Abstract
Background: Recent evidence has indicated that flavanol consumption may have many health benefits in humans, including improved cognitive activities.
Objective: The aim was to evaluate the effect of flavanol consumption on cognitive performance in cognitively intact elderly subjects.
Design: This was a double-blind, controlled, parallel-arm study conducted in 90 elderly individuals without clinical evidence of cognitive dysfunction who were randomly assigned to consume daily for 8 wk a drink containing 993 mg [high flavanol (HF)], 520 mg [intermediate flavanol (IF)], or 48 mg [low flavanol (LF)] cocoa flavanols (CFs). Cognitive function was assessed at baseline and after 8 wk by using the Mini-Mental State Examination (MMSE), the Trail Making Test (TMT) A and B, and the Verbal Fluency Test (VFT).
Results: The changes in MMSE score in response to the 3 different treatments were not different. In contrast, there was a positive impact of the intervention on specific aspects of cognitive function. Mean changes (±SEs) in the time required to complete the TMT A and B after consumption of the HF (−8.6 ± 0.4 and −16.5 ± 0.8 s, respectively) and IF (−6.7 ± 0.5 and −14.2 ± 0.5 s, respectively) drinks significantly (P < 0.0001) differed from that after consumption of the LF drinks (−0.8 ± 1.6 and −1.1 ± 0.7 s, respectively). Similarly, VFT scores significantly improved among all treatment groups, but the magnitude of improvement in the VFT score was significantly (P < 0.0001) greater in the HF group (7.7 ± 1.1 words/60 s) than in the IF (3.6 ± 1.2 words/60 s) and LF (1.3 ± 0.5 words/60 s) groups. Significantly different improvements in insulin resistance (P < 0.0001), blood pressure (P < 0.0001), and lipid peroxidation (P = 0.001) were also observed for the HF and IF groups in comparison with the LF group. Changes in insulin resistance explained ∼17% of changes in composite z score (partial r2 = 0.1703, P < 0.0001).
Conclusions: This dietary intervention study provides evidence that regular CF consumption can reduce some measures of age-related cognitive dysfunction, possibly through an improvement in insulin sensitivity. These data suggest that the habitual intake of flavanols can support healthy cognitive function with age. This trial was registered at www.controlled-trials.com as ISRCTN68970511.
Keywords: blood pressure, cocoa flavanols, cognitive function, insulin resistance, lipid peroxidation
See corresponding editorial on page 423
INTRODUCTION
The question of whether some degree of cognitive deterioration is an inevitable part of aging or should be considered as a pathological prestage of dementia is debated. Aging is usually accompanied by deficits in several domains of cognition, including speed of processing, working memory capacity, inhibitory processes, and long-term memory, whereas other aspects of cognitive function, such as implicit memory and knowledge storage, are less influenced by aging (1). With increasing numbers of elderly adults, evidence-based approaches that can help to slow or prevent cognitive decline in the early stages of cognitive dysfunction are needed (2).
A number of factors have been proposed as potential contributors to brain aging, including hypertension and other cardiovascular disease risk factors (3, 4), as well as vascular changes, metabolic dysfunction, increased oxidative stress and enhanced inflammatory signaling within the aging brain, and nutritional factors (5, 6). Evidence suggestive of neuroprotective effects of flavonoids, polyphenolic compounds that are present in plant-based foods, has increased in recent years. Epidemiologic studies suggest a positive association between flavonoid intake and lower prevalence of cognitive impairment (7) and better cognitive performance (8, 9). Among the flavonoids, flavanols, a subclass abundant in tea, grapes, and cocoa products, have been proposed to have the potential to counteract many aspects of cognitive decline (10, 11). Data from animal and in vitro models show that flavonoids, including flavanols, can interact with the neuronal intracellular signaling pathways mediating neurodegeneration and neuroinflammation and with the cellular and molecular architecture of the brain responsible for memory, learning, and cognitive function (10, 12). Evidence from human studies showed that the consumption of the specific mixture of flavanols found in cocoa can reduce blood pressure and improve insulin sensitivity (13, 14) and induce vasodilation of the peripheral (13, 14) and cerebral (15) vascular system, increasing brain blood flow and perfusion (15, 16) mainly through an improvement in nitric oxide bioavailability in endothelial cells (17). These data suggest that the regular consumption of flavanols may have positive implications for age-related cognitive dysfunction. In support of this concept, in the Cocoa, Cognition, and Aging (CoCoA)5,5 Study, the consumption of a drink enriched with cocoa flavanols (CFs) was shown to improve cognitive function in elderly adults with mild cognitive impairment (MCI) (18). Significant improvements in blood pressure and insulin resistance were also observed (18).
This second part of the CoCoA study was designed to test the hypothesis that the regular consumption of a CF-containing beverage would also be effective in improving cognitive performance in elderly subjects with no evidence of cognitive dysfunction. The impact of this dietary modification on blood pressure, glucose metabolism, and oxidative stress was also studied given consistent evidence of positive effects of flavanols on these outcomes (13, 14, 19–21) and the potentially influential role of these outcomes on cognitive function (3–5).
METHODS
Participants
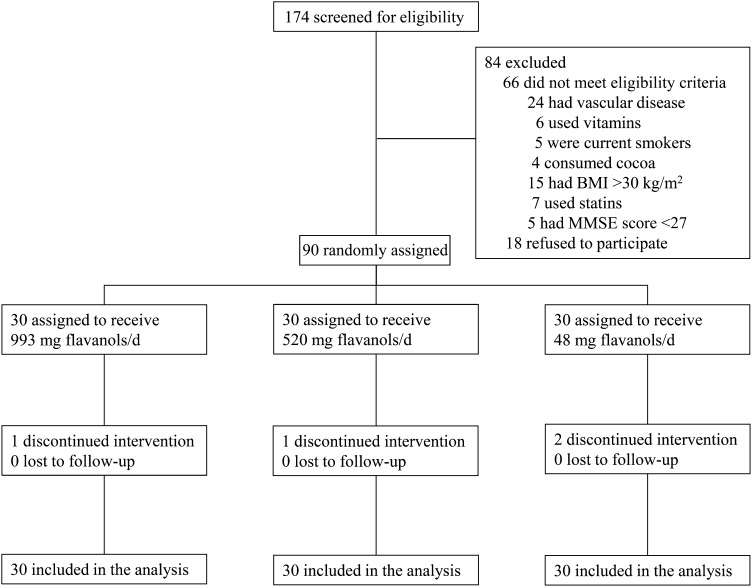
Participants were recruited among those referred to senior centers in the L’Aquila district, in central Italy. Criteria included participants who reported themselves as unconcerned about their own memory functions and with no clinically significant coexisting medical conditions, including known cardiovascular disease, cerebrovascular events, overt dementia [Mini-Mental State Examination (MMSE) score <27] or other neurological disorders, thyroid disorders, or inflammatory diseases. To avoid confounding due to the influence of concomitant depression on the performance on cognitive tests (22), subjects with a score on the Geriatric Depression Scale ≥11 were also excluded (23). Current smokers, habitual users of antioxidant supplements (including vitamins C and E), habitual consumers of chocolate or other cocoa products (daily consumption of any amount), or individuals prescribed medications known to have antioxidant properties (including statins and glitazones) or to interfere with cognitive functions (including benzodiazepines and antidepressants) were all excluded from participation. As long as participants were in stable condition, any other prescribed treatments were kept constant throughout the study period. Individuals with a BMI (in kg/m2) >30 and subjects reporting weight change ±10% body weight within the past 6 mo before entering the study were also excluded. A total of 174 subjects were screened by evaluation of medical history, multidimensional assessment (including neuropsychological testing and physical examination), laboratory variables, and assessment of the individuals’ habitual diet (Figure 1). Sixty-six individuals did not meet eligibility criteria: 24 had vascular disease, 6 regularly used antioxidant-containing vitamin supplements, 5 were current smokers, 4 habitually consumed cocoa-based products, 15 had BMIs >30, 7 used statins, and 5 had MMSE scores <27. Eighteen individuals refused to participate. The remaining 90 individuals were enrolled in the study. The study was approved by the Ethics Committee of the Public Health Agency of L’Aquila, Italy, and written informed consent to participate was provided for each of the participants. This trial was registered at www.controlled-trials.com as ISRCTN68970511.
