Abstract
Since 2006, waitlist candidates with portopulmonary hypertension (POPH) have been eligible for standardized Model for End-Stage Liver Disease (MELD) exception points. However, there are no data evaluating the current POPH exception policy and its implementation. We used Organ Procurement and Transplantation Network (OPTN) data to compare outcomes of patients with approved POPH MELD exceptions from 2006 to 2012 to all nonexception waitlist candidates during this period. Since 2006, 155 waitlist candidates had approved POPH MELD exceptions, with only 73 (47.1%) meeting the formal OPTN exception criteria. Furthermore, over one-third of those with approved POPH exceptions either did not fulfill hemodynamic criteria consistent with POPH or had missing data, with 80% of such patients receiving a transplant based on receiving exception points. In multivariable multistate survival models, waitlist candidates with POPH MELD exceptions had an increased risk of death compared to nonexception waitlist candidates, regardless of whether they did (hazard ratio [HR]: 2.46, 95% confidence interval [CI]: 1.73–3.52; n = 100) or did not (HR: 1.60, 95% CI: 1.04–2.47; n = 55) have hemodynamic criteria consistent with POPH. These data highlight the need for OPTN/UNOS to reconsider not only the policy for POPH MELD exceptions, but also the process by which such points are awarded.
Introduction
Pulmonary arterial hypertension (PAH) is termed portopulmonary hypertension (POPH) when it occurs in the setting of portal hypertension and is not due to other identifiable causes (1). POPH occurs in up to 5% of all patients with cirrhosis and portal hypertension, but with a higher frequency in patients evaluated for liver transplantation (2). Transthoracic echocardiography is used to screen for POPH, but the diagnosis requires right heart catheterization parameters consistent with PAH: mean pulmonary artery pressure (mPAP) >25 mmHg, pulmonary vascular resistance (PVR) >3 Wood units and normal left-sided filling pressure (pulmonary capillary wedge pressure [PCWP] or left ventricular end-diastolic pressure ≤15 mmHg) (1). As cirrhotic patients may also have volume overload resulting in a PCWP > 15 mmHg, the presence of POPH in this situation may also be suggested by an elevated trans-pulmonary gradient (TPG; mPAP-PCWP ≥12 mmHg) (1–3). However, the ultimate diagnosis of POPH is a clinical one that requires meeting hemodynamic parameters, while also ruling out other potential etiologies of pulmonary hypertension, including chronic obstructive pulmonary disease (4), sleep-disordered breathing and left ventricular systolic or diastolic dysfunction.
POPH is associated with significant morbidity and mortality, with estimates of 60% 1-year survival without treatment (1,2,5). While medical treatment for POPH includes endothelin receptor antagonists, phosphodiesterase 5 inhibitors and prostacyclin analogs, similar to that for other forms of PAH, liver transplantation can be curative, but only in select cases. Significant POPH is generally associated with dramatically increased perioperative mortality with liver transplantation (1,2).
Since 2006, liver transplant waitlist candidates with POPH have been eligible to receive waitlist priority upgrades (Model for End-Stage Liver Disease [MELD] exceptions) based on formalized criteria set forth by the Organ Procurement and Transplantation Network (OPTN). These criteria for POPH MELD exceptions are: (1) diagnosis based on “initial mPAP and PVR levels,” (2) documentation of treatment and (3) posttreatment mPAP < 35 mmHg and PVR <5 Wood units (6–9). However, the data to develop this policy derived from small single-center studies, and while in place to guide regional review boards, do not mandate that exception points be restricted only to patients meeting these criteria. Recent work has demonstrated that despite the adoption of formal exception policies (i.e. hepatopulmonary syndrome (10)) or consensus recommendations (i.e. primary sclerosing cholangitis and recurrent bacterial cholangitis (11) or hepatocellular carcinoma beyond Milan criteria (12)) for allocating exception points, the data used to award such points and the compliance with guidelines or recommendations are suboptimal. The goal of this study was to evaluate the current POPH exception policy and its implementation.
