Abstract
Chikungunya fever (CHIKV), a viral disease transmitted by mosquitoes, is currently affecting several areas in the Caribbean. The vector is found in the Americas from southern Florida to Brazil, and the Caribbean is a highly connected region in terms of population movements. There is therefore a significant risk for the epidemic to quickly expand to a wide area in the Americas. Here, we describe the spread of CHIKV in the first three areas to report cases and between areas in the region. Local transmission of CHIKV in the Caribbean is very effective, the mean number of cases generated by a human case ranging from two to four. There is a strong spatial signature in the regional epidemic, with the risk of transmission between areas estimated to be inversely proportional to the distance rather than driven by air transportation. So far, this simple distance-based model has successfully predicted observed patterns of spread. The spatial structure allows ranking areas according to their risk of invasion. This characterisation may help national and international agencies to optimise resource allocation for monitoring and control and encourage areas with elevated risks to act.
Introduction
Chikungunya fever is caused by the chikungunya virus, an alphavirus that is transmitted by several species of mosquitoes, including Aedes albopictus and Ae. aegypti [1]. In the last decade, large outbreaks of chikungunya fever have been reported in the Indian Ocean region [2], with millions of people experiencing incapacitating arthralgia, fever and rashes [3,4]. Transmission was sustained even in places with high standards of sanitary organisation [5].
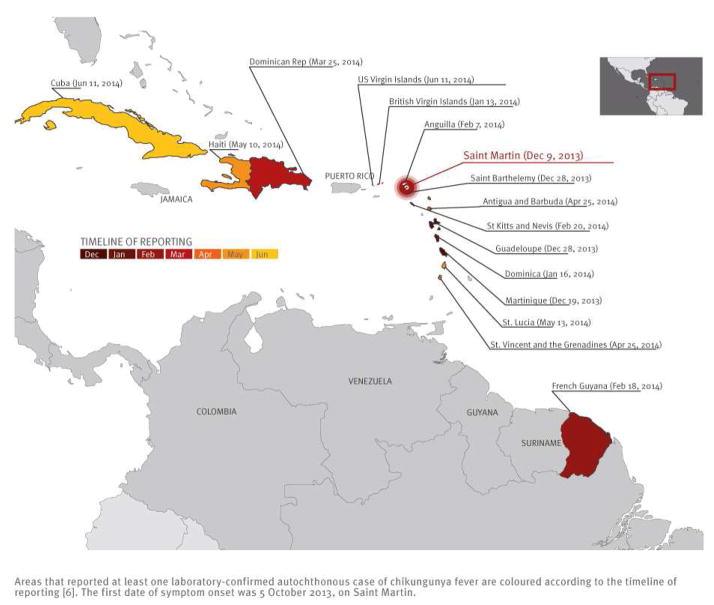
An outbreak of chikungunya fever is currently affecting an increasing number of areas in the Caribbean [6–8]. Figure 1 shows areas that reported at least one autochthonous case by 15 June 2014. The figure also shows the timeline of reporting. The first area reporting cases was Saint Martin (9 December 2013) with symptom onset of the first documented case on 5 October 2013. Further reports quickly followed from two other French territories, Martinique on 19 December 2013 and Guadeloupe on 28 December 2013. By 15 June 2014, 16 areas had reported at least one autochthonous case.
Figure 1.
Chikungunya fever in the Caribbean, as of 15 June 20141
This rapid expansion constitutes a source of concern for public health in the Americas [8]. The mosquito vector is found in a wide geographical zone that goes from South Florida to Brazil [10]. The potential for geographical expansion is therefore considerable and extends far beyond the areas currently affected. Moreover, the Caribbean is a highly connected area with frequent exchanges among the islands in the region, with mainland America and with Europe: more than 10 million international visits are reported each year by the World Tourism Organization, including 25% from Europe [11]. These important connections increase the risk of the current epidemic expanding quickly to a wider area in the Americas. Furthermore, the epidemic generates importations of cases into Europe, where the mosquito species Ae. albopictus is well established in many countries, primarily around the Mediterranean [9,12]. As of 1 July 2014, 98 imported laboratory-confirmed cases have been reported for metropolitan France alone [13].
