Abstract
Background: Weight loss (WL) negatively affects bone mineral density (BMD) in older populations and has specifically been shown in women.
Objective: In this prospective controlled trial, we examined variables of bone quality and endocrine changes after intentional WL in men.
Design: Thirty-eight overweight and obese [mean ± SD body mass index (in kg/m2): 31.9 ± 4.4; age: 58 ± 6 y] men were recruited to either WL through caloric restriction or weight maintenance (WM) for 6 mo.
Results: There was a −7.9 ± 4.4% and +0.2 ± 1.6% change in body weight in the WL and WM groups, respectively. There was a greater increase in femoral neck and total body BMD and bone mineral content (BMC) in the WM group than in the WL group (P-interaction effect < 0.05). In contrast, there was a trend for the tibia cortical thickness and area to decrease more in the WM group than in the WL group (P ≤ 0.08). There was a decrease in the periosteal circumference in both groups over time (P < 0.01) and no statistically significant changes in trabecular bone. Circulating total, free, and bioavailable estradiol decreased in the WL group compared with the WM group, and changes were different between groups (P < 0.05). Serum total and bioavailable testosterone increased in both groups (P < 0.01). Serum 25-hydroxyvitamin D increased to a similar extent in both groups (P < 0.05).
Conclusions: Moderate WL in overweight and obese men did not decrease BMD at any anatomical site or alter cortical and trabecular bone and geometry. Also, despite increased BMD at some sites when maintaining excess body weight, cortical bone showed a trend in the opposite direction. This trial was registered at clinicaltrials.gov as NCT00472745.
Keywords: bone, caloric restriction, men, obesity, sex steroids
INTRODUCTION
The promotion of weight loss (WL) in a rising number of overweight and obese individuals seems the rational way to improve health outcomes. However, despite numerous health benefits in older adults (1), WL can also promote bone and muscle loss with older age (3, 4). In addition, changes in hormonal status with aging in both sexes have direct effects on skeletal health (4). Postmenopausal women undergoing voluntary WL experienced a 1–2.5% bone loss compared with that of a weight-stable group (5, 6). Similarly, epidemiologic studies of older men showed that WL (both voluntary and involuntary) was associated with bone loss and increased fracture risk (7, 8). A delay in age-related sex-steroid decline (9) in men compared with women might explain the later onset of bone loss in this population. As a result, WL-induced bone loss in aging men may differ compared with that observed in older women.
Weight-reduction studies in men examining multiple bone sites, compartments, and geometry are currently lacking. A 1-y intervention in middle-aged overweight men examined dieters who lost 6.4% body weight and 1.5% total body bone mineral density (BMD), but specific bone sites were not measured (10). Another 1-y trial that examined hip BMD and included elderly men and women (∼1:3) showed that a 9.7% WL resulted in a 2.6% BMD loss and negatively affected bone geometry (3, 11). Other smaller intervention studies of WL conducted in older adults that suggested bone loss either did not have a control group or examine primarily a female population (63–98%) (12, 13). Nevertheless, a large observational prospective study showed hip bone loss in elderly men (age: 74 y) who experienced 5% WL, and this WL was even greater in men who decreased serum testosterone concentrations (14).
Although a higher body weight is associated with higher bone mass, there is evidence that bone quality is compromised in the obese (15–17). It is possible that these heavy individuals undergo more dieting or weight cycling, which may be detrimental to bone (2, 18). Hence, an understanding of whether there are changes in bone quality because of WL is important to better predict fracture risk especially in this population. In this controlled trial, the effects of caloric restriction on BMD, geometry, and strength were examined to determine the risk-benefit ratio of WL in middle-aged and older obese and overweight men. Because WL alters sex steroids, 25-hydroxyvitamin D [25(OH)D], and parathyroid hormone (PTH), these hormones were investigated to determine whether they can explain changes in bone parameters.
METHODS
Subjects
Overweight and obese men [BMI (in kg/m2) range: 25–39; age range: 50–72 y] were recruited in the nutrition and WL laboratory at Rutgers University by using local newspaper, electronic, and radio station advertisements. Subjects were recruited during winter months to either maintain or lose weight. Telephone interviews were conducted by trained staff to determine whether subjects met the experimental criteria. Men who were undergoing treatment known to influence calcium or bone metabolism or diagnosed with diseases known to influence calcium metabolism (i.e., metabolic bone disease; hyperparathyroidism; untreated thyroid disease; significant immune, hepatic, or renal disease; kidney stone in the past 5 y; diabetes; significant cardiac disease; active malignancy; or cancer therapy within the past year) were excluded. Men who experienced WL 3 mo before recruitment were also excluded. All eligible volunteers underwent biochemical and physical screening including a comprehensive chemistry panel, complete blood count, and physical examination to ensure they were healthy and had no evidence of undiagnosed diseases (i.e., diabetes and anemia). Subjects signed an informed consent approved by the Rutgers University Institutional Review Board for the protection of human subjects in research before the initiation of the study protocol. The trial was also monitored by an external advisory review board and registered at clinicaltrials.gov as NCT00472745. The protocol met the ethical standards in accordance with the Helsinki Declaration.
Study design
Subjects were recruited to either lose or maintain weight over a 6-mo study period by using the same criteria. Subjects who volunteered for the WL treatment were counseled and offered weekly classes for the first 2 mo of the study followed by biweekly classes until 6 mo. Subjects followed a standard behavior modification nutrition education WL program that has been shown to be effective to increase compliance in our previous studies conducted at our WL unit and at the NY Obesity and Nutrition Research Center as described previously (6). Caloric intake was individualized, consisted of a 500–600-kcal deficit/d, and was designed to achieve a moderate WL.
Participants were instructed to provide detailed information on portion sizes and methods of food preparation to complete food diaries. Adherence to diets was assessed through at least three 24-h recalls twice during the intervention. The dietitian reviewed these diaries with subjects to increase compliance and ensure accuracy. In this prospective adaptive study design, individuals originally in the WL group who were not compliant and did not lose weight within the first 7 wk were eligible for the WM group. If they agreed to continue in the WM group and still met the eligibility criteria, the WM group was considered their final group. Subjects recruited for the weight-maintenance (WM) group were asked to maintain their weights during the intervention. In addition, these individuals were offered free nutrition-education classes with the dietitian at the end of the study. WM was defined as a <2.5% change in body weight from baseline to the end of the 6-mo study. Similar to the WL group, WM subjects were weighed and measured at the same intervals (baseline and months 1, 3, and 6). At these time points, we collected food diaries and assessed for calcium intake and adverse events.