Methods
Study sample
We evaluated all adult (≥18 years of age) waitlist candidates who applied for a “POPH” MELD exception from December 1, 2006 until December 15, 2012 based on OPTN/United Network for Organ Sharing (UNOS) coding. We reviewed the exception narrative for those waitlist candidates with at least one approved POPH MELD exception. We categorized waitlist candidates as meeting hemodynamic criteria for POPH if there was a documented pretreatment PVR > 3 Wood units and mPAP > 25 mmHg, necessary data for the diagnosis of PAH. PCWP and TPG data were not included since these data are not required per OPTN/UNOS policy. When these data were available, we required that patients with a PCWP ≥ 15 mmHg have a corresponding TPG ≥ 12 mmHg to be categorized as having hemodynamic data consistent with POPH. In analyses evaluating pre- and posttransplant outcomes of waitlist candidates with approved POPH MELD exceptions, all other candidates waitlisted during the study period, excluding retransplant candidates and those with other MELD exceptions, were included as the comparator group (n = 34 180).
Outcome
The outcome was overall survival, which included both pre- and posttransplant survival. Pretransplant mortality was defined as waitlist removal for death or clinical deterioration: (1) UNOS removal code of “died”; (2) UNOS removal code “too sick to transplant” or (3) UNOS removal code of “other” in the setting of a confirmed Social Security Death Master File death date within 90 days of waitlist removal (13,14). All waitlist candidates with the removal code of “too sick” were considered as waitlist dropouts for clinical deterioration as such removals are equivalent to death (15,16), and in the case of patients with POPH, may have been removed for progressive POPH and/or POPH unresponsive to medical therapy. Death posttransplantation was defined using OPTN/UNOS data.
Statistical analysis
We evaluated overall survival, including time on the waitlist and posttransplant survival time among those transplanted. Waitlist candidates with approved POPH MELD exceptions were compared to all other nonexception candidates on the liver transplant waitlist during the study period using multistate survival models. These models are considered the best approach to studying outcomes in transplant candidates, as they account for transitions from pre- to posttransplant states (10,17). We assumed proportional baseline hazards, and fit Cox regression models as Markov proportional hazard models (18). The transition state of transplantation was fit as an interaction term to account for variable survival time in the preversus posttransplant states (18). Waitlist candidates were categorized as: (1) waitlist candidates without POPH or any other MELD exception points (general nonexception waitlist population); (2) POPH MELD exception point recipients with documented hemodynamic criteria consistent with POPH (n = 100) or (3) POPH MELD exception point recipients with missing/incomplete hemodynamic data and/or data not consistent with POPH (n = 55).
All analyses used Stata 13.0 (College Station, TX).
Results
There were 174 waitlist candidates who applied for a MELD exception for “POPH” from December 1, 2006 through December 15, 2012. Of these individuals, 155 (89.1%) had at least one POPH exception application approved. Approximately half of waitlist candidates with an approved POPH MELD exception were males and the most common diagnosis was hepatitis C (n = 72, 46.5%; Table 1). Over one-half of the 155 candidates with an approved POPH exception were from UNOS regions 4, 5 or 7, compared with fewer than 40% of nonexception waitlist candidates during the study period (Table 1).
Table 1.