In order to support preparedness and response planning in affected areas and those at risk of invasion (i.e. arrival of the disease in the area), it is important that we understand better the local and regional dynamics of spread of chikungunya fever in the Caribbean. Firstly, how effective is transmission of the disease in the Caribbean? Answering this question is important to assess the potential for large and explosive outbreaks as seen previously in the Indian Ocean region. Secondly, we need to understand the regional dynamics of spread and their determinants to assess which areas currently are at risk of invasion, to help national and international agencies with resource allocation, technical support and planning, and to encourage areas with elevated risks to act. This is essential in order to reduce disease burden in the Americas, but also to reduce the number of imported cases in Europe.
Here, we provide the first assessment of the effectiveness of transmission of the virus in the Caribbean and of the factors explaining the spread at the regional level.
Methods
Data collection
We selected 40 areas (countries or territories) around the Caribbean which overlap with areas infested by Ae. aegypti mosquito [10] and where dengue is present [14,15] in central America (Box).
Box. List of areas included in the assessment of chikungunya virus transmission (n=40).
Anguilla, Antigua and Barbuda, Aruba, Bahamas, Barbados, Belize, British Virgin Islands, Cayman Islands, Colombia, Costa Rica, Cuba, Curacao, Dominica, Dominican Republic, El Salvador, Florida, French Guiana, Grenada, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Jamaica, Martinique, Mexico, Netherlands Antilles, Nicaragua, Panama, Puerto Rico, Saint Barthelemy, Saint Kitts and Nevis, Saint Lucia, Saint Martin, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago, Turks and Caicos islands, United States Virgin Islands, Venezuela.
We defined areas officially affected by chikungunya fever as those reported to have had at least one laboratory-confirmed autochthonous case of chikungunya fever in the ProMED-mail alerts [6], the Pan American Health Organization [16] or the Caribbean Public Health Agency [17]. The date of the first report was also recorded.
In the French overseas territories (Saint Martin, Martinique and Guadeloupe), detailed data were collected by Cire Antilles-Guyane, using different approaches as the health authorities adapted to the situation. At first, an investigation was started around suspected or clinical cases with retrospective identification of other suspected cases in the neighbourhood. Virological confirmation was undertaken for most of the clinically suspected cases by the two laboratories of the national reference centre (Marseille and Cayenne). As the number of cases increased, existing surveillance networks based on general practitioners (GP) were asked to monitor clinical cases according to the case definition (patient with onset of acute fever >38.5 °C and severe arthralgia of hands or feet not explained by another medical condition). The surveillance network comprised 100% of the GPs on Saint-Martin (15 of 15) and around 20% on Martinique and Guadeloupe. Virological confirmation was no longer systematically undertaken as the number of cases increased.
Commercial air connections and 2013 data for volume of passengers between airports of the region were obtained from the International Air Transport Association [18,19]. These data correctly captured multi-leg flight trajectories, i.e. if a person flew from Florida to Jamaica via Puerto Rico, the recorded itinerary would be the Florida to Jamaica journey. Distances between the centroids of the areas were computed.
Characterising local transmission on Saint-Martin, Martinique and Guadeloupe
The human-to-human initial reproduction number R (mean number of secondary cases generated by a human case) was computed using the exponential growth method [20]. We explored the variability of these estimates by analysing all time periods of four weeks or more in the epidemic curves and reporting the 10 periods for which our exponential growth model had the best fit to the data (as measured by the deviance R-squared statistic [21]). Additional details can be found in the supplementary material* that can be accessed at https://docs.google.com/file/d/0B0pDXBmlKKGMRW9ucWRpaVV5bDQ/edit?pli=1.
Characterising regional spread
The transmission paths between areas were analysed under the hypotheses that the risk of invasion arose from previously invaded areas with data available as of 15 June 2014 [22]. We considered that Saint-Martin was the first invaded territory, with a first case on 5 October 2013. For other areas, a delay of on average 30 days was allowed between invasion and reporting. Different mathematical models were developed in which the instantaneous risk of transmission between areas depended on population size, distance, air traffic volume or a combination thereof. The models were fitted by Markov chain Monte Carlo sampling [23]. Goodness of fit was assessed by determining how well the models agreed with the set of areas officially affected by the time the analysis was performed. Finally, we used the best model to predict areas with the highest risk of invasion. As we have been using this model since early 2014, we also evaluated retrospectively short-term predictions that were made with data available on 15 January 2014 and on 30 March 2014. Technical details are available in the supplementary material* at [WEBLINK].