Supplements and nutrient analysis
Calcium intake was assessed through food-frequency questionnaires during the initial screening with a goal to achieve a daily intake of 1.2 g Ca for subjects in both groups beginning 1 mo before and throughout the intervention. Our goal was to examine how caloric restriction under controlled intakes of calcium and vitamin D affected bone variables. A multivitamin and mineral tablet with 400 IU vitamin D3 and 200 mg Ca (Nature Made Multi 50+ Pharmavite, LLC) was given to all the volunteers. As needed, if dietary calcium plus the supplement in the multivitamin did not reach 1.2 g/d, calcium intake was adjusted accordingly by using 200-mg Ca tablets (calcium citrate; Bayer Inc.). All subjects were asked to refrain from taking any other supplements during the study. Dietary and supplemental calcium intakes were assessed for WL and WM groups at baseline and months 1, 3, and 6 by using calcium-intake questionnaires. To assess nutrient intakes, food diaries were analyzed in all study participants from 2 nonconsecutive weekdays and one weekend day at baseline and twice during the intervention with FoodWorks software (version 11; FoodWorks).
Physical activity
Subjects were encouraged to follow their usual exercise regimen throughout the study. Men were given a physical activity questionnaire to assess their physical activity level (PAL) (19). A numerical score was used to calculate PAL with a range from 0 to 3 (0 = inactivity, 1 = low activity, 2 = moderate activity, and 3 = high activity) to reflect an estimation of energy expenditure in metabolic equivalent minutes per week (19). In addition, subjects were asked to record the amount and type of physical activity on a specified area of their food-diary forms.
Body weight and height
A balance-beam scale and stadiometer (Detecto) were used for anthropometric measures. Body weight was obtained at regular morning visits while wearing light clothing. BMI (in kg/m2) was calculated as weight divided by height squared.
Soft tissue and BMD
Dual-energy X-ray absorptiometry (DXA) measurements were performed with a total body scanner (Lunar Prodigy Advanced; GE-Lunar) (CV ≤1% for all sites) at baseline and 6 mo. Total hip, lumbar spine, femoral neck, trochanter, and total body BMD and bone mineral content (BMC) were measured. In addition, soft tissues were evaluated including total tissue, fat (android and gynoid), and fat-free soft tissue compartments. Vertebral exclusion criteria and corrections to DXA lumbar spine measurements (specified by the International Society of Clinical Densitometry) (20) were performed by a trained physician. Vertebrae that showed signs of a local structural change or anatomic abnormality or artifact were excluded if the T-score difference between the abnormal vertebra and adjacent one was >1.0.
Peripheral quantitative computed tomography
Peripheral quantitative computed tomography (pQCT) (Stratec XCT 3000; Orthometrix Inc.) was used for bone measurements performed at specific sites (4% and 38%) at the distal nondominant tibia and included volumetric total BMD, trabecular, cortical BMD and BMC, geometry, and strength indexes at the tibia. Scans were analyzed with Stratec XCT software (Orthometrix, Inc., version 5.4). A scout view allowed the positioning of cross-sectional measurements along the tibia. The voxel size for all the scans was 0.5 mm, and the slice thickness was 2.4 mm. The precision error (CV) was <1.7% for all measurements.
Laboratory methods
After a 12-h overnight fast, venous blood and a spot urine sample was taken from each participant in the study at baseline and 1, 3, and 6 mo. Samples were analyzed in a batch analysis for the hormones 25(OH)D (radioimmunoassay; DiaSorin) (CV <12.5%) and intact PTH (immunoradioassay; Scantibodies) (CV <6.8%) and the sex hormones ultrasensitive estradiol (radioimmunoassay; DSL) (CV <8.9%) and total testosterone and free testosterone (ELISA; Alpco Diagnostics) (CV <9.6% and <12.4, respectively). Sex hormone binding globulin (SHBG) (ELISA; Alpco Diagnostics) (CV <12.1%) and osteocalcin (radioimmunoassay; Biomedical Technologies) (CV <9%) were measured at baseline and after 6 mo.
Calculations
Concentrations of total testosterone, total estradiol, SHBG, and measured albumin were used to calculate bioavailable testosterone and free and bioavailable estradiol according to the algorithm described previously by Vermeulen et al. (21). The algorithm for the free sex hormone calculation assumes that the concentration of the free sex steroids in blood is the result of the interaction between SHBG/albumin and the total hormone concentration (testosterone or estradiol) through different affinity constants of the peptides for these sex hormones without any interaction with other hormones in the blood that could influence the equilibrium
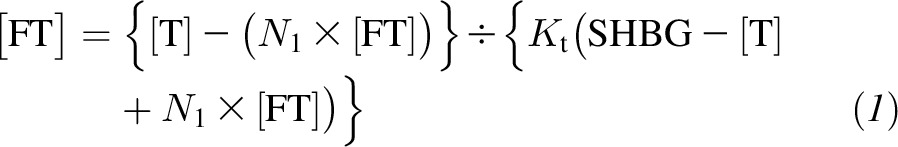 |
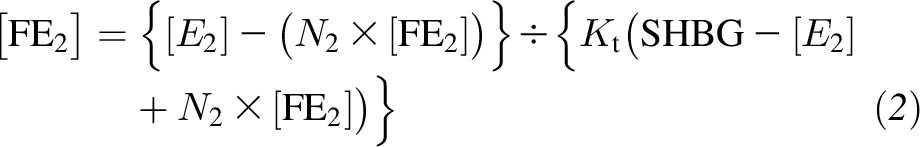 |
where [T] and [E2] are total testosterone and total estradiol concentrations, respectively; [fT] and [fE2] are fT and fE2 concentrations; KtT and KtE2 are the affinity constants of SHBG for testosterone and estradiol, respectively;
 |
and
 |
where Ca is the albumin concentration (BSA, Fisher Scientific, CV <2.9%), and KaT and KaE2 are affinity constants of albumin for testosterone and estradiol, respectively.
Safety
Serious adverse events and adverse symptoms were recorded throughout the study duration. Adverse symptoms included the following: headaches, pain in legs, swelling in legs, pain or heaviness in the chest, nausea, dizziness, fatigue, muscle weakness, urinary frequency, abdominal pain, or muscle aches. The presence of a symptom included the categories sometimes, often, or always, whereas the absence of a symptom required one of the 2 following responses: never or rarely. Volunteers were asked to fill out an adverse symptoms form during baseline measurements and months 1, 3, and 6 at study meetings.