Demographics of waitlist candidates during the study period of 2006–2012
| Hemodynamic criteria consistent with POPH, n=1001 |
Hemodynamic data missing or not consistent with POPH, n=551 |
Nonexception waitlist cohort, n=34 180 |
p-Value | |
|---|---|---|---|---|
| Age at listing | 52 (48–57) | 54 (49–58) | 55 (49–60) | 0.009 |
| Male gender | 57 (57.0) | 31 (56.4) | 21 508 (62.9) | 0.29 |
| Race/ethnicity | 0.12 | |||
| White | 81 (81.0) | 43 (78.2) | 24 685 (72.2) | |
| Black | 3 (3.0) | 3 (5.5) | 2 876 (8.4) | |
| Hispanic | 10 (10.0) | 8 (14.6) | 5 258 (15.4) | |
| Other | 6 (6.0) | 1 (1.8) | 1 361 (4.0) | |
| Diagnosis | <0.001 | |||
| Hepatitis C | 50 (50.0) | 22 (40.0) | 12 987 (38.0) | |
| Alcohol | 23 (23.0) | 8 (14.6) | 7 441 (21.8) | |
| NASH/cryptogenic | 10 (10.0) | 11 (20.0) | 6 515 (19.1) | |
| Autoimmune | 10 (10.0) | 4 (7.3) | 1 840 (5.4) | |
| Other | 7 (7.0) | 10 (18.2) | 5 397 (15.8) | |
| UNOS region | <0.001 | |||
| 1 | 5 (5.0) | 6 (10.9) | 1 686 (4.9) | |
| 2 | 2 (2.0) | 3 (5.5) | 4 163 (12.2) | |
| 3 | 7 (7.0) | 2 (3.6) | 3 895 (11.4) | |
| 4 | 20 (20.0) | 11 (20.0) | 3 939 (11.5) | |
| 5 | 18 (18.0) | 7 (12.7) | 6 251 (18.3) | |
| 6 | 0 (0.0) | 0 (0.0) | 714 (2.1) | |
| 7 | 19 (19.0) | 5 (9.1) | 2 929 (8.6) | |
| 8 | 8 (8.0) | 11 (20.0) | 2 365 (6.9) | |
| 9 | 5 (5.0) | 0 (0.0) | 2 523 (7.4) | |
| 10 | 3 (3.0) | 4 (7.3) | 2 772 (8.1) | |
| 11 | 13 (13.0) | 6 (10.9) | 2 943 (8.6) | |
| Listing MELD score | 12 (10–15) | 14 (11–15) | 17 (13–23) | <0.001 |
MELD, Model for End-Stage Liver Disease; mPAP, mean pulmonary artery pressure; NASH, nonalcoholic steatohepatitis; POPH, portopulmonary hypertension; PVR, pulmonary vascular resistance; UNOS, United Network for Organ Sharing.
Hemodynamic criteria consistent with POPH include documentation of an mPAP>25 mmHg and a PVR>3 Wood units on right heart catheterization prior to initiation of vasodilator therapy.
Exception approval based on UNOS guidelines
We compared the available OPTN data (age, diagnosis, mPAP, PVR) on waitlist candidates with POPH exceptions from UNOS region 7 to previously published center-level data from a center in this region (7). The available hemodynamic data (mPAP and PVR) in the OPTN data set matched precisely to the published data on mPAP, PVR, age and diagnosis with that from the published series from region 7.
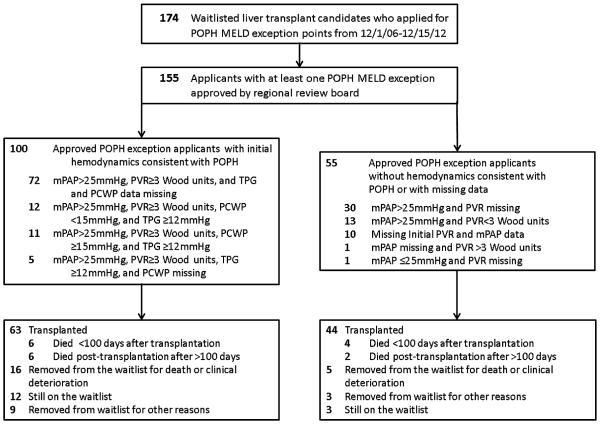
Based on the available data, 47.1% (73/155) of waitlist candidates with an approved POPH MELD exception met OPTN criteria for standardized POPH exception points (Table 2, Figure 1). In addition to these 73, there were 27 patients who met OPTN criteria for initial hemodynamic criteria consistent with POPH, but had: (1) a posttreatment mPAP ≥ 35 mmHg (n = 16); (2) no treatment or posttreatment data (n = 5); (3) posttreatment PVR ≥ 5 Wood units (n = 3) or (4) posttreatment mPAP ≥ 35 mmHg and post-treatment PVR ≥ 5 Wood units (n = 3). Of the 100 patients with initial hemodynamic criteria consistent with POPH, only 23 had data for PCWP and 28 for TPG (although these data are not required under OPTN/UNOS policy).
Table 2.
