Abstract
Tendon is a strong connective tissue that transduces muscle-generated forces into skeletal motion. In fulfilling this role, tendons are subjected to repeated mechanical loading and high stress, which may result in injury. Tissue engineering with stem cells offers the potential to replace injured/damaged tissue with healthy, new living tissue. Critical to tendon tissue engineering is the induction and guidance of stem cells towards the tendon phenotype. Typical strategies have relied on adult tissue homeostatic and healing factors to influence stem cell differentiation, but have yet to achieve tissue regeneration. A novel paradigm is to use embryonic developmental factors as cues to promote tendon regeneration. Embryonic tendon progenitor cell differentiation in vivo is regulated by a combination of mechanical and chemical factors. We propose that these cues will guide stem cells to recapitulate critical aspects of tenogenesis and effectively direct the cells to differentiate and regenerate new tendon. Here, we review recent efforts to identify mechanical and chemical factors of embryonic tendon development to guide stem/progenitor cell differentiation toward new tendon formation, and discuss the role this work may have in the future of tendon tissue engineering.
Keywords: Embryonic tendon development, Tendon, Stem cells, Tissue engineering, Mechanical properties, Elastic modulus, Dynamic loading, Growth factors
1. Introduction
Tendon is a specialized connective tissue that serves a unique and critical role in the musculoskeletal system. As the mechanical link between muscle and bone, tendon transduces muscle-generated forces into skeletal motion. In fulfilling this role, tendon experiences high mechanical stress and repeated loading, which may lead to injury. Unfortunately, when tendon injuries occur, the native healing process results in scar tissue with altered extracellular matrix (ECM) composition and organization, and impaired mechanical properties compared to healthy tendon (Astrom and Rausing, 1995; Lin et al., 2004). Significant tendon ruptures require surgical repair (Del Buono et al., 2013; Wills et al., 1986), but surgery has its limitations. For example, a recent review of rotator cuff repairs found that initial tears of 5 cm or larger suffered re-tearing within a year in at least 43% of surgeries (Duquin et al., 2010). Grafting with autologous or allogeneic tissue is the current standard of care, but surgical replacement with one of these grafts is associated with numerous drawbacks. Consequently, these types of challenges have motivated efforts to engineer new tendons for replacement.
Tissue engineering holds great potential as a treatment for human injuries and disease. A classic tissue engineering approach is to culture stem cells in a biodegradable scaffold and treat the engineered construct with mechanical and chemical factors to direct stem cell differentiation and guide new tissue formation. Ultimately, new tissue would form in place of the scaffold, and be used to replace the injured or diseased tendon. In other tissue systems, differentiation of stem cells toward the desired lineage (e.g., osteogenic or adipogenic) is typically induced via a cocktail of culture medium and other soluble chemical supplements. Studies have explored various growth factors to promote tenogenesis (differentiation of cells towards the tendon lineage) (Barsby and Guest, 2013; Lee et al., 2011; Maeda et al., 2011; Pryce et al., 2009), but a well-defined set of soluble factors has yet to be established. In line with the mechano-active nature of tendon, it seems that mechanical factors also play a role in promoting tenogenesis. A number of recent studies have emphasized the importance of mechanical regulation of tenogenesis using mesenchymal stem cells (MSCs) (Doroski et al., 2010; Kuo and Tuan, 2008; Scott et al., 2011; Sharma and Snedeker, 2010; Thomopoulos et al., 2011). However, while these studies have made significant advancements, an engineered tendon has yet to be achieved.