Statistical analysis
Analyses were conducted with the SAS statistical package (v 9.2; SAS Institute). Groups were compared for baseline characteristics by using 1-factor ANOVA. Variables considered clinically important, even if not significantly different, were used as covariates in analyses (i.e., body weight). Additional covariates included age, season, PAL, sex hormones (total testosterone, free testosterone, and estradiol), SHBG, and 25(OH)D. A mixed-model ANCOVA was used to analyze the 2 main-effect determinants of group and time (6-mo intervention period) on bone variables, fat, fat-free soft tissue, hormones, and nutrient intake. When the interaction group-by-time was significant, a post hoc analysis was conducted with the Bonferroni correction for multiple comparisons. For hormones with >2 time-point measurements (0, 1, 3, and 6 mo), repeated measures ANCOVA using mixed-models with age, season, PAL and baseline weight as covariates was also conducted followed by pairwise comparisons with Bonferroni correction. Pearson correlation coefficients were used to assess the relation between changes in outcomes and changes in the measured variables. A stepwise multiple linear regression analysis (forward selection technique) was performed to select explanatory variables that would be considered the most-important predictors for bone changes. We controlled for micronutrients including calcium and vitamin D during these studies, and thus, micronutrient intake was not included in the model.
In a previous study conducted in overweight and obese men, a 6.4-kg body WL resulted in a total body BMC loss of 42 ± 35 g (10). With a power of 90% and α = 0.05, a sample size of 13/group was required to detect significant differences for total body BMC between groups. Secondary outcomes were bone regulating hormones and sex steroids in response to WL. To allow for one covariate and because of anticipated dropouts and noncompliance, we recruited additional subjects in each group. An evaluation of the success or failure to lose weight (22) was not the goal in this study but, rather, to examine whether successful WL affects bone, and hence, we report data for subjects who met final eligibility criteria and completed the study. A separate analysis of all subjects (n = 44) by using the success or failure of WL as a covariate in the originally assigned groups was conducted to assess how WL affected changes in bone variables. Values are reported as means ± SDs and figures are means ± SEMs. Categorical values are expressed in percentages to represent a portion of the sample. Significance was considered at P < 0.05.
RESULTS
Participants
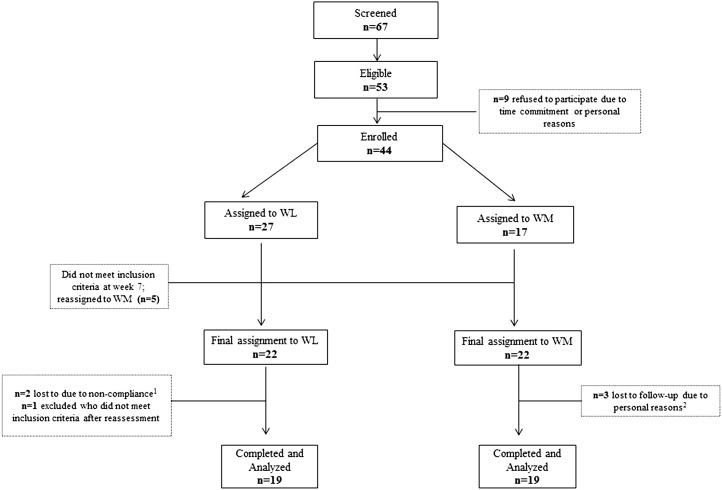
Of 67 men who were screened, 44 men met the inclusion and exclusion criteria and were in either the WM (n = 22) or WL (n = 22) group. Three men dropped out within the first month of the study in the WM group because of personal reasons. One recruited volunteer, who underestimated his weight in the telephone screen, was excluded because, after weight measurement, he no longer met BMI inclusion criteria. Two subjects dropped out because of noncompliance. After 7 wk of dietary counseling, 5 subjects were unsuccessful at losing weight and, therefore, were excluded from the WL group. These men were asked to maintain weight and be part of the WM group. Thirty-eight men (BMI: 31.9 ± 4.4; age: 58 ± 6 y) who included 36 Caucasians, 1 African American, and 1 Asian completed the study (Figure 1).
FIGURE 1.
Flowchart of study participants. 1Noncompliance was defined as weight loss <2.5% of initial body weight; 2personal reasons included distance and time commitment. WL, weight loss; WM, weight maintenance.
Weight, body composition, and BMD
Weight, body composition, and bone results at baseline and after 6 mo of diet intervention are presented in Table 1. There were no significant differences at baseline (Table 1). Subjects in the WL group lost 7.9 ± 4.4% of weight, 16.1 ± 19.5% of total body fat, and 2.2 ± 3.9% of fat-free soft tissue and had a 4.1 ± 6.6% loss of total body BMC that differed significantly compared with in the WM group (P ≤ 0.02). There was an interaction between group and time for femoral neck and total body BMD (P < 0.05) (Table 1). In addition, the change in total body BMD in the WL group (−1.0 ± 2.5%) differed compared with in the WM group (1.5 ± 2.7%) (P < 0.05). The interaction between group and time was NS for radius, lumbar spine, and total hip BMD (Table 1). Hip BMD indicated a significant time effect (P ≤ 0.02) in both groups. In the analysis that used the original groups, which included men who did not lose weight in the WL group, there were no significant differences at baseline or changes between groups over time.
TABLE 1.