Right-heart catheterization and POPH treatment data on 100 waitlist candidates with approved POPH exceptions with hemodynamics consistent with POPH1
| Variable | Median (IQR)** |
|---|---|
| Pretreatment hemodynamics at time of MELD exception | |
| PVR, Wood units | 5.6 (4.6–5.6) |
| mPAP, mmHg | 47 (41–55) |
| PCWP, mmHg | 7 (5–15); (n=23) |
| TPG, mmHg | 32 (26–36); (n=28) |
| Posttreatment values | |
| PVR, Wood units; n=93 | 2.4 (1.7–3.2); (n=93) |
| mPAP, mmHg; n=97 | 30 (26–34); (n=97) |
| Response to treatment | |
| Posttreatment decrease | −3.2 (−1.9, −4.4); (n=93) |
| in PVR, Wood units | |
| Posttreatment decrease | −17 (−11, −24); (n=97) |
| in mPAP, mmHg | |
| Meet posttreatment OPTN/UNOS guidelines † | |
| Proportion with PVR | 89 (95.7) |
| <5 Wood units, n (%) | |
| Proportion with mPAP | 76 (78.4) |
| < 35 mmHg, n (%) | |
IQR, interquartile range; MELD, Model for End-Stage Liver Disease; OPTN, Organ Procurement and Transplantation Network; POPH, portopulmonary hypertension; PVR, pulmonary vascular resistance; mPAP, mean pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; TPG, trans-pulmonary gradient; UNOS, United Network for Organ Sharing.
Unless otherwise noted.
Reference (9).
Hemodynamic criteria consistent with POPH include an mPAP >25mmHg and PVR>3 Wood units prior to initiation of vasodilator therapy.
Figure 1.
Flow diagram for inclusion and outcomes of waitlist candidates with approved portopulmonary hypertension Model for End-Stage Liver Disease exception points.
Fifty-five waitlist candidates with an approved POPH MELD exception either did not have sufficient data to assess whether their pretreatment hemodynamic data were consistent with POPH, or had hemodynamic features that were not consistent with POPH, but rather a volume overload state (low PVR in the setting of an elevated mPAP; Figure 1). Thirty-one (60%) of these exceptions were approved from 2010 to 2012. Notably, there were no significant clinical or demographic differences among waitlist candidates with an approved POPH exception who had initial hemodynamic data consistent with POPH (n = 100) versus those with either missing data or values inconsistent with POPH (n = 55; Table 1).
Waitlist outcomes
Waitlist candidates with hemodynamics consistent with POPH were significantly less likely to be transplanted (63% [63/100] vs. 80% [44/55]; p = 0.03) and more likely to be removed from the waitlist for death or clinical deterioration (23% [23/100] vs. 9% [5/55]; p = 0.03) when compared with waitlist candidates with approved POPH exceptions who did not have hemodynamics consistent with POPH or had insufficient data. Cause of death or waitlist removal data are limited in the OPTN/UNOS data set to further explore the differences in waitlist removal between the two groups. The remaining waitlist candidates are either still waitlisted (n = 15), or removed for reasons other than death or clinical deterioration (i.e. lost to follow-up; n = 5). By contrast, a much smaller proportion of nonexception waitlist candidates (37.9%, n = 12 947) underwent transplantation during the study period, while 5547 (16.2%) were removed from the waitlist for death or clinical deterioration.
Posttransplant outcomes
Unadjusted posttransplant survival was not significantly different when comparing transplant recipients with POPH MELD exception points and nonexception transplant recipients (log-rank test p = 0.96). There were 18 (16.7%) posttransplant deaths among the 107 transplant recipients with POPH MELD exceptions—12 among those with hemodynamics consistent with POPH and 6 deaths in recipients with insufficient hemodynamic data. Of the 12 deaths among transplant recipients with hemodynamics consistent with POPH, 3 (25%) occurred on the same day of transplantation; 1 (16.7%) death occurred in a patient with insufficient hemodynamic data on postoperative day #1. Five additional transplant recipients died between post-transplant days 8–35, three of whom had hemodynamic criteria consistent with POPH.
There were six deaths within the first 16 days posttrans-plantation (four with hemodynamic criteria consistent with POPH, two with insufficient hemodynamic data), all of which were due to cardiac arrest/right heart failure. Among the 14 deaths of transplant recipients with POPH MELD exception points within the first 6 months of transplantation, one-half (n = 7) either did not meet treatment response criteria yet maintained exception points (n = 3) or did not have hemodynamic data to document a treatment response (n = 4).