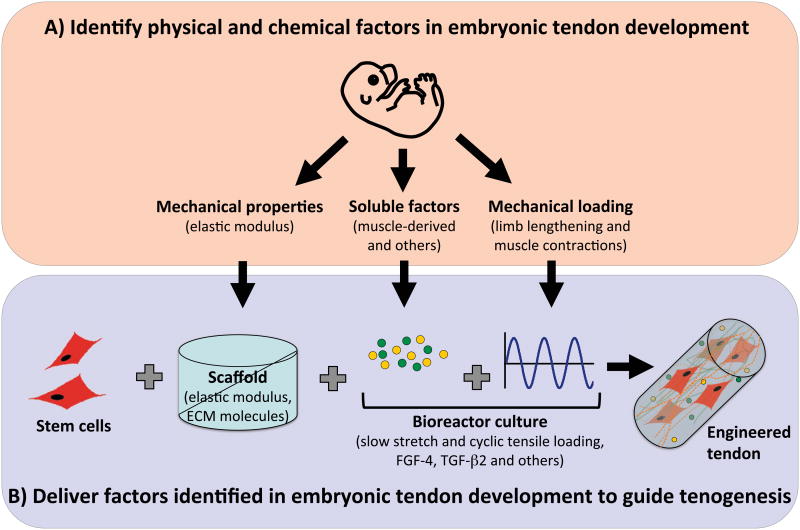
Interestingly, mechanical and chemical factors used in many tissue engineering strategies are those identified in adult tendon homeostasis and healing, even though those factors direct adult tendon healing toward scar formation rather than tissue regeneration. In contrast, we propose environmental factors that are present during embryonic tendon formation will induce a developmental response from stem cells and direct these cells to regenerate new, normal tissue. Specifically, physical and biochemical cues in the embryonic tissue microenvironment may be delivered via scaffolds and bioreactors to effectively direct stem cell tenogenesis and new tissue formation (Figure 1). In this review, we discuss recent efforts to characterize the embryonic tendon microenvironment, and to use embryonic cues to guide progenitor cell tenogenesis, with a specific focus on dynamic mechanical stimulation (section 2) and matrix stiffness (section 3). We also discuss the identification of functional markers that may be used to assess tendon formation (section 4). Finally, we close with considerations for embryonically inspired tissue engineering approaches to regenerate new tendon from adult stem cells (section 5).
Figure 1.
Embryonically inspired tissue engineering strategy. A) Characterize physical and chemical properties of developing embryonic tendon and its microenvironment. B) Deliver these factors to stem cells via the scaffold (e.g., elastic modulus, bound ECM molecules) and bioreactor culture (e.g., mechanical loading, soluble factors). Synergistic combinations of these cues may enhance and accelerate stem cell tenogenesis and neotissue formation.
2. Dynamic Mechanical Stimulation
Once the myotendinous junction is formed to connect muscle to bone, tendons become essential components of force transfer in the musculoskeletal system and experience static and dynamic mechanical tension. Muscle paralysis and removal studies implicate muscle-derived forces (e.g., contraction) as significant contributors to tendon formation during embryonic development (Edom-Vovard et al., 2002; Germiller and Goldstein, 1997; Kardon, 1998; Kieny and Chevallier, 1979; Mikic et al., 2000a; Mikic et al., 2000b). Embryonic tendon cells may also experience changes in tensile loading as embryonic limbs increase in length and the developing tendons are slowly stretched (Hamburger and Hamilton, 1951). Taken together, it is likely that these applied mechanical stimuli experienced by embryonic tendon act as physical cues to guide development.
To better understand how slow tensile stretch may influence tendon formation, chick embryonic tendon cells were seeded in a fibrin gel, which was then gradually stretched at 2 mm per day over 4 days to double the gel length (Kalson et al., 2011). This slow stretch resulted in increased collagen fibril diameter and volume fraction, collagen type I gene expression, and cell nuclei length. Mechanical evaluation showed an increase in the ultimate tensile stress and elastic modulus of the gel with stretch. This study suggests that tensile stretch provides a mechanical cue for embryonic tendon cells to regulate collagen and enhance tissue mechanical properties. However, the influence of slow tensile stretch on expression of tenogenic markers by stem/progenitor cells was not reported.
Embryonic muscle activation and the influence of muscle paralysis on embryonic tendon development was recently reviewed (Schiele et al., 2013), and thus will not be discussed at length here. Briefly, muscle paralysis results in tendon degeneration and significant musculoskeletal deformities (Germiller and Goldstein, 1997; Mikic et al., 2000a; Mikic et al., 2000b; Roddy et al., 2011). These studies suggest that dynamic mechanical loading resulting from muscle contractions is a significant influencer of tendon development, as embryonic tendon cells likely experience dynamic loading during muscle contractions. However, a significant challenge of studying the role of mechanical loading on developing tendon in vivo is to isolate muscle-derived mechanical influences from chemical influences. In addition to imposing mechanical strains on the tendon, muscle secretes soluble factors that may influence tendon development. Scleraxis, a transcription factor considered an early marker for tendon fate (Schweitzer et al., 2001), is regulated in part by fibroblastic growth factors (FGFs), such as FGF-4, that are secreted by developing muscles (Brent et al., 2005; Brent and Tabin, 2004; Edom-Vovard et al., 2002). Removal or disruption of muscle results in diminished scleraxis expression, while ectopic FGF-4 application rescues scleraxis expression (Brent et al., 2003; Edom-Vovard et al., 2002). These results implicate muscle-derived chemical signaling in the control of tendon differentiation, but these effects cannot be isolated from the effects of altered mechanical loading due to muscle removal or disruption. Taken together, these studies suggest muscle-derived physical and chemical cues are acting in concert to influence embryonic tendon development. While numerous in vitro studies have focused on the ability for mechanical loading to induce and sustain tenogenic differentiation of stem cells (Kuo and Tuan, 2008; Scott et al., 2011), it is likely that a combination of loading and soluble factor treatments are necessary to effectively guide tenogenic lineage commitment and differentiation.