Body composition and areal BMD1
| WL (n = 19) |
WM (n = 19) |
P |
|||||
| Baseline | Final | Baseline | Final | Group | Time | Group × time | |
| Age, y | 57.7 ± 6.6 | — | 59.7 ± 4.9 | — | — | — | — |
| BMI, kg/m2 | 32.4 ± 4.7 | 30.0 ± 4.8 | 30.6 ± 3.0 | 30.6 ± 2.9 | 0.15 | <0.001 | <0.001 |
| Weight, kg | 103.1 ± 18.5 | 95.1 ± 18.5 | 94.4 ± 11.6 | 94.2 ± 11.6 | 0.39 | <0.001 | <0.001 |
| Total body fat, kg | 34.2 ± 10.9 | 28.7 ± 12.4 | 29.2 ± 7.4 | 29.3 ± 6.9 | 0.22 | <0.003 | <0.001 |
| Fat-free soft tissue, kg | 64.0 ± 8.7 | 62.5 ± 8.6 | 59.9 ± 6.4 | 60.3 ± 7.2 | 0.46 | 0.11 | 0.01 |
| Bone mineral content, g | 3184.8 ± 416.9 | 3069.7 ± 451.4 | 3121.4 ± 480.3 | 3113.6 ± 464.8 | 0.94 | 0.04 | 0.02 |
| BMD, g/cm2 | |||||||
| Ultradistal radius | 0.439 ± 0.044 | 0.431 ± 0.048 | 0.413 ± 0.056 | 0.415 ± 0.058 | 0.87 | 0.30 | 0.19 |
| One-third radius2 | 0.804 ± 0.075 | 0.798 ± 0.075 | 0.759 ± 0.087 | 0.754 ± 0.091 | 0.70 | 0.23 | 0.81 |
| Lumbar spine3 | 1.238 ± 0.148 | 1.258 ± 0.151 | 1.312 ± 0.251 | 1.321 ± 0.233 | 0.10 | 0.22 | 0.21 |
| Trochanter | 0.891 ± 0.159 | 0.888 ± 0.155 | 0.908 ± 0.150 | 0.905 ± 0.151 | 0.11 | 0.30 | 0.91 |
| Femoral neck | 0.965 ± 0.137 | 0.958 ± 0.143 | 0.980 ± 0.155 | 0.989 ± 0.154a | 0.10 | 0.82 | 0.04 |
| Total hip | 1.041 ± 0.152 | 1.033 ± 0.155 | 1.071 ± 0.163 | 1.070 ± 0.163 | 0.06 | 0.02 | 0.11 |
| Total body | 1.285 ± 0.094 | 1.272 ± 0.084 | 1.263 ± 0.114 | 1.283 ± 0.118a | 0.13 | 0.56 | 0.004 |
All values are means ± SDs. A mixed-model ANCOVA analysis was performed with time (0 and 6 mo) and group (WL or WM) as independent variables. The following covariates were included in the analysis: age, season, physical activity level, body weight, sex hormones (total testosterone, free testosterone, and estradiol), sex hormone binding globulin, and 25-hydroxyvitamin D. Covariates tested in the model had no significant influence on bone variables at any site. Baseline characteristics were not significantly different between the WM and WL groups. Fat-free soft tissue and bone mineral content reflect total body measurements. aBaseline differed from final WM, P < 0.05. BMD, bone mineral density; WL, weight loss; WM, weight maintenance.
Radial BMD at one-third the distance from the distal end.
Lumbar spine BMD scans were analyzed according to International Society for Clinical Densitometry exclusion criteria (20).
Trabecular and cortical bone at the tibia
There were NS changes in trabecular variables (Table 2). There was an interaction between group and time that approached significance for cortical thickness (P ≤ 0.06) (Table 2) that showed a change of −0.8 ± 2.9% and 0.7 ± 2.3% in WM and WL groups, respectively. A trend was also observed for the interaction between group and time for the cortical area (P < 0.08). In addition, there were trends for the endosteal circumference and polar moment of inertia to decrease over time in both groups (P ≤ 0.08). There were no other changes in cortical variables between groups or over time (Table 2). In the analysis that used the original groups, the change over 6 mo in cortical volumetric bone mineral density differed between WL (−0.1 ± 0.5%) and WM (0.6 ± 1.2%) groups (P-interaction effect < 0.05). However, the cortical area and polar moment of inertia decreased more in the WM group (−0.6 ± 4.0% and −2.4 ± 4.2%, respectively) than WL group (−0.1 ± 2.1% and −1.6 ± 2.7%, respectively) (P-interaction effect < 0.05).
TABLE 2.
Trabecular and cortical volumetric bone mineral density, geometry, and strength at the tibia1
| WL (n = 19) |
WM (n = 19) |
P |
|||||
| Baseline | Final | Baseline | Final | Group | Time | Group × time | |
| Total | |||||||
| vBMD, mg/cm3 | 331.0 ± 45.2 | 333.2 ± 44.5 | 327.6 ± 44.9 | 329.0 ± 44.4 | 0.95 | 0.27 | 0.59 |
| BMC, mg | 407.6 ± 49.9 | 406.7 ± 51.43 | 406.4 ± 48.1 | 404.4 ± 47.9 | 0.06 | 0.89 | 0.98 |
| Area, mm2 | 1266.4 ± 128.1 | 1258.4 ± 118.9 | 1267.2 ± 161.6 | 1257.8 ± 159.5 | 0.53 | 0.25 | 0.93 |
| Trabecular | |||||||
| vBMD, mg/cm3 | 241.8 ± 28.4 | 242.5 ± 29.3 | 249.4 ± 36.4 | 249.9 ± 36.9 | 0.11 | 0.39 | 0.94 |
| BMC, mg | 135.5 ± 23.6 | 134.6 ± 23.9 | 141.0 ± 20.1 | 140.3 ± 20.6 | 0.06 | 0.22 | 0.72 |
| Cortical | |||||||
| vBMD, mg/cm3 | 1149.9 ± 21.5 | 1148.6 ± 20.2 | 1145.2 ± 32.7 | 1149.3 ± 32.1 | 0.94 | 0.32 | 0.13 |
| BMC, mg | 414.4 ± 47.6 | 414.9 ± 50.6 | 377.5 ± 75.2 | 374.3 ± 75.8 | 0.26 | 0.20 | 0.12 |
| Area, mm2 | 360.0 ± 38.2 | 360.9 ± 40.4 | 328.9 ± 60.8 | 324.2 ± 60.2 | 0.20 | 0.17 | 0.08 |
| Thickness, mm | 5.8 ± 0.6 | 5.9 ± 0.7 | 5.2 ± 1.3 | 5.1 ± 1.3 | 0.06 | 0.69 | 0.06 |
| Periosteal, mm | 80.2 ± 4.2 | 80.0 ± 4.2 | 81.2 ± 4.1 | 80.7 ± 4.3 | 0.20 | 0.01 | 0.60 |
| Endosteal, mm | 43.5 ± 5.6 | 43.0 ± 5.4 | 48.6 ± 10.6 | 48.4 ± 10.9 | 0.10 | 0.06 | 0.32 |
| Polar moment of inertia, mm4 | 40,180 ± 8283 | 40,082 ± 8718 | 37,873 ± 6643 | 37,074 ± 6439 | 0.57 | 0.08 | 0.28 |
| SSI, mm3 | 2214 ± 369 | 2209 ± 377 | 2169 ± 278 | 2138 ± 299 | 0.93 | 0.15 | 0.42 |
All values are means ± SDs. A mixed-model ANCOVA analysis was performed with time (0 and 6 mo) and group (WL or WM) as independent variables. The following covariates were included in the analysis: age, season, physical activity level, body weight, sex hormones (total testosterone, free testosterone, and estradiol), sex hormone binding globulin, and 25-hydroxyvitamin D. Covariates tested in the model had no significant influence on bone variables at any site. Baseline characteristics were not significantly different between the WM and WL groups. BMC, bone mineral content, SSI; stress-strain index; vBMD, volumetric bone mineral density; WL, weight loss; WM, weight maintenance.