Overall survival
Overall unadjusted 3-year survival was numerically but not statistically significantly lower (p = 0.08) in the cohort with hemodynamic criteria consistent with POPH (64.3%, 95% confidence interval (CI): 52.1–74.1), compared to waitlist candidates with approved POPH exceptions and insufficient hemodynamic data (77.3%, 95% CI: 62.5–86.9) or nonexception waitlist candidates (69.8%, 95% CI: 69.1–70.6). In multivariable multistate survival models that accounted for all survival time and transitions from pre- to posttransplant states, both cohorts of patients with POPH MELD exception points had greater mortality compared to all nonexception waitlist candidates during the study period (Table 3). Specifically, the hazard ratio (HR) for POPH patients without hemodynamic criteria consistent with POPH was: HR: 1.60, 95% CI: 1.04–2.47; compared to: HR: 2.46, 95% CI: 1.73–3.52 for cohort with hemodynamic data consistent with POPH.
Table 3.
Multistate Cox regression model comparing the risk of death of POPH MELD exception point recipients to non-POPH, nonexception waitlist candidates from the time of listing/receiving exception points
| Variable | Multivariable HR | p-Value1 |
|---|---|---|
| Nonexception waitlist candidates, n=34 180 |
1 | |
| POPH MELD exceptions with hemodynamic criteria consistent with POPH, n=100 † |
2.46 (1.73–3.52) | <0.001 |
| POPH MELD exceptions without hemodynamic criteria consistent with POPH, n=55 † |
1.60 (1.04–2.47) | 0.03 |
| Male gender | 0.91 (0.85–0.98) | 0.01 |
| Race/ethnicity | <0.001 | |
| White | 1 | |
| Black | 1.00 (0.90–1.10) | |
| Hispanic | 0.93 (0.85–1.03) | |
| Asian | 0.94 (0.82–1.08) | |
| Other | 0.89 (0.74–1.07) | |
| Age at listing | 1.36 (1.32–1.39) | <0.001 |
| Primary diagnosis | <0.001 | |
| Hepatitis C | 1 | |
| Alcohol | 0.74 (0.70–0.78) | |
| Hepatitis B | 0.66 (0.57–0.76) | |
| NASH/cryptogenic | 0.79 (0.75–0.84) | |
| Cholestatic | 0.74 (0.69–0.80) | |
| Autoimmune | 0.83 (0.72–0.94) | |
| Other | 0.97 (0.88–1.06) | |
| Listing laboratory MELD score | 1.09 (1.08–1.10) | <0.001 |
| Blood type | <0.001 | |
| O | 1 | |
| A | 1.05 (0.99–1.12) | |
| B | 0.93 (0.84–1.02) | |
| AB | 0.83 (0.73–0.94) |
HR, hazard ratio; MELD, Model for End-Stage Liver Disease; POPH, portopulmonary hypertension.
Reference (9).
Adjusted for age, sex, race/ethnicity, age at listing, primary diagnosis, listing laboratory MELD score and blood type.
Discussion
Since December 2006, 155 liver transplant waitlist candidates have been approved for a POPH MELD exception. Despite standardized criteria for the approval of POPH exception applications, less than one-half of waitlist candidates with an approved POPH exception met these criteria. Furthermore, over one-third of those with an approved POPH exception either did not fulfill hemodynamic criteria that would be consistent with POPH or had missing data, with 80% of such patients receiving a transplant based on receiving exception points. Ethical issues of justice and equity in the distribution of this scarce resource (transplantable livers) may be compromised by the current implementation of the POPH MELD exception policy. Beyond the question of the appropriateness of the current criteria, the prioritization of a patient group yielding preferential allocation of organs without adherence to the required policy of OPTN/UNOS is concerning. In addition, the overall mortality, accounting for the transitions from pre- to posttransplant states, of the patients granted exception points for POPH was substantial and significantly higher than that of patients listed without exception points. These data suggest that patients with POPH exception points, irrespective of the degree of characterization of their pulmonary hypertension, have an increased risk of death regardless of transplantation.