Results
Local transmission on Saint-Martin, Martinique and Guadeloupe
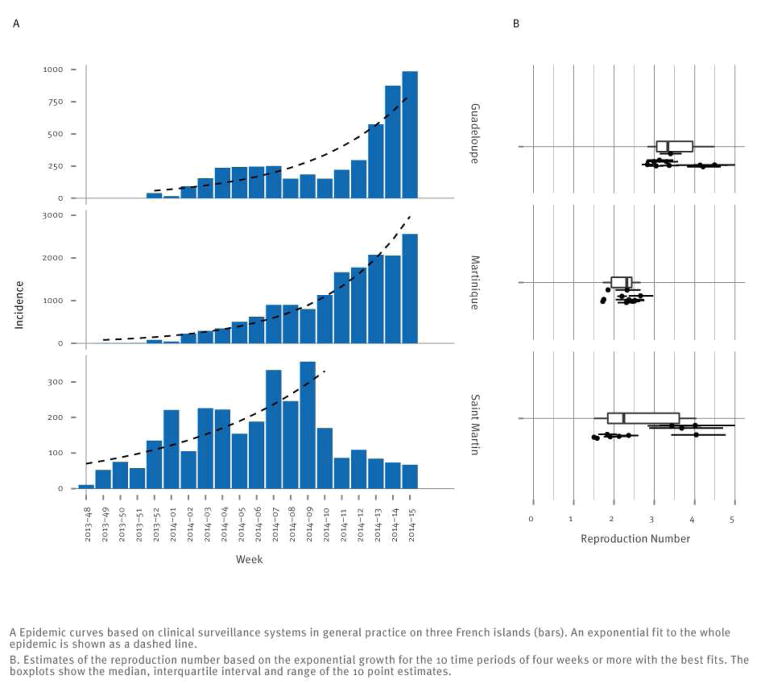
Surveillance of clinically suspected cases started in weeks 48, 49 and 52 of 2013 on Saint-Martin, Martinique and Guadeloupe, respectively. The fit of an exponential increase to the first weeks of each outbreak was reasonable, leading to estimates of the reproduction number in the range 2 to 4 (Figure 2). The reproduction number was estimated to be slightly higher on Guadeloupe than on Martinique, due to a renewed outbreak starting in week 10 of 2014 on Guadeloupe.
Figure 2.
Reproduction number of chikungunya fever in the Caribbean, 2014
Regional spread
A marked geographical pattern of the spread was apparent (Figure 1), as 12 of 16 officially affected areas were situated in a relatively small geographical zone between the British Virgin Islands in the north-west and Saint Vincent and the Grenadines in the south-east.
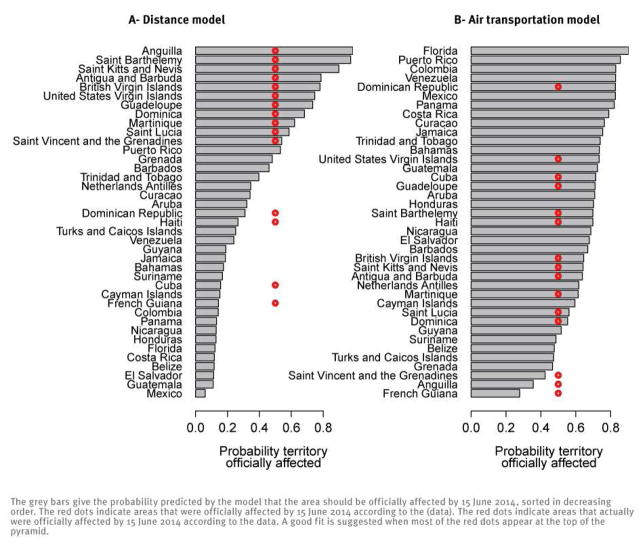
We found that this pattern was best explained by making the risk of transmission between areas inversely proportional to distance. If we exclude the seed location Saint Martin, 15 areas were officially affected. Of these 15, 11 were at the top of the list of areas predicted to be at highest risk of invasion by this simple model based on distance (Figure 3A). In contrast, only one of 15 officially affected areas was at the top of the list if the risk of transmission was instead assumed to depend on air passenger flows, indicating that air passenger flow was a poor predictor of transmission (Figure 3B). Population sizes of areas were not found to significantly affect transmission (see supplementary material*[WEBLINK]).
