward adult obesity.
Supplementary Material
Acknowledgments
We thank our statistician, Rocco Paluch, for his assistance with the data analyses; Katelyn Carr, Tinuke Daniels, Jen Scheid, and Elizabeth Sengupta for their assistance with designing the pilot study; April Seelbinder, Brigid Walsh, Neha Sharma, Elizabeth Woodworth, and Molly Walsh for assistance with subject recruitment, data entry, and execution of the study protocol; and Xiaozhong Wen for the use of the infantometer and infant scale.
The authors' responsibilities were as follows—KLK, DMF, RDE, and LHE: study design, data analysis, interpretation, and manuscript preparation; and KLK and DMF: data collection. None of the authors declared any conflicts of interest related to this study.
Footnotes
Abbreviations used: BUB, playing with bubbles; BUB Pmax, reinforcing value of nonfood alternative (bubbles); DVD, Baby Einstein–Baby MacDonald shows; DVD Pmax, reinforcing value of nonfood alternative (Baby Einstein–Baby MacDonald shows); Food Pmax, reinforcing value of food; FR, fixed ratio; FRR, food reinforcing ratio; FRR-BUB, food reinforcing ratio of favorite food in study 2 [food/(food + bubbles)]; FRR-DVD, food reinforcing ratio of favorite food in study 1 [food/(Food + DVD)].
REFERENCES
- 1.Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite 1996;27:41–50. [DOI] [PubMed] [Google Scholar]
- 2.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: a multilevel analysis. Psychol Bull 2007;133:884–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rollins BY, Loken E, Savage JS, Birch LL. Measurement of food reinforcement in preschool children: associations with food intake, BMI, and reward sensitivity. Appetite 2014;72:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr 2008;87:1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci 2007;121:877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill C, Saxton J, Webber L, Blundell J, Wardle J. The relative reinforcing value of food predicts weight gain in a longitudinal study of 7 10-y-old children. Am J Clin Nutr 2009;90:276–81. [DOI] [PubMed] [Google Scholar]
- 7.Epstein LH, Yokum S, Feda DM, Stice E. Food reinforcement and parental obesity predict future weight gain in non-obese adolescents. Appetite 2014;82:138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron N, Pettifor J, De Wet T, Norris S. The relationship of rapid weight gain in infancy to obesity and skeletal maturity in childhood. Obes Res 2003;11:457–60. [DOI] [PubMed] [Google Scholar]
- 9.Ekelund U, Ong K, Linne Y, Neovius M, Brage S, Dunger DB, Wareham NJ, Rossner S. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES). Am J Clin Nutr 2006;83:324–30. [DOI] [PubMed] [Google Scholar]
- 10.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics 2002;109:194–9. [DOI] [PubMed] [Google Scholar]
- 11.Agras WS, Kraemer HC, Berkowitz RI, Korner AF, Hammer LD. Does a vigorous feeding style influence early development of adiposity? J Pediatr 1987;110:799–804. [DOI] [PubMed] [Google Scholar]
- 12.Stunkard AJ, Berkowitz RI, Stallings VA, Schoeller DA. Energy intake, net energy output, is a determinant of body size in infants. Am J Clin Nutr 1999;69:524–30. [DOI] [PubMed] [Google Scholar]
- 13.Strauss RS, Knight J. Influence of the home environment on the development of obesity in children. Pediatrics 1999;103:e85. [DOI] [PubMed] [Google Scholar]
- 14.Best JR, Theim KR, Gredysa DM, Stein RI, Welch RR, Saelens BE, Perri MG, Schechtman KB, Epstein LH, Wilfley DE. Behavioral economic predictors of overweight children's weight loss. J Consult Clin Psychol 2012;80:1086–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shelov SP; American Academy of Pediatrics. Caring for your baby and young child: birth to age 5. New, rev. 5th ed. New York: Bantam; 2009. [Google Scholar]
- 16.Epstein LH, Wright SM, Paluch RA, Leddy J, Hawk LW, Jr, Jaroni JL, Saad FG, Crystal-Mansour S, Lerman C. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiol Behav 2004;81:511–7. [DOI] [PubMed] [Google Scholar]
- 17.Rolls BJ. The volumetrics eating plan: techniques and recipes for feeling full on fewer calories. New York: Harper; 2007. [Google Scholar]
- 18.Llewellyn CH, van Jaarsveld CHM, Johnson L, Carnell S, Wardle J. Development and factor structure of the Baby Eating Behaviour Questionnaire in the Gemini birth cohort. Appetite 2011;57:388–96. [DOI] [PubMed] [Google Scholar]
- 19.Audrain-McGovern J, Rodriguez D, Rodgers K, Cuevas J. Declining alternative reinforcers link depression to young adult smoking. Addiction 2011;106:178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav 2002;73:663–71. [DOI] [PubMed] [Google Scholar]
- 21.Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol 2010;92:572–92. [DOI] [PubMed] [Google Scholar]
- 22.Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav 2009;92:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, Harkness JH. Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behav Pharmacol 2008;19:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein LH, Roemmich JN, Stein RI, Paluch RA, Kilanowski CK. The challenge of identifying behavioral alternatives to food: clinic and field studies. Ann Behav Med 2005;30:201–9. [DOI] [PubMed] [Google Scholar]
- 25.Bickel WK, Johnson MW, Koffarnus MN, MacKillop J, Murphy JG. The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annu Rev Clin Psychol 2014;10:641–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viana V, Sinde S, Saxton JC. Children's Eating Behaviour Questionnaire: associations with BMI in Portuguese children. Br J Nutr 2008;100:445–50. [DOI] [PubMed] [Google Scholar]
- 27.French SA, Epstein LH, Jeffery RW, Blundell JE, Wardle J. Eating behavior dimensions: associations with energy intake and body weight: a review. Appetite 2012;59:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carnell S, Wardle J. Appetite and adiposity in children: evidence for a behavioral susceptibility theory of obesity. Am J Clin Nutr 2008;88:22–9. [DOI] [PubMed] [Google Scholar]
- 29.Agras WS, Kraemer HC, Berkowitz RI, Hammer LD. Influence of early feeding style on adiposity at 6 years of age. J Pediatr 1990;116:805–9. [DOI] [PubMed] [Google Scholar]
- 30.van Jaarsveld CH, Boniface D, Llewellyn CH, Wardle J. Appetite and growth: a longitudinal sibling analysis. JAMA Pediatr 2014;168:345–50. [DOI] [PubMed] [Google Scholar]
- 31.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 1998;28:309–69. [DOI] [PubMed] [Google Scholar]
- 32.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci 2003;26:507–13. [DOI] [PubMed] [Google Scholar]
- 33.Gredebäck G, Johnson S, von Hofsten C. Eye tracking in infancy research. Dev Neuropsychol 2010;35:1–19. [DOI] [PubMed] [Google Scholar]
- 34.Haas SA. Health selection and the process of social stratification: the effect of childhood health on socioeconomic attainment. J Health Soc Behav 2006;47:339–54. [DOI] [PubMed] [Google Scholar]
- 35.Wen X, Shenassa ED, Paradis AD. Maternal smoking, breastfeeding, and risk of childhood overweight: findings from a national cohort. Matern Child Health J 2013;17:746–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.