In 2000, the largest published study of liver transplantation in patients with POPH demonstrated a considerable risk of early postoperative mortality, occurring exclusively in patients with an mPAP ≥ 35 mmHg (19). However, 84% of the cases included in this publication were based on previously published case reports or case series, and more importantly, 65% were diagnosed in the operating room at the time of transplantation, including 11 of the 14 deaths (19). With the advent of screening by echocardiography and medical treatments, these data may be difficult to generalize to the current day. Even so, many transplant teams have solely considered an mPAP ≥ 35 mmHg as the cutpoint to determine whether a patient with POPH is “high-risk” versus acceptable for transplant, and the results of this study are the basis for the current POPH exception criteria (1,3,8,20). Three US centers have published small case series of successful transplantation in patients with POPH in the MELD era (7,20,21). Although the results of these small series suggest acceptable posttransplant outcomes in certain recipients with POPH, the sample sizes were small and the outcomes for POPH remain unknown.
In 2006, the MELD Exception Study Group and Conference (MESSAGE) identified conditions that were underserved by the MELD score (6). The goal was to standardize exception points, and identify areas that merited further study, while having expert consensus opinion on which conditions were associated with increased waitlist mortality, or worse posttransplant outcomes if transplant was delayed (i.e. hepatocellular carcinoma or familial amyloid polyneuropathy). In the case of POPH, there are conflicting data as to whether liver transplantation can reverse POPH, and no data demonstrating that earlier transplantation is associated with improved outcomes (6). In fact, the MESSAGE investigators concluded that, “Because there is little data on the magnitude of increased risk related to mPAP, quantification of increased need on the basis of mPAP remains arbitrary at this time” (6, p. S133). Nonetheless, MESSAGE investigators recommended that exception points be awarded to patients with: (1) mPAP >35 mmHg; (2) a minimum of 12 weeks of an FDA-approved PAH therapy, resulting in a hemodynamic profile of mPAP < 35 mmHg and PVR < 5 Wood units and (3) satisfactory right ventricular function (defined by the individual transplant center) (8). However, when the OPTN formalized their policy for POPH exception points, the restrictions for data were less stringent, without a prespecified time period on therapy, and without limitations on right ventricular function (9).
Our data highlight the limitations of the current OPTN policy on POPH exceptions and define several key areas that should be urgently addressed. First, current OPTN policy does not require documentation of all the clinical and hemodynamic criteria needed to diagnose POPH. Pulmonary hypertension in advanced liver disease may result from causes other than POPH, most commonly pulmonary venous hypertension from volume overload, high cardiac output, parenchymal lung disease and sleep-disordered breathing. The diagnosis of POPH is ultimately a clinical one, requiring pulmonary function testing, complete hemodynamics (including the PCWP), chest imaging and other data. None of the exception narratives included all of these data. Such data should be mandatory to ensure POPH exception points are awarded to those who in fact have POPH, and not another disorder. This issue takes on added importance beyond accurate diagnosis, as other parameters such as right ventricular function (reflected by cardiac output and index) may be an indicator of the ability to tolerate liver transplant, and access to such data would improve our ability to risk-stratify POPH patients prior to transplantation. For example, a recent series of patients transplanted with POPH suggested that patients with extremely high cardiac output going into surgery survived, whereas those with lower cardiac output died perioperatively (22). Not only do the majority of patients with POPH MELD exceptions lack the minimal data requirements documenting both initial and posttreatment hemodynamics set forth by the OPTN/UNOS policy for POPH exception points, but over one-third do not even have pretreatment hemodynamic findings to meet minimal criteria for PAH.
Second, given that there are limited data from small case series to demonstrate acceptable posttransplant outcomes in patients with POPH and the potential for publication bias, a national registry of patients with POPH listed for liver transplantation is the only means to accurately capture patient characteristics and outcomes. A multicenter cohort is needed to determine: (1) the safety, feasibility and proper indications for transplantation in patients with POPH; (2) hemodynamic criteria that accurately risk stratify waitlist candidates with POPH; (3) optimal therapies and dosages for treating POPH; (4) best practices of peri- and posttrans-plant management of transplant recipients with POPH and (5) long-term patient outcomes, including accurate cause-of-death data. The importance of such a registry is highlighted by the current data showing that there is a continued risk of early postoperative mortality among waitlist candidates with hemodynamic criteria consistent with POPH who received POPH MELD exception points.