FIGURE 1.
Flow of participants through the phases of the study. MMSE, Mini-Mental State Examination.
Study design and outcomes
To investigate the impact of regular CF consumption on cognitive function in cognitively intact elderly individuals, an 8-wk double-blind, randomized, parallel-arm study was conducted between December 2006 and July 2008. After initial enrollment, study participants met with a dietitian to evaluate current dietary habits, correct any nutritional insufficiencies, and discuss the incorporation of the low-calorie test products into their habitual diet. Participants were then instructed to maintain their usual lifestyle and intake of fruit and vegetables and to avoid or, to the maximum extent possible, limit the intake of specific flavanol-containing foods and beverages including tea, red wine, fruit and vegetable juices, and chocolate. A detailed list of foods was given to each participant to allow appropriate dietary choices without imposing restrictions on the total consumption of fruit, vegetables, and beverages (i.e., the total consumption of fruit and vegetables was not restricted; the recommendation was simply made to modify the types of these foods consumed). Dietary total flavanol intake was estimated by a validated semiquantitative dietary questionnaire (24) at the time of enrollment and after a 1-wk run-in period; total flavanol intake was recorded as the intake of free and gallated flavanol monomers and their structurally related oligomeric compounds known as proanthocyanidins. Results of this assessment are shown in Table 1; total flavanol intakes were in the range of what was previously reported for an Italian population (25, 26).
TABLE 1.
Mean dietary total flavanol intakes at enrollment and after the 1-wk run-in period in the 3 treatment groups1
| Treatment group, mg/d |
P |
||||
| Total flavanols2 | HF (n = 30) | IF (n = 30) | LF (n = 30) | ANOVA | Time × treatment interaction |
| Enrollment | 216.60 ± 7.13 | 210.67 ± 5.39 | 203.80 ± 6.18 | 0.357 | 0.9243 |
| End of run-in | 201.70 ± 4.39 | 197.37 ± 3.99 | 192.70 ± 4.34 | ||
Values are means ± SEs. Differences between groups were analyzed by ANOVA. HF, high flavanol intake; IF, intermediate flavanol intake; LF, low flavanol intake.
Total flavanols = the sum of free and gallated flavanol monomers and their structurally related oligomeric compounds.
Time effect, P = 0.0012; treatment effect, P = 0.1843.
All participants were encouraged to continue with their usual physical activity throughout the study period. After a 1-wk run-in period, participants were randomly assigned to consume once daily a dairy-based cocoa drink containing CFs either at high flavanol (HF; 993 mg flavanols/serving), intermediate flavanol (IF; 520 mg flavanols/serving), or low flavanol (LF; 48 mg flavanols/serving) amounts for 8 wk (Table 2). Throughout the intervention, drinks were provided in individual sachets containing a dry beverage mix; the mix was reconstituted with water according to provided instructions just before consumption. Frequent participant monitoring by weekly phone contact or prescheduled meetings, according to participant preference, was used to maximize compliance with the intervention and to minimize changes in overall diet and lifestyle. All participants were instructed to return excess and empty sachets to check compliance. Compliance was calculated by using the amount of servings ingested divided by the amount the participants should have ingested and multiplied by 100.
TABLE 2.
Chemical composition of the cocoa test drinks1
| HF | IF | LF | |
| Nutrient content per serving (58 g) | |||
| Calories | 218 | 216 | 215 |
| Total fat, g | 3 | 3 | 3 |
| Saturated fat, g | 1 | 1 | 1 |
| Cholesterol, mg | 9 | 10 | 10 |
| Total carbohydrates, g | 32 | 31 | 31 |
| Dietary fiber, g | 6 | 6 | 7 |
| Sugars, g | 18 | 17 | 17 |
| Protein, g | 17 | 17 | 17 |
| Caffeine, g | 41 | 44 | 46 |
| Theobromine, mg | 458 | 429 | 400 |
| Sodium, mg | 380 | 415 | 450 |
| Potassium, mg | 980 | 1088 | 1195 |
| Calcium, mg | 458 | 455 | 451 |
| Iron, mg | 5 | 7 | 9 |
| Phosphorus, mg | 521 | 509 | 496 |
| Magnesium, mg | 158 | 149 | 141 |
| Zinc, mg | 3 | 3 | 3 |
| Copper, mg | 0.7 | 0.8 | 0.8 |
| Manganese, mg | 1.1 | 1.1 | 1.0 |
| Flavanol and procyanidin composition, mg | |||
| Epicatechin | 185 | 95 | 5 |
| Catechin | 62 | 35 | 8 |
| Dimers | 182 | 96 | 10 |
| Trimers | 141 | 72 | 4 |
| Tetramers | 126 | 64 | 2 |
| Pentamers-decamers | 297 | 158 | 17 |
| Total flavanols (monomers-decamers) | 993 | 520 | 48 |
HF, high flavanol intake; IF, intermediate flavanol intake; LF, low flavanol intake.
Randomization ensured that the groups at baseline were approximately equal, averaging out between-subject differences on potential confounding due to baseline nutritional habits. Neither the treating physicians nor the patients were aware of treatment allocation.
Main outcome measures examined were changes in cognitive function after 8 wk of regular CF consumption. Secondary outcome measures examined included changes in blood pressure, metabolic variables, and plasma markers of lipid peroxidation.
Cocoa drinks
The dairy-based cocoa drinks were supplied as a dry beverage mix in individual anonymized sachets. The food products used in this study were specially designed, calorically balanced, carefully nutrient- and alkaloid-matched, and indistinguishable in appearance and had a flavanol content that was not obvious on the basis of flavor. The 3 beverage mixes contained similar macronutrient, mineral, theobromine, and caffeine content, varying significantly only in the content of CFs (Table 2). To achieve the higher content of flavanols, the HF and IF drink mixes were made with a flavanol-rich cocoa powder (Cocoapro processed cocoa powder; Mars Inc), whereas the LF drink was made with a highly processed, alkalized cocoa powder. The flavanol composition of the cocoa drinks is also shown in Table 2. In this study, the term “cocoa flavanols” is used to define the sum of all monomeric flavanols and their oligomeric derivatives (procyanidins) up to and including decamers (10 monomeric subunits). To prepare the drinks, the contents of the packets were mixed by a hand-held mixer with 250 mL warm water and immediately consumed. The assigned drink was consumed once a day, in the morning, during the entire length of the study, with the final drink consumed ∼24 h before the final evaluation. Participants were instructed to promptly inform researchers of any adverse events, including intolerance.