Figure 3.
Areas in the Caribbean officially affected by chikungunya fever on 15 June 2014 and prediction in the distance model (A) and the air transportation model (B)
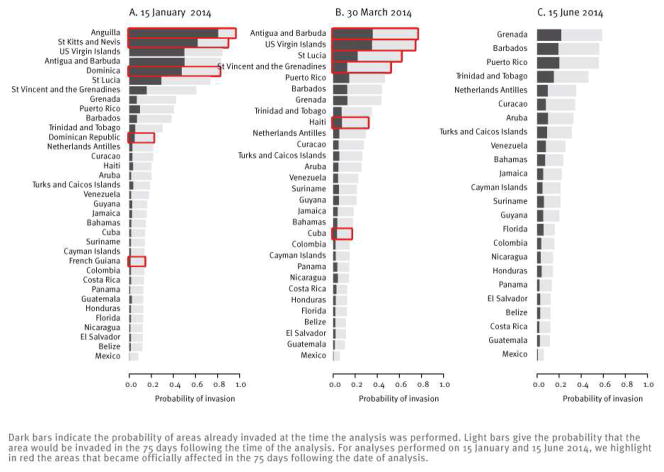
Figure 4 presents predictions made with this model on 15 January 2014 (Figure 4A) and on 30 March 2014 (Figure 4B). It shows the risk of being already invaded at the time of the analysis or of being invaded in the following 75 days, based on data available at the time. Overall, performance of the model has been good, as most areas officially affected in the following 75 days were among those that had the highest predicted risk of invasion. Of 11 areas officially affected during this period, French Guiana and Cuba were the only two with low predicted risks.
Figure 4.
Short-term predictions of the distance model performed on different dates in the chikungunya fever epidemic in the Caribbean with data as available on these dates
Figure 4C shows predictions of the model with data available on 15 June 2014. Grenada, Barbados and Puerto Rico currently have the largest predicted probability of being invaded in the 75 days following the analysis (36%). We note that heterogeneity in the predicted risk of invasion has decreased as Chikungunya has expanded in the region, with the standard deviation in the predicted risk declining from 27% on 15 January 2014 to 15% on 15 June 2014.
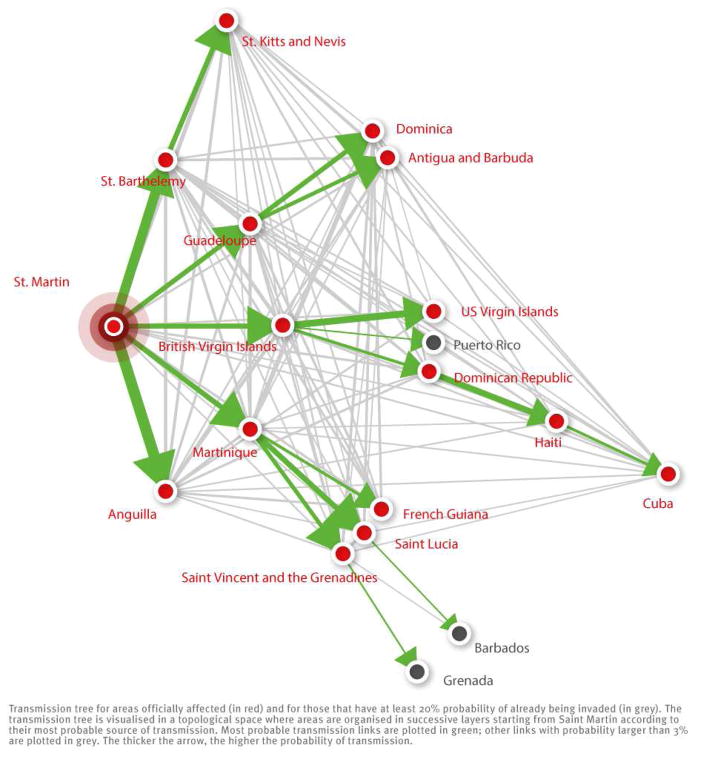
Assuming that Saint Martin was the seed of infection in the region, Figure 5 shows the most likely path of transmission for areas that were either officially affected or likely to be already invaded although autochtonous cases had not been reported. The first round of invasion included Martinique, Guadeloupe, Saint Barthélemy, British Virgin Islands and Anguilla. The second round of invasion eventually led to eight new invaded areas, including Dominica and French Guiana. Four rounds were necessary for the disease to reach Cuba. Looking at the reconstructed transmission tree and restricting the analysis to areas that were officially affected, we found that the median distance between two areas predicted to have transmitted chikungunya to each other was 476 km (95% CI: 16–2,040). It was 173 km (95% CI: 16–451) and 626 km (95% CI: 54–2,043), respectively, for areas in the first and in subsequent rounds of the regional epidemic.