Several potential modifications should be considered. First, similar to hepatocellular carcinoma, there should be an electronic submission process for POPH exception points, whereby all clinical and hemodynamic data needed for the diagnosis of POPH would be required. This would ensure that exception points are awarded to those who in fact have POPH, and serve to prospectively collect data to use for research and policymaking purposes. Second, regional review boards should not deviate from approved exception guidelines unless there is another compelling reason/ indication. Third, we need to better understand the impact of liver transplant in POPH. Without a clear overall improvement in outcomes conferred by prioritizing these patients for transplant, we may jeopardize the ethical allocation of liver allografts. Currently available data preclude us from making a determination of specific cutpoints for awarding and maintaining MELD exception points for POPH. While an argument can be made for awarding exception points for patients with POPH, prospective data are needed to verify whether posttransplant outcomes are acceptable in such patients, and more importantly, which patients should be awarded such exceptions.
In conclusion, these data demonstrate that since the implementation of a formalized MELD exception policy for POPH, the majority of patients awarded such points have not met OPTN criteria for such exception points due to missing or incomplete data, with nearly one-third not having hemodynamic data consistent with POPH. In this subset of patients with POPH MELD exceptions, there was a sizable risk of waitlist mortality, more so in those with hemodynamic criteria consistent with POPH, with several early posttransplant deaths in both groups attributable to right heart failure/persistent pulmonary hypertension. These data highlight the need for OPTN/UNOS to revise not only the policy for POPH MELD exceptions, but also the process by which such points are awarded. Future research is needed to define features to better risk stratify waitlist candidates with POPH in order to minimize peri- and postoperative deaths related to pulmonary hypertension and right heart failure, and can only be achieved with improved prospective data collection.
Acknowledgments
These funding agencies played no role in the development, analysis or manuscript preparation for this study. The relevant grant information is as follows: Michael Fallon: HL 116886, HL113988; Steven Kawut: NIH K24 HL 103844, HL113988; David Goldberg: NIH K08 DK098272-01A1. This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
Abbreviations
- CI
confidence interval
- HR
hazard ratio
- LVEDP
left ventricular end-diastolic pressure
- MELD
Model for End-Stage Liver Disease
- MESSAGE
MELD Exception Study Group and Conference
- mPAP
mean pulmonary artery pressure
- OPTN
Organ Procurement and Transplantation Network
- PAH
pulmonary arterial hypertension
- PCWP
pulmonary capillary wedge pressure
- POPH
portopulmonary hypertension
- PVR
pulmonary vascular resistance
- TPG
trans-pulmonary gradient
- UNOS
United Network for Organ Sharing
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Fritz JS, Fallon MB, Kawut SM. Pulmonary vascular complications of liver disease. Am J Respir Crit Care Med. 2013;187:133–143. doi: 10.1164/rccm.201209-1583CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krowka MJ, Mandell MS, Ramsay MA, et al. Hepatopulmonary syndrome and portopulmonary hypertension: A report of the multicenter liver transplant database. Liver Transpl. 2004;10:174–182. doi: 10.1002/lt.20016. [DOI] [PubMed] [Google Scholar]
- 3.Passarella M, Fallon MB, Kawut SM. Portopulmonary hypertension. Clin Liver Dis. 2006;10:653–663. doi: 10.1016/j.cld.2006.08.023. x. [DOI] [PubMed] [Google Scholar]
- 4.Rybak D, Fallon MB, Krowka MJ, et al. Risk factors and impact of chronic obstructive pulmonary disease in candidates for liver transplantation. Liver Transpl. 2008;14:1357–1365. doi: 10.1002/lt.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadengue A, Benhayoun MK, Lebrec D, Benhamou JP. Pulmonary hypertension complicating portal hypertension: Prevalence and relation to splanchnic hemodynamics. Gastroenterology. 1991;100:520–528. doi: 10.1016/0016-5085(91)90225-a. [DOI] [PubMed] [Google Scholar]
- 6.Freeman RB, Jr, Gish RG, Harper A, et al. Model for end-stage liver disease (MELD) exception guidelines: Results and recommendations from the MELD Exception Study Group and Conference (MESSAGE) for the approval of patients who need liver transplantation with diseases not considered by the standard MELD formula. Liver Transpl. 2006;12(12 Suppl 3):S128–S136. doi: 10.1002/lt.20979. [DOI] [PubMed] [Google Scholar]
- 7.Hollatz TJ, Musat A, Westphal S, et al. Treatment with sildenafil and treprostinil allows successful liver transplantation of patients with moderate to severe portopulmonary hypertension. Liver Transpl. 2012;18:686–695. doi: 10.1002/lt.23407. [DOI] [PubMed] [Google Scholar]
- 8.Krowka MJ, Fallon MB, Mulligan DC, Gish RG. Model for end-stage liver disease (MELD) exception for portopulmonary hypertension. Liver Transpl. 2006;12(12 Suppl 3):S114–S116. doi: 10.1002/lt.20975. [DOI] [PubMed] [Google Scholar]
- 9.OPTN/UNOS Policies and Bylaws. Available at: http://optn. transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_8.pdf. Accessed September 2, 2013.