Cognitive function assessment
Study participants arrived on the morning of evaluation after a small breakfast had been consumed at home but before taking the daily cocoa drink. The participants were tested during a morning visit in a quiet room by neuropsychologically trained research assistants who were unaware of the aim of the study. The same research assistant administered the neuropsychological tests to the same subject during the study to minimize examiner bias. Cognitive testing was performed at baseline and after 8 wk (±2 d) by using a combination of 4 well-validated standardized tests: the MMSE (27), the Trail Making Test (TMT) A and B (28), and the verbal fluency test (VFT) (29). As per the predefined procedure, an integrated measure of overall cognitive function—composite cognitive z score—was also constructed for each participant by converting the log-transformed raw scores from the individual tests to standardized scores (z score) that were based on the means and pooled SDs of the whole cohort at baseline.
Blood pressure measurement
Before neuropsychological testing, clinical systolic and diastolic blood pressures were recorded in the morning with the use of a validated oscillometric device with appropriately sized cuffs (Omron 705 CP; Omron Matsusaka) on the nondominant upper arm. These evaluations were performed by staff blinded to the study protocol. At each visit, participants rested 15 min in a seated position, the first blood pressure measurement was taken but discarded, and the subsequent 3 consecutive blood pressure readings, taken at 3-min intervals, were recorded. The average of these latter measures was considered for statistical analysis.
Laboratory analysis
Within 24 h of neuropsychological testing, blood samples were drawn from each participant after an overnight fasting period for determinations of lipid profile and fasting plasma glucose and insulin. The HOMA-IR index [fasting serum insulin (mU/L) × fasting plasma glucose (mmol/L)/22.5] was calculated from fasting glucose and insulin concentrations as a marker of insulin resistance. Plasma concentrations of total 8-iso-prostaglandin F2α, as an index of oxidative stress–related lipid peroxidation (30), were assessed by enzyme immunoassay (Assay Design Inc) according to the manufacturer’s instructions.
Statistical analysis
The statistical analysis was conducted according to the intention-to-treat principle, and a per protocol analysis was performed as a sensitivity analysis. Accurate prestudy sample-size calculation was hindered by the lack of available data on the effect of CF administration on cognitive function in healthy elderly subjects. Thus, the study was based on an estimated sample size of 90 subjects, with a ratio 1:1:1 for the 3 treatment groups, which was calculated to be adequate to achieve 90% power to detect a moderate effect size (Cohen’s f: 0.25) with 2 df and an α of 0.05 on the global cognitive z score between HF and LF groups at week 8. SAS program version 9.2 was used to perform a 2-factor mixed-design ANOVA using the repeated statement with the general linear model procedure. Post hoc analysis between treatment groups was performed by Tukey’s studentized range honestly significant difference test. A 2-tailed P < 0.05 was considered significant. η2 was computed as measure of the magnitude of treatment effects (31). A chi-square test was used to compare categorical variables; ANOVA was used for continuous variables. Spearman nonparametric correlation was used to evaluate correlations between variables. Change in composite cognitive z score was entered as the dependent variable in a hierarchical multiple regression analysis in which changes in HOMA-IR, isoprostanes, and blood pressure were entered sequentially in the order suggested by the pathophysiologic hypothesis of a driving effect of improvements in insulin resistance on cognitive outcomes. Analysis was performed on variables logarithmically transformed to enhance symmetry of measures. If not otherwise specified, data are presented as means ± SEs.
RESULTS
Study cohort
General characteristics of the study cohort are shown in Table 3. According to the selection criteria, none of participants had a BMI >30, were current smokers, or were receiving statin treatment. Hypertension was the most prevalent cardiovascular disease risk factor. The 3 study groups were comparable with regard to sex, age, anthropometric characteristics, cardiovascular disease risk factor prevalence, and pharmacologic treatments (Table 3, Table 4). Dietary total flavanol intake was also similar in the 3 study groups at enrollment and slightly but significantly decreased in the whole cohort during the run-in period to a similar degree in all of the groups, a consequence of the dietary recommendations to limit as much as possible the intake of specific flavanol-containing foods, without significant differences between the 3 study groups at the end of follow-up (Table 1).
TABLE 3.
Baseline general characteristics of the study participants according to the assigned treatment1
| General characteristics | HF (n = 30) | IF (n = 30) | LF (n = 30) | P |
| Sex (M/F), n | 12/18 | 11/19 | 14/16 | 0.725 |
| Age, y (range) | 70.00 ± 0.882 (65–85) | 68.67 ± 0.67 (61–77) | 69.97 ± 0.77 (62–79) | 0.391 |
| BMI range, kg/m2 | 19–30 | 21–30 | 20–30 | |
| Hypertension, n (%) | 16 (53) | 19 (63) | 15 (50) | 0.557 |
| Treated | 12 (75) | 13 (68) | 11 (73) | 0.870 |
| Pharmacologic treatments | ||||
| ACE-Is | 7 (58) | 8 (62) | 6 (54) | 0.830 |
| ARBs | 2 (17) | 0 (0) | 2 (18) | 0.351 |
| CCBs | 5 (42) | 6 (46) | 4 (36) | 0.787 |
| Diuretics | 3 (25) | 4 (31) | 3 (27) | 0.894 |
| Diabetes, n (%) | 5 (16) | 5 (16) | 7 (23) | 0.748 |
| Oral agents | 4 (80) | 4 (80) | 6 (86) | 0.713 |
| Insulin | None | None | None | |
| Hypercholesterolemia, n (%) | 3 (10) | 7 (23) | 6 (20) | 0.372 |
| Former smoker, n (%) | 4 (13) | 3 (10) | 6 (20) | 0.533 |
ANOVA was used for continuous variables; chi-square test was used for categorical variables. ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II type 1 receptor blocker; CCB, calcium channel blocker; HF, high flavanol intake; IF, intermediate flavanol intake; LF, low flavanol intake.
Mean ± SE (all such values).
TABLE 4.