Figure 5.
Most probable source of transmission for areas that are officially affected by chikungunya fever and for those that may already be invaded but have not yet reported cases
Discussion
The chikungunya virus has found a propitious environment for transmission in the Caribbean. All areas of the Caribbean and Central America are at risk of invasion, although with important heterogeneities in their predicted risks. Our analysis provides a quantitative basis for informed policy making and planning.
Transmission of chikungunya fever was consistently estimated to be effective in the three French territories that first reported cases (Saint-Martin, Martinique and Guadeloupe). Estimates of the reproduction number R ranged from 2 to 4, similar to what was reported in the Indian Ocean region [5,24], making large and fast-growing outbreaks possible. With the largest estimate, Guadeloupe may end up with the largest attack rate if transmission goes on unchanged. Interestingly, incidence there showed sustained increase only after the epidemic entered the largest city (Pointe à Pitre), suggesting heterogeneity in transmission. In Saint-Martin, incidence has notably slowed down in the last weeks, despite large growth at first. Further investigation is required to find out how vector abundance, heterogeneity in population mixing and exposure explain these outcomes. These estimates of R were obtained under the assumption that the serial interval was 23 days (see supplementary material* [WEBLINK]). Using a shorter duration for the gonotrophic cycle (three days vs four days) led to little change in the serial interval distribution (two days) and less than 5% variation on the estimates of R. With higher daily mortality in mosquitoes (15% instead of 10%), the serial interval was shorter, and the estimates of R were reduced by ca 20%.
Sustained transmission in the French islands has been in contrast with the limited number or absence of cases reported in some nearby areas. This could partly be explained if French territories were invaded first so that they had more time to build up large numbers of cases. However, heterogeneity in reporting is also likely to be involved, as some areas only reported the disease when it had already been responsible for hundreds of cases.
Indeed, a difficulty in the analysis of the regional diffusion of chikungunya fever has been the imperfect documentation of areas that were affected and of the dates when they were invaded. This is due to variable delays between (unobserved) dates of invasion and reporting of the first autochthonous cases. We did not model heterogeneities in the capabilities of the different areas to identify cases, as supporting data are lacking and this would therefore have been mostly subjective and added uncertainty to the analysis. But we used state-of-the-art data augmentation techniques [25–27] to overcome uncertainty about timing. In our baseline scenario, we assumed an average 30-day reporting delay but analysed alternative scenarios with shorter and longer delays in the supplementary material*[WEBLINK]. Reducing the reporting delay did not change the relative order of areas by risk of invasion but led to reduced probabilities of invasion in the near future. Unfortunately, we did not have independent data to back up the baseline assumption of an average 30-day delay in reporting.
To understand and predict regional spread, we postulated that importation of infected humans or mosquitoes by usual transportation routes was likely to be responsible for invasion of new areas. Most islands are served by air carriers, but travelling by boat, ferries and cruisers is also very common. Up to now, areas officially affected by chikungunya fever have presented smaller air passenger flows than those not yet affected (daily average: 797 as opposed to 2,476). It is therefore not surprising that air transportation data could not reproduce the patterns of spread seen so far (Figure 3B). A direct assessment of alternative modes of transportation, including boats and cruises, was not possible due to a lack of detailed data on these routes. To overcome this limitation, we used standard geographical models where connections between areas depend on distance and population sizes [28–30]. We found that the spatial structure of the epidemic was most consistent with a model in which the strength of a connection was inversely proportional to the distance. Overall, our results suggest that short-range transportation such as boats and cruises hopping between islands are likely to have played a substantial role in the spread observed in the early phase of the chikungunya outbreak in the Carribean.
The good fit of this distance model to current data (Figure 3A) and its successful predictions so far (Figure 4, panels A and B) give us some confidence in the short-term predictions of this model (Figure 4C). However, the relative importance of the transmission routes may change as the epidemic spreads, which could increase the risk to more distant areas in the longer term. In that respect, we note an apparent increase in the median distance of transmission between the first and subsequent waves in the regional epidemic. Given the current absence of correlation between available long-range air transportation data and disease spread, long-term predictions for international spread are harder to make.