Despite these studies, specific dynamic loading parameters (e.g., strain rate, strain magnitude, duration, and frequency) experienced by tenogenically differentiating cells during embryonic development in vivo are unknown. Similarly, soluble factor regulation of embryonic tendon during development is mostly unknown. The in vivo environment consists of numerous unknown factors, making it difficult to isolate specific factor effects based on animal studies. To circumvent this challenge, we are utilizing in vitro culture systems to study the effects of mechanical and chemical cues on tenogenesis of embryonic tendon progenitor cells (TPCs) in isolation of unknown confounding factors. We proposed that mechanical and chemical factors that are tenogenic during embryonic tendon development will promote or sustain tendon marker expression in embryonic TPCs in vitro. Embryonic mouse TPCs were isolated from axial (e.g., tail, spine, etc.) and limb (e.g., Achilles, patellar, flexor, etc.) tendons at different embryonic days (E) 13-17 of development and mechanically loaded or treated with FGF-4 or transforming growth factor (TGF)-β2 in vitro (Brown et al., 2014). FGF-4 and TGF-β2 have been shown to be critical for embryonic tendon development (Brent and Tabin, 2004; Kuo et al., 2008; Pryce et al., 2009), though their functions are not yet entirely known. For all stages, dynamic mechanical tensile loading (1% strain, 0.5 Hz) and FGF-4 each minimally influenced tenogenic gene expression when applied individually, while TGF-β2 significantly upregulated scleraxis gene expression in TPCs as a function of developmental stage and anatomical origin. Interestingly, when E16.5 limb TPCs were treated with the combination of TGF-β2, FGF-4 and loading (1% strain, 0.5 Hz), scleraxis gene expression was further enhanced. These results demonstrate that while certain individual embryonic chemical factors and mechanical loading are not sufficiently tenogenic, multiple cues may collaborate synergistically to regulate tenogenesis. This study also demonstrated that differentiation protocols should be tailored for tendons as a function of anatomical origin.
In a subsequent study, we also compared the response of adult MSCs to that of embryonic TPCs when treated with embryonic factors TGF-β2, FGF-4 and mechanical loading (manuscript in preparation). Interestingly, MSCs either lagged in magnitude of response or failed to respond to specific factors in comparison to TPCs. For instance, TGF-β2 treatment significantly upregulated scleraxis gene expression in both cell types, but approximately three times greater in embryonic limb TPCs than in MSCs. Additionally, the tenogenic response of TPCs to TGF-β2 was enhanced by all combinations with FGF-4 and mechanical loading, whereas MSCs responded tenogenically to fewer specific combinations.
Taken together, these studies identified TGF-β2 as a potent tenogenic factor for both primary embryonic TPCs and adult MSCs. Our results also demonstrated synergistic effects between loading and chemical factor treatment, suggesting that specific combinations of physical and chemical cues will effectively induce and guide tenogenesis of stem cells. This makes sense since both growth factors and muscle-derived mechanical loading are present during tenogenic differentiation in vivo. Further study will be required to continue to optimize loading parameters, as scleraxis expression in murine C310T1/2 mesenchymal progenitor cells was shown to increase in a strain magnitude and strain repetition-dependent fashion (Scott et al., 2011). Finally, a deeper understanding of the mechanisms of MSC response to physical and chemical cues, and why it deviates from that of TPCs, will certainly advance tendon tissue engineering and regeneration efforts.