Changes in body weight, BMI, blood pressure, and metabolic variables during the study period in the 3 treatment groups1
| Treatment group |
P |
||||
| Variable | HF (n = 30) | IF (n = 30) | LF (n = 30) | ANOVA | Time × treatment interaction |
| Body weight, kg | |||||
| Week 0 | 68.10 ± 2.05 | 72.53 ± 1.76 | 72.73 ± 1.54 | 0.126 | 0.5112 |
| Week 8 | 68.03 ± 2.09 | 72.23 ± 1.78 | 72.87 ± 1.55 | ||
| Change | −0.07 ± 0.24 | −0.30 ± 0.34 | 0.13 ± 0.20 | ||
| BMI, kg/m2 | |||||
| Week 0 | 25.83 ± 0.58 | 26.43 ± 0.44 | 27.23 ± 0.47 | 0.15 | 0.6093 |
| Week 8 | 25.67 ± 0.58 | 26.33 ± 0.46 | 27.23 ± 0.49 | ||
| Change | −0.17 ± 0.13 | −0.10 ± 0.12 | 0.00 ± 0.11 | ||
| SBP, mm Hg | |||||
| Week 0 | 138.0 ± 1.28 | 137.0 ± 1.37 | 136.1 ± 1.11 | 0.56 | <0.0001 |
| Week 8 | 130.2 ± 1.21a | 130.2 ± 1.28a | 134.5 ± 1.37b | 0.028 | |
| Change | −7.83 ± 0.56a | −6.80 ± 0.59a | −1.60 ± 1.06b | <0.0001 | |
| P (ANOVA) | <0.0001 | <0.0001 | 0.14 | ||
| DBP, mm Hg | |||||
| Week 0 | 83.5 ± 0.95 | 83.5 ± 1.09 | 82.4 ± 1.13 | 0.68 | <0.0001 |
| Week 8 | 78.8 ± 0.82 | 80.3 ± 1.04 | 80.8 ± 1.25 | 0.37 | |
| Change | −4.77 ± 0.37a | −3.20 ± 0.36a | −1.57 ± 0.61b | <0.0001 | |
| P (ANOVA) | <0.0001 | <0.0001 | 0.016 | ||
| Glucose, mmol/L | |||||
| Week 0 | 5.38 ± 0.15 | 5.21 ± 0.14 | 5.60 ± 0.19 | 0.26 | <0.0001 |
| Week 8 | 4.79 ± 0.15a | 4.72 ± 0.13a | 5.55 ± 0.19b | 0.0005 | |
| Change | −0.59 ± 0.04a | −0.49 ± 0.04a | −0.05 ± 0.04b | <0.0001 | |
| P (ANOVA) | <0.0001 | <0.0001 | 0.26 | ||
| Insulin, mU/L | |||||
| Week 0 | 9.59 ± 0.91 | 10.14 ± 1.05 | 9.91 ± 1.32 | 0.94 | <0.0001 |
| Week 8 | 6.33 ± 0.74a | 5.73 ± 0.79a | 10.18 ± 1.40b | 0.005 | |
| Change | −3.27 ± 0.21a | −4.40 ± 0.30b | 0.28 ± 0.14c | <0.0001 | |
| P (ANOVA) | <0.0001 | <0.0001 | 0.05 | ||
| HOMA-IR | |||||
| Week 0 | 2.37 ± 0.25 | 2.36 ± 0.25 | 2.65 ± 0.40 | 0.76 | <0.0001 |
| Week 8 | 1.41 ± 0.18a | 1.21 ± 0.17a | 2.64 ± 0.41b | 0.0008 | |
| Change | −0.95 ± 0.08a | −1.15 ± 0.09a | −0.01 ± 0.05b | <0.0001 | |
| P (ANOVA) | <0.0001 | <0.0001 | 0.90 | ||
| TC, mmol/L | |||||
| Week 0 | 5.15 ± 0.14 | 5.28 ± 0.20 | 5.24 ± 0.16 | 0.86 | <0.0001 |
| Week 8 | 4.68 ± 0.14 | 4.84 ± 0.18 | 5.20 ± 0.18 | 0.07 | |
| Change | 0.48 ± 0.02a | 0.44 ± 0.04a | 0.04 ± 0.04b | <0.0001 | |
| P (ANOVA) | <0.0001 | <0.0001 | 0.25 | ||
| LDL cholesterol, mmol/L | |||||
| Week 0 | 3.15 ± 0.13 | 3.33 ± 0.17 | 3.30 ± 0.16 | 0.67 | <0.0001 |
| Week 8 | 2.67 ± 0.13a | 2.92 ± 0.14a,b | 3.26 ± 0.17b | 0.02 | |
| Change | −0.48 ± 0.02a | −0.42 ± 0.04a | −0.04 ± 0.04b | <0.0001 | |
| P (ANOVA) | <0.0001 | <0.0001 | 0.39 | ||
| HDL cholesterol, mmol/L | |||||
| Week 0 | 1.34 ± 0.05 | 1.24 ± 0.06 | 1.21 ± 0.05 | 0.20 | 0.027 |
| Week 8 | 1.38 ± 0.06 | 1.27 ± 0.06 | 1.22 ± 0.05 | 0.11 | |
| Change | 0.04 ± 0.01a | 0.03 ± 0.01a,b | 0.01 ± 0.01b | 0.027 | |
| P (ANOVA) | 0.0001 | 0.0014 | 0.083 | ||
| HDL:LDL cholesterol | |||||
| Week 0 | 0.44 ± 0.03 | 0.40 ± 0.03 | 0.41 ± 0.03 | 0.495 | <0.0001 |
| Week 8 | 0.54 ± 0.03 | 0.47 ± 0.03 | 0.42 ± 0.04 | 0.065 | |
| Change | 0.10 ± 0.01a | 0.07 ± 0.01a | 0.02 ± 0.01b | <0.0001 | |
| P (ANOVA) | <0.0001 | <0.0001 | 0.044 | ||
| Triglycerides, mmol/L | |||||
| Week 0 | 1.45 ± 0.06 | 1.54 ± 0.07 | 1.61 ± 0.06 | 0.22 | <0.0001 |
| Week 8 | 1.41 ± 0.06 | 1.44 ± 0.07 | 1.58 ± 0.06 | 0.13 | |
| Change | −0.04 ± 0.01a | −0.10 ± 0.01b | −0.03 ± 0.01a | <0.0001 | |
| P (ANOVA) | 0.0028 | <0.0001 | 0.073 | ||
| 8-Iso-PGF2α, pg/mL | |||||
| Week 0 | 296.8 ± 15.3 | 307.6 ± 16.9 | 303.6 ± 17.3 | 0.90 | 0.0015 |
| Week 8 | 228.7 ± 10.9a | 267.1 ± 13.3a | 327.5 ± 17.4b | <0.0001 | |
| Change | −68.2 ± 16.04a | −40.6 ± 13.83a | 23.9 ± 22.64b | 0.001 | |
| P (ANOVA) | 0.0002 | 0.0065 | 0.299 | ||
Values are means ± SEs. Differences within groups were analyzed by ANOVA. Differences between groups were analyzed by ANOVA followed by Tukey’s honestly significant difference test. Values within a row not sharing a common superscript letter are significantly different, P < 0.05. DBP, diastolic blood pressure; HF, high flavanol intake; IF, intermediate flavanol intake; LF, low flavanol intake; SBP, systolic blood pressure; TC, total cholesterol; 8-Iso-PGF2α, 8-iso-prostaglandin F2α.
Time effect, P = 0.619; treatment effect, P = 0.128.
Time effect, P = 0.199; treatment effect, P = 0.120.