Sex steroids and bone regulating hormones
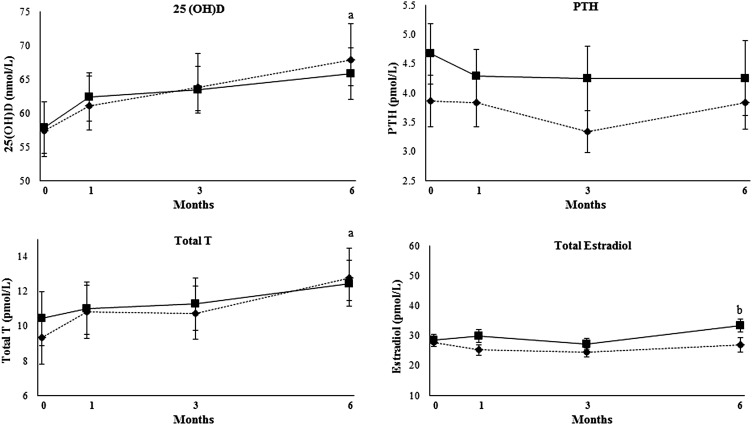
Serum sex steroids and bone regulating hormones are shown in Table 3. Forty-two percent of men had serum 25(OH)D concentrations <50 nmol/L, and 21% of men had serum 25(OH)D concentrations >75 nmol/L. The interaction between group and time was significant for serum total, free, and bioavailable estradiol (P < 0.05; Table 3). In addition, compared with at baseline, there was an increase in serum free and bioavailable estradiol (P < 0.001). Serum 25(OH)D and total testosterone concentrations increased over time in both groups (P < 0.05) (Figure 2). The increase in the total serum estradiol concentration in the WM group differed compared with the small decrease in the WL group (P-interaction < 0.05) (Figure 2). In addition, there was no change in serum PTH (Figure 2). Serum osteocalcin concentrations were 1.4 ± 0.6 and 1.2 ± 0.4 nmol/L in WL and WM groups, respectively, and did not differ significantly between groups or change over time (data not shown).
TABLE 3.
Sex steroid and bone regulating hormones1
| WL (n = 19) |
WM (n = 19) |
P |
|||||
| Baseline | Final | Baseline | Final | Group | Time | Group × time | |
| Total estradiol, pmol/L | 101.6 ± 30.4 | 99.0 ± 33.0 | 104.3 ± 21.0 | 122.5 ± 35.7a | 0.20 | 0.14 | 0.03 |
| Free estradiol, pmol/L | 2.9 ± 1.7 | 2.2 ± 1.2 | 2.6 ± 1.0 | 4.2 ± 2.4a | 0.08 | 0.48 | 0.006 |
| Bioavailable estradiol, pmol/L | 58.4 ± 40.8 | 42.2 ± 49.4 | 52.5 ± 26.8 | 73.7 ± 48.4a | 0.14 | 0.93 | 0.02 |
| Total testosterone, nmol/L | 11.2 ± 3.8 | 15.3 ± 5.5 | 11.7 ± 6.1 | 13.9 ± 4.2 | 0.31 | 0.005 | 0.13 |
| Free testosterone, pmol/L | 33.0 ± 10.7 | 33.8 ± 11.6 | 22.0 ± 9.3 | 25.7 ± 13.1 | 0.001 | 0.20 | 0.16 |
| Bioavailable testosterone, nmol/L | 5.0 ± 2.7 | 7.1 ± 6.2 | 5.7 ± 4.1 | 7.3 ± 6.4 | 0.65 | 0.03 | 0.97 |
| SHBG, nmol/L | 60.1 ± 24.7 | 70.4 ± 39.1 | 54.8 ± 23.1 | 58.7 ± 20.4 | 0.03 | 0.06 | 0.32 |
| 25(OH)D, nmol/L | 57.5 ± 19.7 | 68.0 ± 24.2 | 57.9 ± 17.7 | 65.9 ± 17.8 | 0.94 | 0.005 | 0.70 |
| Intact PTH, ng/L | 36.4 ± 19.1 | 36.1 ± 19.2 | 46.5 ± 20.5 | 44.4 ± 25.4 | 0.66 | 0.58 | 0.67 |
All values are means ± SDs. A mixed-model ANCOVA analysis was performed with time (0 and 6 mo) and group (WL or WM) as independent variables. The following covariates were included in the analysis: age, season, physical activity level, and body weight. Covariates tested in the model had no significant influence on bone variables at any site. Baseline characteristics were not significantly different between the WM and WL groups aBaseline differed from final WM, P < 0.05. PTH, parathyroid hormone; SHBG, sex hormone binding globulin; WL, weight loss; WM, weight maintenance; 25(OH)D, 25-hydroxyvitamin D.
FIGURE 2.
Mean (±SEM) changes in serum 25(OH)D, PTH, total T, and estradiol during the intervention in men adhering to WL and WM (n = 38). A repeated-measures ANCOVA analysis that used mixed models was performed with time (0, 1, 3, and 6 mo) and group (WL or WM) as independent variables. The following covariates were included in the analysis: age, season, physical activity level, and body weight. aTime effect in WL and WM groups with no difference between groups, P < 0.05; b WM group differed from WL group (interaction effect over time and at the 6-mo post hoc test), P < 0.05. Diamond dotted lines represent WL, and square solid lines represent WM. PTH, parathyroid hormone; T, testosterone; WL, weight loss; WM, weight maintenance; 25(OH)D, 25-hydroxyvitamin D.
Nutrient intake and PAL
As expected, during the intervention, there was lower intake of total calories in the WL group (1595 ± 182 kcal/d) than WM group (2097 ± 248 kcal/d) (P < 0.05) (Supplemental Table 1). In addition, the WL group had lower protein intake (81 ± 8 g/d) and fat intake (61 ± 18 g/d) than the WM group did (87 ± 11 g protein/d and 89 ± 11 g fat/d) (P < 0.05). The daily calcium intake from the diet and supplement was 1179 ± 251 and 1176 ± 89 mg/d in WL and WM groups, respectively (Supplemental Table 1). Compliance with supplemental multivitamin and calcium intake was 90% ± 22% (WL: 92 ± 22%; WM: 89 ± 22%). Diaries indicated that most of the men were sedentary, there was no difference in the PAL change between groups, and the score rose slightly over time in both groups (1.1 ± 1.0, –1.2 ± 1.1, P-time effect < 0.05).
Pearson correlations and stepwise regression
Body composition
Changes in total body BMD correlated positively to changes in body weight (r = 0.43, P < 0.01), fat-free soft tissue (r = 0.44, P = 0.005), and total body fat (r = 0.31, P ≤ 0.05). In contrast, total body fat tended to inversely correlate with the stress-strain index (r = −0.30, P = 0.06).