The propensity of an area to get invaded and to transmit is expected to depend on vector activity and case numbers, respectively. Here, we used qualitative data on the presence of the Ae. aegypti mosquito [10], which are supported by recent reports on dengue virus circulation [14,15], to characterise vector activity. The vector was present in all areas included in our analysis [10,14,15]. Due to the lack of adequate data, we were unable to modulate the risk of invasion with more quantitative indicators of vector activity. Efforts to construct quantitative maps of vector activity should be a priority to improve model predictions. If they become available, data on incidence of cases in the invaded areas may improve the fit further, although this was not shown to be the case in the spatial analysis of other outbreaks [22]. Despite these limitations, short-term predictions of the model have been good (Figure 4, panels A and B). Improved predictions may require taking seasonality into account, as vector abundance may change with the seasons. The range of temperature is limited in the Caribbean islands (between 26 °C and 29 °C in Saint-Martin), but larger changes are expected as we move away from the equator. Seasonal changes in the number of passengers to and from the Caribbean must also be considered when studying the risk of importation to Europe.
In conclusion, we have shown that chikungunya fever is an important threat in the Americas. The high transmissibility may lead to fast-growing and large outbreaks. Regional dissemination is under way, so far with a simple geographical pattern, which is relevant for optimising the monitoring of areas.
Supplementary Material
Acknowledgments
We thank IMMI, EU FP7 PREDEMICS, Labex IBEID, NIH MIDAS and HARMSflu for research funding.
Footnotes
Note: Supplementary information made available by the authors on an independent website is not edited by Eurosurveillance, and Eurosurveillance is not responsible for the content. The material can be accessed at: https://docs.google.com/file/d/0B0pDXBmlKKGMRW9ucWRpaVV5bDQ/edit?pli=1.
Conflict of interest
None declared.
Authors’ contributions
ML, PQ, HDV provided the data. SC, CP, VC, PYB analysed the data. SC and PYB designed the analysis and wrote the first draft. All authors edited and commented the paper.
References
- 1.Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7(5):319–27. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 2.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet. 2012;379(9816):662–71. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- 3.Gerardin P, Guernier V, Perrau J, Fianu A, Le Roux K, Grivard P, et al. Estimating Chikungunya prevalence in La Reunion Island outbreak by serosurveys: Two methods for two critical times of the epidemic. BMC Infect Dis. 2008;8:99. doi: 10.1186/1471-2334-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soumahoro MK, Boelle PY, Gauzere BA, Atsou K, Pelat C, Lambert B, et al. The Chikungunya epidemic on La Reunion Island in 2005–2006: a cost-of-illness study. PLoS Negl Trop Dis. 2011;5(6):e1197. doi: 10.1371/journal.pntd.0001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boelle PY, Thomas G, Vergu E, Renault P, Valleron AJ, Flahault A. Investigating transmission in a two-wave epidemic of Chikungunya fever, Reunion Island. Vector Borne Zoonotic Dis. 2008;8(2):207–17. doi: 10.1089/vbz.2006.0620. [DOI] [PubMed] [Google Scholar]
- 6.ProMED-mail. Chikungunya (36): Caribbean. Archive Number 20140614.2539532. 2014 Jun 14; Available from: http://www.promedmail.org.