Adherence to the study protocol
One participant assigned to HF treatment discontinued beverage consumption after 3 wk because of personal reasons, one participant assigned to IF treatment discontinued beverage consumption after 3 wk because of reported gastric discomfort, and 2 participants assigned to LF treatment discontinued beverage consumption after 4 wk because of personal reasons. Aside from the single reported event of gastric discomfort, all interventions were well tolerated, with no other mild or more severe adverse events reported throughout the trial. All of these 4 participants were followed up during the entire study period, and the data from the last scheduled measurements according to the study protocol were included in the database for statistical analysis according to an intention-to-treat procedure. The overall compliance, assessed by monitoring the number of returned drink sachets, was high, with compliance rates of 99.3% at week 4 and 98.7% at week 8, without any differences between the HF, IF, and LF interventions. Adherence to the dietary requirements was evaluated at randomization and at the end of follow-up by a checklist questionnaire of specified food items and by monitoring body weight. Compliance with the dietary restrictions was good because none of the subjects reported regular consumption of the restricted flavanol/procyanidin-containing foods and beverages. Furthermore, indications on overall diet and lifestyle were adequately followed by participants throughout the study because no significant changes in body weight or BMI in the 3 study groups were observed at the end of follow-up (Table 4).
Cognitive function measures
Baseline performance on the cognitive function tests was similar for the 3 treatment groups, indicating an adequate randomization procedure (Table 5). The response of MMSE scores to the 3 different treatments during the study period did not differ (P = 0.52) (Table 5). In contrast, there was an impact of the intervention on specific aspects of cognitive function. The changes throughout the study period in the time required to complete TMT A for HF and IF groups significantly differed from those for the LF group (P < 0.0001), with significant time reductions observed in subjects assigned to HF and IF interventions but not in those assigned to the LF intervention (Table 5). Similarly, the changes throughout the study period in the time required to complete TMT B for the HF and IF groups were significantly different in comparison to those for the LF group (P < 0.0001), with significant time reductions again observed among HF and IF subjects but not in those assigned to the LF intervention (Table 5).
TABLE 5.
Changes in neuropsychological test scores during the study period in the 3 treatment groups1
| Treatment group |
P |
||||
| Neuropsychological tests | HF (n = 30) | IF (n = 30) | LF (n = 30) | ANOVA | Time × treatment interaction |
| MMSE | |||||
| Week 0 | 29.07 ± 0.20 | 29.27 ± 0.20 | 29.23 ± 0.22 | 0.76 | 0.522 |
| Week 8 | 29.20 ± 0.17 | 29.27 ± 0.18 | 29.20 ± 0.19 | ||
| Change | 0.13 ± 0.13 | 0.00 ± 0.10 | −0.03 ± 0.09 | ||
| TMT A, s | |||||
| Week 0 | 33.87 ± 1.81 | 32.27 ± 1.67 | 33.07 ± 1.75 | 0.81 | <0.0001 |
| Week 8 | 25.25 ± 1.45a | 25.58 ± 1.53a | 32.13 ± 1.82b | 0.0035 | |
| Change | −8.57 ± 0.38a | −6.67 ± 0.45a | −0.77 ± 1.57b | <0.0001 | |
| P (ANOVA) | <0.0001 | <0.0001 | 0.63 | ||
| TMT B, s | |||||
| Week 0 | 79.23 ± 3.37 | 78.27 ± 3.43 | 77.45 ± 4.17 | 0.94 | <0.0001 |
| Week 8 | 62.71 ± 2.80a | 63.99 ± 3.14a | 76.43 ± 4.21b | 0.01 | |
| Change | −16.50 ± 0.8a | −14.20 ± 0.49a | −1.10 ± 0.68b | <0.0001 | |
| P (ANOVA) | <0.0001 | <0.0001 | 0.12 | ||
| VFT, words/60 s | |||||
| Week 0 | 24.87 ± 1.08 | 26.13 ± 1.11 | 23.72 ± 1.28 | 0.38 | <0.0001 |
| Week 8 | 32.58 ± 1.60a | 29.62 ± 1.35a,b | 25.05 ± 1.44b | 0.002 | |
| Change | 7.70 ± 1.09a | 3.57 ± 1.23b | 1.33 ± 0.45b | <0.0001 | |
| P (ANOVA) | <0.0001 | 0.007 | 0.01 | ||
| z Score | |||||
| Week 0 | −0.058 ± 0.10 | 0.083 ± 0.10 | −0.026 ± 0.17 | 0.72 | <0.0001 |
| Week 8 | 0.656 ± 0.10a | 0.582 ± 0.10a | 0.063 ± 0.15b | 0.0013 | |
| Change | 0.714 ± 0.03a | 0.498 ± 0.05b | 0.089 ± 0.7c | <0.0001 | |
| P (ANOVA) | <0.0001 | <0.0001 | 0.19 | ||
Values are means ± SEs. Differences within groups were analyzed by ANOVA. Differences between groups were analyzed by ANOVA followed by Tukey’s honestly significant difference test. Values within a row not sharing a common superscript letter are significantly different, P < 0.05. HF, high flavanol intake; IF, intermediate flavanol intake; LF, low flavanol intake; MMSE, Mini-Mental State Examination; TMT, Trail Making Test; VFT, Verbal Fluency Test.
Time effect, P = 0.594; treatment effect, P = 0.878.
VFT scores significantly improved in the study among all treatment groups, but to various degrees (P < 0.0001). Consistent with the TMT scores, significant improvements in verbal fluency were observed in the HF and IF groups. A significant improvement, although to a lesser extent than that seen in the HF and IF groups, was also observed after 8 wk among those assigned to the LF group; this was the only cognitive test for which a significant cognitive improvement was observed in this treatment group. The magnitude of improvement in VFT score was significantly greater in HF subjects in comparison to those assigned to the IF and LF groups (Table 5).
As an integrated measure of overall cognitive function, a composite overall z score was calculated. Consistent with the results detailed earlier, changes in the composite cognitive z score differed during the study period between the 3 treatment groups (P < 0.0001), with the HF and IF groups showing significant improvements after 8 wk of regular consumption of the defined intervention; no significant variations in the composite z score were observed in the LF group (Table 5). In a comparison across groups, despite no differences at baseline, the composite cognitive z score at the end of follow-up was significantly (P < 0.0001) better in subjects assigned to the HF and IF interventions than at week 0 and was significantly (P < 0.05) better in comparison to those assigned to the LF intervention (Table 5).
η2 Values as measures of the magnitude of treatment effects are presented as online supplemental material (Supplemental Table 1). All of the above results were confirmed by per protocol analysis (HF, n = 29; IF, n = 29; LF, n = 28) with the exception of the loss of statistical significance in the difference in the time required to complete the TMT B at 8 wk between IF and LF groups (data not shown).
Blood pressure and metabolic variables
Baseline blood pressure and metabolic variables were similar for the 3 treatment groups (Table 4), with no significant differences in any of these variables between any of the groups at baseline.
Blood pressure
The response of systolic blood pressure to the 3 treatments during the study period differed (P < 0.0001), with significant reductions among HF and IF subjects (Table 4). Systolic blood pressure at the end of follow-up was significantly (P < 0.05) lower in subjects assigned to the HF and IF treatments compared with those assigned to the LF treatment (Table 4). The response of diastolic blood pressure to the 3 different treatments during the study period also differed (P < 0.0001), with significant reductions among HF and IF subjects (Table 4). A significant reduction in diastolic blood pressure was also observed in subjects assigned to the LF group, but this decrease was lower in magnitude than the changes observed in the HF and IF groups (Table 4). Diastolic blood pressure did not differ significantly between the 3 treatment groups at week 8 (Table 4).