Hormones
Total serum testosterone and total body BMD changes were inversely correlated (r = −0.34, P < 0.05). Changes in serum 25(OH)D concentrations were positively correlated with changes in femoral neck BMD (r = 0.39, P < 0.01) and tibia cortical thickness (r = 0.39, P ≤ 0.01). Changes in serum free estradiol inversely correlated with changes in cortical thickness (r = −0.49, P ≤ 0.002), polar moment of inertia (r = −0.33, P < 0.05), and the stress-strain index (r = −0.32, P ≤ 0.05) and tended to positively correlate with the endosteal circumference (r = 0.30, P = 0.06). A stepwise regression was performed with bone outcomes as dependent variables and hormonal and body-composition changes as independent variables. Only independent variables that reached significance were included in the final model (Table 4). Changes in body weight and 25(OH)D serum concentrations explained 25% (model R2) of the variance in femoral neck BMD. Changes in fat-free soft tissue explained 19% of the changes in total body BMD, whereas the changes in free estradiol explained 14% of the changes in cortical thickness at the tibia with pervious variables controlled for. Models constructed to explain changes in femoral neck, total body BMD, and cortical thickness for each group individually did not reach significance.
TABLE 4.
Multiple linear stepwise regression analysis of the explanatory variables for changes in femoral neck BMD, total body BMD, and tibia cortical thickness1
| Variable | R2 | β | P |
| Femoral neck BMD | |||
| Body weight | 0.15 | 0.32 | 0.01 |
| 25(OH)D | 0.10 | 0.42 | 0.03 |
| Total body BMD | |||
| Fat-free soft tissue | 0.19 | 0.44 | 0.05 |
| Cortical thickness | |||
| Free estradiol | 0.14 | −0.36 | 0.02 |
Analysis was done for change in variables over 6 mo (n = 38). The final model did not include variables that did not reach significance (physical activity level, total body fat, parathyroid hormone, and total and free testosterone). A separate analysis was conducted for each individual group (not shown in the table). In the WL group, there was a trend for the change in fat-free soft tissue to explain 18% of the change in total body BMD (β = 0.42, P = 0.06), and there were no other significant explanatory variables for the femoral neck BMD or cortical thickness. There were no explanatory variables for the WM group. BMD, bone mineral density; 25(OH)D, 25-hydroxyvitamin D.
Adverse events and symptoms
There were no serious adverse events recorded during the study. The frequency of nonserious adverse symptoms did not change from baseline or differ between groups.
DISCUSSION
Osteoporosis and bone loss in older men is an increasing public health burden in an aging society (23), and a history of WL in older men or women is known to increase fracture risk (8, 24, 25). The use of calorie restriction to prevent obesity complications was shown to result in bone loss in postmenopausal women (2). The current study was designed to examine how voluntary weight reduction in moderately older men affected BMD, geometry, and strength, and to our knowledge, these effects were not examined previously in a controlled trial. In addition, a secondary goal was to examine if the endocrine response to caloric restriction could explain bone changes with weight reduction. In this WL study, even though the change in femoral neck and total body BMD differed from that in weight-stable men, the decrease was NS, and there were no changes in cortical or trabecular bone variables.
Men who were 50–72 y of age and lost 8% of their body weight showed little or no change in BMD, geometry, or strength. These outcomes differed from the significant decrease in BMD shown in older women by using a similar protocol in our laboratory (6) and in other WL studies (2). One early study examined WL in an overweight middle-aged male population (43 y of age) and only measured total body BMD (10) and showed a 1.5% loss with 6 kg WL. Bone loss that is associated with WL might be greater if there is a lower initial weight, or greater loss of lean body mass such as when there is lower protein intake (26) or less physical activity (10, 13, 27). In this study, the absence of femoral neck BMD loss with WL differed from previous WL findings in women and mixed-sex populations (2). There were no changes in trabecular or cortical bone compartments with WL in men in this study, but yet there was a trend to decreased cortical thickness and area only in overweight and obese men who maintained their body weights. This finding was consistent with evidence that obesity is associated with altered cortical bone that is detrimental to bone microarchitecture (15–17). In previous studies of WL in women, there was also no reported change in cortical and trabecular volumetric bone mineral density, strength, or geometry (26, 28). In the study by Uusi-Rasi et al. (28), the amount of WL (range: 2–19%) did not predict the loss of bone after 3 mo of caloric restriction in premenopausal women nor did it predict bone loss after an additional 9 mo of WM measured by using pQCT. The absence of bone loss in premenopausal women (28) might have been explained by their younger age and higher estrogen status and was consistent with no areal BMD loss shown in another study of premenopausal women (29). However, in a more-recent study, we also showed that postmenopausal women (mean age: 58 y) showed no change in trabecular and cortical bone variables with 7% WL after 1 y despite a decrease in areal BMD at a few central bone sites (18). In another study of elderly men and women (72 y of age), it was shown that hip geometric properties including cortical thickness calculated from areal BMD decreased with moderate WL after 12 mo (11). It may be that central bone sites respond differently than peripheral sites, or bone in the elderly is more vulnerable to a decline in cortical bone properties.
In the current study, with moderate WL, there was a significant difference in total estradiol change between groups although the decline from baseline was not as great as reported in elderly men (age: 74 y) who decreased weight (14). Note that serum estradiol concentrations reported in the current study (102 pmol/L) were slightly higher than those shown in the The Osteoporotic Fracture in Men Study of elderly men (66–92 pmol/L) (30). A younger age or higher adiposity might explain the higher estradiol concentrations in the current study. In addition, the cause for a slight rise in serum estradiol in weight-stable obese men was not entirely clear. Possible explanations included aging during the study, an increase in aromatase enzyme concentrations (31), or a small rise or redistribution of adipose tissue (32). Studies showed a positive effect of estradiol on areal BMD in men (30, 33, 34) but a negative association between free estradiol and cortical bone size (35), similar to findings in this study. Also, other factors associated with excess adiposity such as altered cytokines, growth factors, and calciotropic hormones attenuate the protective effect of estrogen on cortical bone in obesity (2, 36).
Circulating testosterone is a positive determinant of BMD, and lower concentrations are associated with faster bone loss (30, 37). In this current study, the absolute concentration of testosterone would have been negatively influenced by the advanced age and excess adiposity of subjects (37, 38). Concentrations of testosterone in these obese men averaged 11.4 ± 5.0 nmol/L, which were at the lower end of the normal range (10.4–34.6 nmol/L) (39). Men who lost weight in this study showed a 35% increase in testosterone that could be considered physiologically significant (40–42). A rise in serum testosterone possibly combined with the rise in 25(OH)D (6) might have attenuated bone changes in this study.