- 7.Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, De Lamballerie X. Chikungunya in the Americas. Lancet. 2014;383(9916):514. doi: 10.1016/S0140-6736(14)60185-9. [DOI] [PubMed] [Google Scholar]
- 8.Chikungunya - coming to America. Lancet. 2014;383(9916):488. doi: 10.1016/S0140-6736(14)60167-7. [DOI] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control (ECDC) Rapid risk assessment: Chikungunya outbreak in Caribbean region, 25 June 2014. Stockholm: ECDC; 2014. Available from: http://ecdc.europa.eu/en/publications/Publications/chikungunya-caribbean-june-2014-risk-assessment.pdf. [Google Scholar]
- 10.United Nations Environment Programme (UNEP) GEO4 – Global Environment Outlook 4: environment for development. Nairobi: UNEP; 2007. Available from: http://www.unep.org/geo/geo4.asp. [Google Scholar]
- 11.Dehoorne O, Murat C, Petit-Charles N. International tourism in the Caribbean area: current status and future prospects. Etudes caribéennes. 2010:16. doi: 10.4000/etudescaribeennes.4713. Available from: http://etudescaribeennes.revues.org/4713. [DOI]
- 12.Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, Zeller H, et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonot Dis. 2012;12(6):435–47. doi: 10.1089/vbz.2011.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Institut de Veille Sanitaire (InVS) Chikungunya et dengue - Données de la surveillance renforcée en 2014. [Chikungunuya and dengue – enhanced surveillance data in 2014]. Paris: InVS; 2014. French. Available from: http://www.invs.sante.fr/Dossiers-thematiques/Maladies-infectieuses/Maladies-a-transmission-vectorielle/Chikungunya/Donnees-epidemiologiques/France-metropolitaine/Chikungunya-et-dengue-Donnees-de-la-surveillance-renforcee-en-2014. [Google Scholar]
- 14.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healthmap. [Accessed 25 Apr 2014];Denguemap. Available from: http://healthmap.org/dengue/en/
- 16.Pan American Health Organization (PAHO) Epidemiological updates. PAHO; [Accessed 11 Jul 2014]. Chikungunya. Available from: www.paho.org/hq/index.php?option=com_topics&view=article&id=343&Itemid=40931&lang=enhttp://www.paho.org/hq. [Google Scholar]
- 17.Caribbean Public Health Agency (CARPHA) Chikungunya alert. CARPHA; 2013. Available from: http://carpha.org/articles/ID/10/Chikungunya-Alert. [Google Scholar]
- 18.International Air Transport Association (IATA) [Accessed 25 Mar 2014]; Available from: www.iata.org.
- 19.Balcan D, Colizza V, Goncalves B, Hu H, Ramasco JJ, Vespignani A. Multiscale mobility networks and the spatial spreading of infectious diseases. Proc Natl Acad Sci USA. 2009;106(51):21484–9. doi: 10.1073/pnas.0906910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallinga J, Lipsitch M. How generation intervals shape the relationship between growth rates and reproductive numbers. Proc Biol Sci. 2007;274(1609):599–604. doi: 10.1098/rspb.2006.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boelle PY, Bernillon P, Desenclos JC. A preliminary estimation of the reproduction ratio for new influenza A(H1N1) from the outbreak in Mexico, March-April 2009. Euro Surveill. 2009;14(19) doi: 10.2807/ese.14.19.19205-en. pii=19205. [DOI] [PubMed] [Google Scholar]
- 22.Eggo RM, Cauchemez S, Ferguson NM. Spatial dynamics of the 1918 influenza pandemic in England, Wales and the United States. J R Soc Interface. 2011;8(55):233–43. doi: 10.1098/rsif.2010.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilks WR, Richardson S, Spiegelhalter DJ. Markov Chain Monte Carlo in Practice. London: Chapman and Hall; 1996. [Google Scholar]
- 24.Yakob L, Clements ACA. A Mathematical Model of Chikungunya Dynamics and Control: The Major Epidemic on Reunion Island. Plos One. 2013:8. doi: 10.1371/journal.pone.0057448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cauchemez S, Carrat F, Viboud C, Valleron AJ, Boelle PY. A Bayesian MCMC approach to study transmission of influenza: application to household longitudinal data. Stat Med. 2004;23(22):3469–87. doi: 10.1002/sim.1912. [DOI] [PubMed] [Google Scholar]
- 26.Cauchemez S, Temime L, Guillemot D, Varon E, Valleron A, Thomas G, et al. Investigating heterogeneity in pneumococcal transmission: A Bayesian-MCMC approach applied to a follow-up of schools. J Am Stat Assoc. 2006;101:946–58. [Google Scholar]
- 27.O’Neill PD, Roberts GO. Bayesian inference for partially observed stochastic epidemics. J Roy Statist Soc Ser A. 1999;162:121–9. [Google Scholar]
- 28.Haynes KE, Fotheringham AS. Gravity and spatial interaction models. Sage publications; 1984. [Google Scholar]
- 29.Ortuzar J, Willumsen L. Modelling transport. 3. Wiley; 2001. [Google Scholar]
- 30.Zipf G. The P1 P2/D hypothesis: on the intercity movement of persons. Am Sociol Rev. 1946;11:677–89. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.