Metabolic variables
The response of plasma glucose and insulin to the 3 treatments during the study period differed (P < 0.0001), with significant reductions observed in subjects assigned to the HF and IF groups. A significant reduction in insulin concentrations was also observed in the LF group, but this decrease was lower in magnitude than changes in the HF and IF groups (Table 4). Plasma glucose and insulin concentrations at the end of follow-up were significantly (P < 0.05) lower in subjects assigned to the HF and IF groups in comparison to those assigned to the LF intervention (Table 4). The estimation of insulin resistance was determined through the calculation of an HOMA-IR score. Given the different reductions in fasting glucose and insulin concentrations between the 3 treatment groups during the study period, the response of the HOMA-IR score also differed (P < 0.0001), with significant reductions observed in subjects assigned to the HF and IF groups but not in those assigned to the LF group (Table 4). The change values of HOMA-IR for HF and IF treatments significantly differed from those for the LF treatment (Table 4). HOMA-IR at the end of study was significantly (P < 0.05) better in subjects assigned to the HF and IF interventions in comparison to those assigned to the LF intervention (Table 4). Spearman nonparametric correlation revealed significant relations between changes in plasma insulin and HOMA-IR during the study period and their respective values at baseline (ρ = −0.363, P = 0.0004, and ρ = −0.382 and P = 0.0002, respectively) in the study cohort considered as a whole.
With regard to lipid profile, circulating concentrations of total cholesterol, LDL cholesterol, and triglycerides significantly decreased through the study period and HDL-cholesterol concentrations significantly increased among subjects assigned to the HF and IF interventions, but these did not change among those assigned to the LF group (Table 4). Change values in LDL cholesterol and the HDL- to LDL-cholesterol ratio for HF and IF groups significantly differed from those for the LF group (Table 4). Circulating concentrations of LDL cholesterol at the end of the 8-wk intervention were significantly (P < 0.05) lower in subjects assigned to the HF group in comparison to those assigned to the LF group (Table 4). HDL- to LDL-cholesterol ratio improved in the HF and IF groups and, to a lesser extent, in the LF group through the study period (Table 4). Changes in circulating concentrations of triglycerides were significantly different in the IF group in comparison to those observed in the HF and LF groups (Table 4).
The response of plasma total 8-iso-prostaglandin F2α to the 3 treatments during the study period differed (P = 0.0015), with significant reductions observed among subjects assigned to both the HF and IF groups but not in those assigned to the LF group (Table 4). The change values for the HF and IF groups significantly differed from those for the LF group (Table 4). Plasma total 8-iso-prostaglandin F2α concentrations at the end of the study period were significantly (P < 0.05) lower in subjects assigned to the HF and IF groups in comparison to those assigned to the LF intervention (Table 4).
η2 Values as measures of the magnitude of treatment effects are presented in Supplemental Table 1. All of the above results were confirmed by per protocol analysis (HF, n = 29; IF, n = 29; LF, n = 28), with the exception of the loss of statistical significance of the difference in systolic blood pressure at 8 wk between IF and LF groups (data not shown).
Determinant of cognitive changes after flavanol consumption
Because of the collinearity between systolic and diastolic blood pressures, only changes in systolic blood pressure were entered in the hierarchical regression analysis because changes in systolic blood pressure were found to be more strictly correlated with changes in composite cognitive z score than were changes in diastolic blood pressure (ρ = −0.286, P = 0.0063, vs. ρ = −0.224, P = 0.0340). Changes in HOMA-IR were found to be the main determinants of changes in cognitive function, explaining ∼17% of composite z score variability through the study period (r2 = 0.1703, β = −0.2486, P < 0.0001; Table 6). Changes in plasma isoprostane concentrations and systolic blood pressure explained ∼4% and ∼3% of cognitive improvement throughout the study period, respectively (Table 6). Regression analysis with a stepwise approach confirmed this order of relevance of the tested variables on the changes in cognitive function (data not shown).
TABLE 6.
Hierarchical linear regression in the prediction of determinants of composite cognitive z score changes from baseline in the study cohort1
| Variable | r | r2 | r2 change | β (95% confidence limit) | P |
| ΔHOMA-IR | −0.4585* | 0.1703 | — | −0.2486 (−0.36396, −0.13317) | <0.0001 |
| ΔHOMA-IR | — | 0.2109 | 0.0386 | −0.2127 (−0.33119, −0.09422) | 0.0006 |
| Δ8-Iso-PGF2α | −0.4265* | −0.0007 (−0.00142, −0.000026) | 0.0421 | ||
| ΔHOMA-IR | — | 0.2372 | 0.0263 | −0.1641 (−0.29408, −0.03420) | 0.0139 |
| Δ8-Iso-PGF2α | −0.2858* | −0.0007 (−0.00137, 0.000008) | 0.0528 | ||
| ΔSBP | — | −0.0140 (−0.03010, 0.00217) | 0.0890 |
n = 90. *P ≤ 0.006. ΔHOMA-IR, change in HOMA-IR; Δ8-Iso-PGF2α, change in 8-iso-prostaglandin F2α; ΔSBP, change in systolic blood pressure.
DISCUSSION
In this study, daily CF consumption for 8 wk improved cognitive performance in a group of cognitively intact older adults, without major adverse effects. Furthermore, CF consumption significantly improved blood pressure and several metabolic markers. Interestingly, the improvement in cognitive performance was associated with a reduction in insulin resistance, suggesting a possible influential role of glucose metabolism in modulating cognitive function.
The role of cocoa in improving brain health is matter of growing scientific interest (32, 33). Several epidemiologic studies suggest a favorable association between diets rich in flavonoid-containing foods and cognitive function (7–9). Cognitive benefits were also reported in multiple dietary intervention studies (34). Although there has been heterogeneity across studies (34), and some null findings (35), the demonstration of cognitive benefits provides encouraging support for the concept that dietary flavonoids and, specifically, flavanols may play a positive role in maintaining and improving cognitive function, particularly with age.
The current study sheds new light on this relevant topic, providing evidence of a maintained improvement in cognitive performance in response to regular CF consumption; importantly, these benefits were observed at multiple intake amounts. The reported cognitive benefits included improvements in some tasks most affected by age-related cognitive decline (1). One important feature of this study was that final cognitive assessments were performed ∼24 h after the final drink was consumed, allowing evaluation of the chronic intake effects. This study provides evidence that CF-containing foods can be effective in reversing certain aspects of age-related cognitive decline. These effects were similar to those recently described in elderly individuals with MCI (18). Together, these findings suggest a restorative effect of habitual flavanol consumption on different expressions of cognitive dysfunction. Consistent with emerging epidemiologic evidence, these data also suggest that regular flavanol consumption may be protective against the development of age-related cognitive dysfunction.