There were some limitations and strengths of this study. The precision of DXA measurements is influenced by abnormally high amounts of soft tissue surrounding the bone or changes in total body fat (43, 44). For example, it was shown that 6 kg fat (but not less), that overlies bone will affect the precision of areal BMD measurements by DXA but not volumetric BMD measurements by pQCT (44). Note that, although men in the treatment group lost 6.7 kg fat in the current study, the amount of loss at a single anatomical site would have been significantly less. Hence, the precision errors to estimate BMD by using DXA were less likely to occur during the moderate WL that was achieved in this study. In addition, although previous studies showed that a 6-mo intervention induced bone loss, a longer duration may have resulted in greater bone loss (2). However, in the current study, the goal was to examine bone while subjects were still close to their weight nadir rather than after a regain or maintenance of weight. Nevertheless, a future intervention trial to examine the long-term effect of weight change on BMD and bone quality in men is indicated. The separate recruitments for each group may have led to the higher, although insignificant, body weight in the WL than weight-stable group of men. A larger sample size would have limited the influence of weight variability between groups. Other limitations of this study were the lack of random assignment and masking and the multiple comparisons that increased risk of a type I error and reduced the reliability of P values > 0.005. Also, the adaptive study design was used to retain eligibility criteria and cases recruited for the sake of adequate power and thereby reduce problems that arise from a lack of efficacy (45, 46). In addition, our goal was to examine bone changes in response to WL rather than the success or failure of the WL diet, whereas other studies that did not use an adaptive design used motivational interviewing to achieve the goal of WL (47). A strength of the current study was the additional information about bone microarchitecture by using pQCT that was not captured by areal BMD measurements alone, and these measurements are especially important in studies of the obese who have evidence of compromised bone quality (15–17). Another strong point of this study is that, by excluding vertebrae with structural abnormalities such as osteophytes or compression fracture for lumbar spine BMD, which is more common in the obese (48), risk of overestimating values and reporting changes that were because of artifacts was reduced. Also, participants in this study were recruited in the same season (winter) so that the seasonal influence on 25(OH)D concentrations did not differ between individuals or groups.
In conclusion, an 8% WL in overweight and obese men did not decrease areal or volumetric BMD or show evidence of altered bone geometric properties. Also, our findings suggest that maintaining an obese or overweight status may lead to detrimental changes in cortical bone compared with those with WL. These results indicate the need for a larger longitudinal study that specifically examines obese men and factors contributing to changes in bone quality over time.
Supplementary Material
Acknowledgments
We thank R Zurfluh, RD, for invaluable clinical assistance. We appreciate the effort of the external data safety monitoring board (Lester Katzel, Robert Recker, and Bruce Barton). We thank our colleagues Drs. M Watford, D Hoffman, and G Henderson for reviewing our findings.
The authors’ responsibilities were as follows—SAS: study design and conception, data analysis and interpretation, and primary responsibility for the final content of the manuscript; LCP: record keeping, data collection, management, and interpretation, and laboratory and statistical analysis; DS: coordination of the study and laboratory and statistical analysis; KT: data collection, management, and interpretation, and laboratory and statistical analysis; YS: study design and statistical analysis and interpretation; SHS: study design, study physician, interpretation of results, and contribution to the safety report; CLG: imaging analysis and data interpretation; XW: calculations of data and interpretation of results; and all authors: contribution to the interpretation of results, data analysis, and review and writing of the manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: BMC, bone mineral content; BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; PAL, physical activity level; PTH, parathyroid hormone; pQCT, peripheral quantitative computed tomography; SHBG, sex hormone binding globulin; 25(OH)D, 25-hydroxyvitamin D; WL, weight loss; WM, weight maintenance.
REFERENCES
- 1.Darmon P. Intentional weight loss in older adults: useful or wasting disease generating strategy? Curr Opin Clin Nutr Metab Care 2013;16:284–9. [DOI] [PubMed] [Google Scholar]
- 2.Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr 2012;32:287–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khosla S. Pathogenesis of age-related bone loss in humans. J Gerontol A Biol Sci Med Sci 2013;68:1226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen TV, Sambrook PN, Eisman JA. Bone loss, physical activity, and weight change in elderly women: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res 1998;13:1458–67. [DOI] [PubMed] [Google Scholar]
- 6.Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res 2005;20:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knoke JD, Barrett-Connor E. Weight loss: a determinant of hip bone loss in older men and women. The Rancho Bernardo Study. Am J Epidemiol 2003;158:1132–8. [DOI] [PubMed] [Google Scholar]
- 8.Mussolino ME, Looker AC, Madans JH, Langlois JA, Orwoll ES. Risk factors for hip fracture in white men: the NHANES I Epidemiologic Follow-up Study. J Bone Miner Res 1998;13:918–24. [DOI] [PubMed] [Google Scholar]
- 9.Yialamas MA, Hayes FJ. Androgens and the ageing male and female. Best Pract Res Clin Endocrinol Metab 2003;17:223–36. [DOI] [PubMed] [Google Scholar]
- 10.Pritchard JE, Nowson CA, Wark JD. Bone loss accompanying diet-induced or exercise-induced weight loss: a randomised controlled study. Int J Obes Relat Metab Disord 1996;20:513–20. [PubMed] [Google Scholar]
- 11.Armamento-Villareal R, Sadler C, Napoli N, Shah K, Chode S, Sinacore DR, Qualls C, Villareal DT. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res 2012;27:1215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen LB, Quaade F, Sørensen OH. Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Res 1994;9:459–63. [DOI] [PubMed] [Google Scholar]
- 13.Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med 2006;166:2502–10. [DOI] [PubMed] [Google Scholar]
- 14.Ensrud KE, Lewis CE, Lambert LC, Taylor BC, Fink HA, Barrett-Connor E, Cauley JA, Stefanick ML, Orwoll E; Osteoporotic Fractures in Men MrOS Study Research Group. Endogenous sex steroids, weight change and rates of hip bone loss in older men: the MrOS study. Osteoporos Int 2006;17:1329–36. [DOI] [PubMed] [Google Scholar]