- 10.Goldberg DS, Krok K, Batra S, Trotter JF, Kawut SM, Fallon MB. Impact of the hepatopulmonary syndrome MELD exception policy on outcomes of patients after liver transplantation: An analysis of the UNOS database. Gastroenterology. 2014;146:1256–1265. doi: 10.1053/j.gastro.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg D, Bittermann T, Makar G. Lack of standardization in exception points for patients with primary sclerosing cholangitis and bacterial cholangitis. Am J Transplant. 2012;12:1603–1609. doi: 10.1111/j.1600-6143.2011.03969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bittermann T, Niu B, Hoteit MA, Goldberg D. Waitlist priority for hepatocellular carcinoma beyond Milan criteria: A potentially appropriate decision without a structured approach. Am J Transplant. 2014;14:79–87. doi: 10.1111/ajt.12530. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg D, French B, Abt P, Feng S, Cameron AM. Increasing disparity in waitlist mortality rates with increased model for end-stage liver disease scores for candidates with hepatocellular carcinoma versus candidates without hepatocellular carcinoma. Liver Transpl. 2012;18:434–443. doi: 10.1002/lt.23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300:2371–2378. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg D, French B, Thomasson A, Reddy KR, Halpern SD. Waitlist survival of patients with primary sclerosing cholangitis in the model for end-stage liver disease era. Liver Transpl. 2011;17:1355–1363. doi: 10.1002/lt.22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg D, French B, Trotter J, et al. Underreporting of liver transplant waitlist removals due to death or clinical deterioration: Results at four major centers. Transplantation. 2013;96:211–216. doi: 10.1097/TP.0b013e3182970619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey RC, Jia-Yeong Lin M, Krakauer H. Time-to-event modeling of competing risks with intervening states in transplantation. Am J Transplant. 2003;3:192–202. doi: 10.1034/j.1600-6143.2003.30203.x. [DOI] [PubMed] [Google Scholar]
- 18.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: Competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 19.Krowka MJ, Plevak DJ, Findlay JY, Rosen CB, Wiesner RH, Krom RA. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6:443–450. doi: 10.1053/jlts.2000.6356. [DOI] [PubMed] [Google Scholar]
- 20.Sussman N, Kaza V, Barshes N, et al. Successful liver transplantation following medical management of portopulmonary hypertension: A single-center series. Am J Transplant. 2006;6:2177–2182. doi: 10.1111/j.1600-6143.2006.01432.x. [DOI] [PubMed] [Google Scholar]
- 21.Fix OK, Bass NM, De Marco T, Merriman RB. Long-term follow-up of portopulmonary hypertension: Effect of treatment with epoprostenol. Liver Transpl. 2007;13:875–885. doi: 10.1002/lt.21174. [DOI] [PubMed] [Google Scholar]
- 22.Swanson KL, Wiesner RH, Nyberg SL, Rosen CB, Krowka MJ. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445–2453. doi: 10.1111/j.1600-6143.2008.02384.x. [DOI] [PubMed] [Google Scholar]