A slight, but significant improvement in performance on the VFT was evident in the LF group. Given the drink’s very low CF amount, the observed cognitive improvement was likely a consequence of the intrinsic alkaloids (∼50 mg caffeine, 400 mg theobromine). Evidence supports that caffeine, alone (36) and in combination with theobromine (37), can favorably affect cognitive function. It is reasonable then to suggest that the cognitive improvements in the LF group could be attributable to meythlxanthine ingestion, highlighting the importance of controlling alkaloids in cognitive studies. The more consistent cognitive improvements in the HF and IF groups support a direct effect of CFs beyond that simply attributable to the consumption of alkaloids.
Our study provides insights about possible pathways that could underlie the observed cognitive effects. In the HF and IF groups, there was evidence of improvements in glucose metabolism, with a significant reduction in HOMA-IR, which was a result of decreased circulating insulin and glucose concentrations. Similarly, previous work from our group demonstrated that regular CF ingestion could improve cognitive function and insulin sensitivity in an elderly cohort with MCI (18). These improvements are consistent with a recent meta-analysis by Hooper et al. (19), which indicated promising benefits of CFs on insulin sensitivity. The contributory role of the reduction in insulin resistance in the improvement of cognitive performance was 2-fold higher in subjects with MCI than in subjects without MCI (∼40% vs. ∼17%). These findings are of particular relevance given growing evidence that suggests a central role for insulin resistance in certain aspects of brain aging (38, 39). These findings suggest an influential role of insulin resistance in modulating cognitive function in elderly subjects. Furthermore, these data suggest that dietary interventions that positively affect glucose homeostasis may confer important cognitive benefits. More research in this area is needed to explore this hypothesis further.
CF-mediated improvements in cognitive function have been previously reported (18, 40, 41). A study by Scholey et al. (40) showed acute cognitive improvements in healthy adults after the ingestion of drinks with CF amounts comparable to what were used in the current study. A single-blind study from Field et al. (41) showed that the consumption of 720 mg CFs (in dark chocolate) resulted in acute improvements in visual and cognitive function in healthy adults. Because improvements in cognitive function have not always been shown (15, 16, 35, 42), a possible consequence of methodologic differences between studies, more research is needed to understand the effect of CF consumption on cognitive function; however, this study and the related CoCoA study performed in MCI (18) should offer much-needed insight into the benefits of dietary CFs, particularly among the elderly.
One potential mechanism underlying the observed cognitive benefits may be CF-mediated improvements in cerebral endothelial function. The consumption of 450 mg CFs was shown to acutely improve perfusion in the brain (15). Likewise, the consumption of CFs for 2 wk (at an amount similar to that of the HF group) led to a significant increase in cerebral blood flow in healthy elderly individuals (15). It is thus intriguing to suggest that the observed improvements in cognitive performance could be a consequence of improvements in vascular reactivity. This interpretation of the data fits with previously published studies that demonstrated that acute and regular CF consumption can lead to a significant improvement in nitric oxide–dependent endothelial function within the peripheral vasculature (recently reviewed in reference 19), both in younger and older individuals, as well as in individuals with and without established cardiovascular disease (43). In addition to these effects on vascular function, direct neuroprotective effects of flavanols may be involved. Evidence from in vitro and animal studies support that flavonoids, including flavanols, can promote neuronal survival and differentiation, effects that may help promote memory and learning (10). Interestingly, in this study, neither blood pressure nor lipid peroxidation changes were shown to contribute significantly to variations in cognitive performance.
The potential clinical relevance and limitations of this study should be considered. First, the moderate benefits in cognitive performance and insulin resistance are quite intriguing because they were evident in subjects without evidence of cognitive dysfunction and in a relatively short period of time. Because the intervention lasted just 2 months, the full extent of these cognitive benefits and their duration are not yet known. Furthermore, because only the chronic effects on cognitive function were measured, acute/acute-on-chronic effects of CF consumption could not be determined. Second, subjects in this study were generally in good health, without relevant comorbidities, so this cohort was not representative of all elderly individuals. Third, to avoid confounders, habitual consumers of dietary supplements with antioxidant properties were excluded; however, this is likely to have had a limited impact on the generalizability of findings because supplement consumption in our cohort was low (<10%), which is similar to that reported in other cohorts (44). Finally, although measurement of plasma flavanols would have been a better measure of compliance, blood samples were taken after a 12-h fast, well past the expected clearance time of flavanols in plasma (45).
In summary, the results of this study indicate that the regular intake of CFs can improve aspects of cognitive performance among elderly subjects with no evidence of cognitive dysfunction, effects that appear to be dependent on the amount of CF intake. In addition to the possible direct effects of flavanols on cognitive function, general improvements in cardiovascular function and specific metabolic variables could have, alone or in combination, played a role in improving cognitive performance in these subjects. Importantly, these improvements were noted in the context of a typical habitual intake of flavanols (26), as well as in the context of medication use. These findings, together with other published work in a similar cohort (18), provide encouraging evidence that the regular inclusion of flavanol- containing foods may be an effective dietary approach for counteracting cognitive changes associated with brain aging and offer a possible complementary strategy to support cognitive and cardiovascular health with age.
Supplementary Material
Acknowledgments
We thank Liesbeth Bruckers and Francesca Solmi of the Center for Statistics, Hasselt University, for their statistical consultation and contributions.
The authors’ responsibilities were as follows—GD: designed the research and had primary responsibility for final content; DM, DG, AR, LP, RR, and MCL: conducted the research; SN: performed the statistical analysis; SN, CM, and GD: analyzed the data; and CK-U, RB, CF, and GD: interpreted the results and wrote the manuscript. All of the authors read and approved the final manuscript. CK-U is employed by Mars Inc., a company with long-term research and commercial interests in cocoa flavanols. None of the other authors declared a conflict of interest.
Footnotes
Abbreviations used: CF, cocoa flavanol; CoCoA, Cocoa, Cognition, and Aging; HF, high flavanol; IF, intermediate flavanol; LF, low flavanol; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; TMT, Trail Making Test; VFT, Verbal Fluency Test.