- 15.Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res 2012;27:1–10. [DOI] [PubMed] [Google Scholar]
- 16.Premaor MO, Compston JE, Avilés FF, Pagès-Castellà A, Nogués X, Díez-Pérez A, Prieto-Alhambra D. The association between fracture site and obesity in men: a population-based cohort study. J Bone Miner Res 2013;28:1771–7. [DOI] [PubMed] [Google Scholar]
- 17.Sukumar D, Schlussel Y, Riedt CS, Gordon C, Stahl T, Shapses SA. Obesity alters cortical and trabecular bone density and geometry in women. Osteoporos Int 2011;22:635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fogelholm GM, Sievanen HT, Kukkonen-Harjula TK, Pasanen ME. Bone mineral density during reduction, maintenance and regain of body weight in premenopausal, obese women. Osteoporos Int 2001;12:199–206. [DOI] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575–81. [DOI] [PubMed] [Google Scholar]
- 20.Lewiecki EM, Baim S, Binkley N, Bilezikian JP, Kendler DL, Hans DB, Silverman S; International Society for Clinical Densitometry. Report of the International Society for Clinical Densitometry 2007 Adult Position Development Conference and Official Positions. South Med J 2008;101:735–9. [DOI] [PubMed] [Google Scholar]
- 21.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72. [DOI] [PubMed] [Google Scholar]
- 22.Furlow EA, Anderson JW. A systematic review of targeted outcomes associated with a medically supervised commercial weight-loss program. J Am Diet Assoc 2009;109:1417–21. [DOI] [PubMed] [Google Scholar]
- 23.Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, Finkelstein JS. Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:1802–22. [DOI] [PubMed] [Google Scholar]
- 24.Langlois JA, Mussolino ME, Visser M, Looker AC, Harris T, Madans J. Weight loss from maximum body weight among middle-aged and older white women and the risk of hip fracture: the NHANES I epidemiologic follow-up study. Osteoporos Int 2001;12:763–8. [DOI] [PubMed] [Google Scholar]
- 25.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR, Osteoporotic Fractures Research Group. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc 2003;51:1740–7. [DOI] [PubMed] [Google Scholar]
- 26.Sukumar D, Ambia-Sobhan H, Zurfluh R, Schlussel Y, Stahl TJ, Gordon CL, Shapses SA. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized, controlled trial. J Bone Miner Res 2011;26:1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan AS, Nicklas BJ, Dennis KE. Aerobic exercise maintains regional bone mineral density during weight loss in postmenopausal women. J Appl Physiol 1998;84:1305–10. [DOI] [PubMed] [Google Scholar]
- 28.Uusi-Rasi K, Rauhio A, Kannus P, Pasanen M, Kukkonen-Harjula K, Fogelholm M, Sievänen H. Three-month weight reduction does not compromise bone strength in obese premenopausal women. Bone 2010;46:1286–93. [DOI] [PubMed] [Google Scholar]
- 29.Riedt CS, Schlussel Y, von Thun N, Ambia-Sobhan H, Stahl T, Field MP, Sherrell RM, Shapses SA. Premenopausal overweight women do not lose bone during moderate weight loss with adequate or higher calcium intake. Am J Clin Nutr 2007;85:972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cauley JA, Ewing SK, Taylor BC, Fink HA, Ensrud KE, Bauer DC, Barrett-Connor E, Marshall L, Orwoll ES; Osteoporotic Fractures in Men Study (MrOS) Research Group. Sex steroid hormones in older men: longitudinal associations with 4.5-year change in hip bone mineral density–the osteoporotic fractures in men study. J Clin Endocrinol Metab 2010;95:4314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjørnerem A, Straume B, Midtby M, Fønnebø V, Sundsfjord J, Svartberg J, Acharya G, Oian P, Berntsen GK. Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: the Tromsø study. J Clin Endocrinol Metab 2004;89:6039–47. [DOI] [PubMed] [Google Scholar]
- 32.Wake DJ, Strand M, Rask E, Westerbacka J, Livingstone DE, Soderberg S, Andrew R, Yki-Jarvinen H, Olsson T, Walker BR. Intra-adipose sex steroid metabolism and body fat distribution in idiopathic human obesity. Clin Endocrinol (Oxf) 2007;66:440–6. [DOI] [PubMed] [Google Scholar]
- 33.Khosla S, Riggs BL. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin North Am 2005;34:1015–30. [DOI] [PubMed] [Google Scholar]
- 34.Bredella MA, Lin E, Gerweck AV, Landa MG, Thomas BJ, Torriani M, Bouxsein ML, Miller KK. Determinants of bone microarchitecture and mechanical properties in obese men. J Clin Endocrinol Metab 2012;97:4115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorentzon M, Swanson C, Andersson N, Mellström D, Ohlsson C. Free testosterone is a positive, whereas free estradiol is a negative, predictor of cortical bone size in young Swedish men: the GOOD study. J Bone Miner Res 2005;20:1334–41. [DOI] [PubMed] [Google Scholar]
- 36.Donath MY. Inflammation as a sensor of metabolic stress in obesity and type 2 diabetes. Endocrinology 2011;152:4005–6. [DOI] [PubMed] [Google Scholar]
- 37.van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab 2000;85:3276–82. [DOI] [PubMed] [Google Scholar]
- 38.Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril 2006;85:1319–40. [DOI] [PubMed] [Google Scholar]
- 39.Swerdloff R, Wang C. Testosterone treatment of older men–why are controversies created? J Clin Endocrinol Metab 2011;96:62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corona G, Rastrelli G, Monami M, Saad F, Luconi M, Lucchese M, Facchiano E, Sforza A, Forti G, Mannucci E, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol 2013;168:829–43. [DOI] [PubMed] [Google Scholar]
- 41.Eichholzer M, Barbir A, Basaria S, Dobs AS, Feinleib M, Guallar E, Menke A, Nelson WG, Rifai N, Platz EA, et al. Serum sex steroid hormones and frailty in older American men of the Third National Health and Nutrition Examination Survey (NHANES III). Aging Male 2012;15:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO, McGee D. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc 2010;58:1134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knapp KM, Welsman JR, Hopkins SJ, Fogelman I, Blake GM. Obesity increases precision errors in dual-energy X-ray absorptiometry measurements. J Clin Densitom 2012;15:315–9. [DOI] [PubMed] [Google Scholar]
- 44.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res 2012;27:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chow SC, Chang M. Adaptive design methods in clinical trials - a review. Orphanet J Rare Dis 2008;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang M, Chow SC. A hybrid Bayesian adaptive design for dose response trials. J Biopharm Stat 2005;15:677–91. [DOI] [PubMed] [Google Scholar]
- 47.West DS, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care 2007;30:1081–7. [DOI] [PubMed] [Google Scholar]
- 48.Samartzis D, Karppinen J, Chan D, Luk KD, Cheung KM. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: a population-based study. Arthritis Rheum 2012;64:1488–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.