REFERENCES
- 1.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol 2009;60:173–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams KN, Kemper S. Exploring interventions to reduce cognitive decline in aging. J Psychosoc Nurs Ment Health Serv 2010;48:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol 2012;32:2060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uranga RM, Bruce-Keller AJ, Morrison CD, Fernandez-Kim SO, Ebenezer PJ, Zhang L, Dasuri K, Keller JN. Intersection between metabolic dysfunction, high fat diet consumption, and brain aging. J Neurochem 2010;114:344–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris MC. Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc 2012;71:1–13. [DOI] [PubMed] [Google Scholar]
- 7.Kuriyama S, Hozaka A, Ohmori K, Shimazu T, Matsui T, Ebihara S, Awata S, Nagatomi R, Arai H, Tsuji I. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project. Am J Clin Nutr 2006;83:355–61. [DOI] [PubMed] [Google Scholar]
- 8.Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol 2007;165:1364–71. [DOI] [PubMed] [Google Scholar]
- 9.Nurk E, Refsum H, Drevon CA, Tell GS, Nygaard HA, Engedal K, Smith AD. Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J Nutr 2009;139:120–7. [DOI] [PubMed] [Google Scholar]
- 10.Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JPE. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr 2008;3:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt-Schillig S, Schaffer S, Weber CC, Eckert GP, Müller WE. Flavonoids and the aging brain. J Physiol Pharmacol 2005;56(Suppl 1):23–36. [PubMed] [Google Scholar]
- 12.Williams RJ, Spencer JPE. Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med 2012;52:35–45. [DOI] [PubMed] [Google Scholar]
- 13.Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, Blumberg JB, Ferri C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr 2008;138:1671–6. [DOI] [PubMed] [Google Scholar]
- 14.Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, Desideri G, Blumberg JB, Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005;46:398–405. [DOI] [PubMed] [Google Scholar]
- 15.Sorond FA, Lipsitz LA, Hollenberg NK, Fisher NDL. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr Dis Treat 2008;4:433–40. [PMC free article] [PubMed] [Google Scholar]
- 16.Francis ST, Head K, Morris PG, MacDonald IA. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J Cardiovasc Pharmacol 2006;47:S215–20. [DOI] [PubMed] [Google Scholar]
- 17.Heiss C, Keen CL, Kelm M. Flavanols and cardiovascular disease prevention. Eur Heart J 2010;31:2583–92. [DOI] [PubMed] [Google Scholar]
- 18.Desideri G, Kwik-Uribe C, Grassi D, Necozione S, Ghiadoni L, Mastroiacovo D, Raffaele A, Ferri L, Bocale R, Lechiara MC, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) Study. Hypertension 2012;60:794–801. [DOI] [PubMed] [Google Scholar]
- 19.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 2012;95:740–51. [DOI] [PubMed] [Google Scholar]
- 20.Ried K, Sullivan TR, Fakler P, Frank OR, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev 2012;8:CD008893. [DOI] [PubMed] [Google Scholar]
- 21.Wiswedel I, Hirsch D, Kropf S, Gruening M, Pfister E, Schewe T, Sies H. Flavanol-rich cocoa drink lowers plasma F(2)-isoprostane concentrations in humans. Free Radic Biol Med 2004;37:411–21. [DOI] [PubMed] [Google Scholar]
- 22.Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull 1995;117:285–305. [DOI] [PubMed] [Google Scholar]
- 23.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17:37–49. [DOI] [PubMed] [Google Scholar]
- 24.Gaddi A, Cicero AF, Wani FO, Dormi A, Pasquarelli V, D'Addato S. The realization of a project aimed at reducing the plasmatic lipid level in a large Italian population improves the mean calcium daily intake: the Brisighella Study. Eur J Clin Nutr 2001;55:97–106. [DOI] [PubMed] [Google Scholar]
- 25.Knaze V, Zamora-Ros R, Luja’n-Barroso L, Romieu I, Scalbert A, Slimani N, Riboli E, van Rossum CTM, Bas Bueno-de-Mesquita H, Trichopoulou A, et al. Intake estimation of total and individual flavan-3-ols, proanthocyanidins and thea flavins, their food sources and determinants in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr 2012;108:1095–108. [DOI] [PubMed] [Google Scholar]
- 26.Vogiatzoglou A, Mulligan AA, Luben RN, Lentjes MA, Heiss C, Kelm M, Merx MW, Spencer JP, Schroeter H, Kuhnle GG. Assessment of the dietary intake of total flavan-3-ols, monomeric flavan-3-ols, proanthocyanidins and theaflavins in the European Union. Br J Nutr 2014;111:1463–73. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 28.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958;8:271–6. [Google Scholar]
- 29.Borkowski J, Benton A, Spreen O. Word fluency and brain damage. Neuropsychologia 1967;5:135–40. [Google Scholar]
- 30.Patrono C, Fitzgerald GA. Isoprostanes: potential markers of oxidant stress in atherothrombotic disease. Arterioscler Thromb Vasc Biol 1997;17:2309–15. [DOI] [PubMed] [Google Scholar]
- 31.Levine TR, Hullett CR. Eta squared, partial eta squared, and misreporting of effect size in communication research. Hum Commun Res 2002;28:612–25. [Google Scholar]
- 32.Scholey A, Owen L. Effects of chocolate on cognitive function and mood: a systematic review. Nutr Rev 2013;71:665–81. [DOI] [PubMed] [Google Scholar]
- 33.Sokolov AN, Pavlova MA, Klosterhalfen S, Enck P. Chocolate and the brain: neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci Biobehav Rev 2013;37:2445–53. [DOI] [PubMed] [Google Scholar]
- 34.Macready AL, Kennedy OB, Ellis JA, Williams CM, Spencer JPE, Butler LT. Flavonoids and cognitive function: a review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr 2009;4:227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crews WD, Jr., Harrison DW, Wright JW. A double-blind, placebo-controlled, randomized trial of the effects of dark chocolate and cocoa on variables associated with neuropsychological functioning and cardiovascular health: clinical findings from a sample of healthy, cognitively intact older adults. Am J Clin Nutr 2008;87:872–80. [DOI] [PubMed] [Google Scholar]
- 36.Ritchie K, Artero S, Portet F, Brickman A, Muraskin J, Beanino E, Ancelin ML, Carrière I. Caffeine, cognitive functioning, and white matter lesions in the elderly: establishing causality from epidemiological evidence. J Alzheimers Dis 2010;20:S161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smit HJ, Gaffan EA, Rogers PJ. Methylxanthines are the psycho-pharmacologically active constituents of chocolate. Psychopharmacology (Berl) 2004;176:412–9. [DOI] [PubMed] [Google Scholar]
- 38.Neumann KF, Rojo L, Navarrete LP, Faras G, Reyes P, Maccioni RB. Insulin resistance and Alzheimer’s disease: molecular links and clinical implications. Curr Alzheimer Res 2008;5:438–47. [DOI] [PubMed] [Google Scholar]
- 39.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012;122:1316–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scholey AB, French SJ, Morris PJ, Kennedy DO, Milne AL, Haskell CF. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J Psychopharmacol 2010;24:1505–14. [DOI] [PubMed] [Google Scholar]
- 41.Field DT, Williams CM, Butler LT. Consumption of cocoa flavanols results in an acute improvement in visual and cognitive functions. Physiol Behav 2011;103:255–60. [DOI] [PubMed] [Google Scholar]
- 42.Pase MP, Scholey AB, Pipingas A, Kras M, Nolidin K, Gibbs A, Wesnes K, Stough C. Cocoa polyphenols enhance positive mood states but not cognitive performance: a randomized, placebo-controlled trial. J Psychopharmacol 2013;27:451–8. [DOI] [PubMed] [Google Scholar]
- 43.Holt RR, Heiss C, Kelm M, Keen CL. The potential of flavanol and procyanidin intake to influence age-related vascular disease. J Nutr Gerontol Geriatr 2012;31:290–323. [DOI] [PubMed] [Google Scholar]
- 44.Skeie G, Braaten T, Hjartåker A, Lentjes M, Amiano P, Jakszyn P, Pala V, Palanca A, Niekerk EM, Verhagen H, et al. Use of dietary supplements in the European Prospective Investigation into Cancer and Nutrition calibration study. Eur J Clin Nutr 2009;63:S226–38. [DOI] [PubMed] [Google Scholar]
- 45.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 2006;103:1024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.