3. Role of Matrix Stiffness
Another potential tenogenic mechanical cue is tissue elastic modulus. Substrate mechanical properties have been shown to be capable of directing gene expression of specific lineage (e.g., osteogenic, myogenic, neurogenic) markers in MSCs in the absence of chemical cues (Engler et al., 2006). Others have demonstrated that substrate modulus coupled with ECM composition can differentially regulate tenogenic gene expression by stem cells (Sharma and Snedeker, 2010). Based on this, we proposed embryonic tendon mechanical properties may be effective cues for tenogenic differentiation.
To pursue this hypothesis, we quantitatively characterized mechanical properties of embryonic chick tendon, and then used this information to fabricate scaffolds with embryonic tendon elastic moduli and evaluate their ability to influence tenogenic differentiation of chick TPCs. Using force volume-atomic force microscopy (FV-AFM) with both nanoscale and microscale size probes, we measured the elastic modulus of embryonic chick tendon as a function of developmental stage between Hamburger-Hamilton (HH) stages 28 to 43 (equivalent to embryonic developmental days 6 to 17) (Marturano et al., 2013a). Measurements at these length scales evaluate local tissue mechanical properties at a scale that cells may sense (Stolz et al., 2004). The elastic modulus of embryonic tendon was found to increase nonlinearly as a function of embryonic stage at both the nanoscale and microscale (Marturano et al., 2013a) (Figure 2A, saline control).
Figure 2.
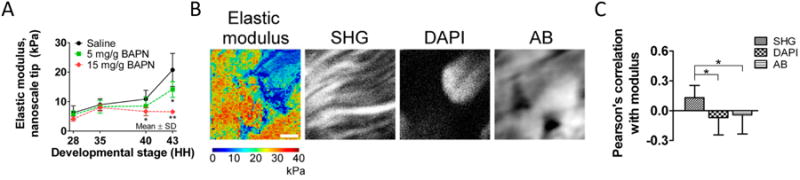
A) Embryonic tendon nanoscale elastic modulus, measured by FV-AFM, increased nonlinearly as a function of developmental stage (black line). To investigate collagen crosslinking as a mechanical contributor to embryonic tendon elastic modulus, we used BAPN treatment to inhibit LOX-mediated collagen crosslinks at each stage of embryonic development (HH 28-43). BAPN treatment of embryonic tendon resulted in a dose-dependent decrease in embryonic tendon elastic modulus at late stages (HH 40 and 43) of development, compared to saline control. B) Spatial mapping of nanoscale elastic modulus and corresponding images of collagen fibrils (SHG), cell nuclei (DAPI) and GAGs (alcian blue) in embryonic chick tendon. C) Pearson's correlation coefficient (r) between FV-AFM modulus maps and multiphoton microscopy images demonstrated elastic modulus was weakly correlated with collagen fibers (r = 0.13, P < 0.05), and there was no correlation with cell nuclei or GAGs. (Figure adapted from Marturano et al., 2013a).
Based on these measurements, we fabricated scaffolds with embryonic tendon elastic modulus and investigated the ability for scaffold modulus to influence tenogenic gene expression (Marturano et al., 2013b). Embryonic TPCs were encapsulated in RGD peptide-functionalized alginate hydrogels with nanoscale mechanical properties of embryonic tendon of various developmental stages. Scaffold moduli were chosen to mimic the elastic modulus of embryonic tendons at earlier, matching or later developmental stages of encapsulated TPCs, and found to regulate expression of scleraxis and other tenogenic genes as a function of modulus. This study suggests that tenogenic differentiation of stem cells such as embryonic TPCs, and perhaps MSCs, can be directed by native and engineered tissue elastic modulus. Ongoing studies are further characterizing the effects of these cues individually and in combination on stem cell differentiation.
4. Potential Functional Markers of Tendon Formation
It is common to presume that the mechanical properties of developing tissues increase with ECM biochemical content and organization. A recent study measured changes in murine Achilles tendon mechanical properties as well as collagen content, fibril diameter and organization (Ansorge et al., 2011). Tendon elastic modulus increased with post-natal age along with increased collagen content and fibril diameter, which suggests a correlation between mechanical properties and ECM content and composition. Based on this and other studies (Ansorge et al., 2011; Silver et al., 2003), we investigated relationships between embryonic chick tendon elastic modulus and ECM composition (Marturano et al., 2013a). Quantitatively, tendon elastic modulus increased nonlinearly while collagen biochemical content increased exponentially as a function of developmental stage, suggesting some correlation between the two parameters. However, analysis of spatial correlations between FV-AFM modulus maps and histological and second-harmonic generation (SHG) images demonstrated that collagen fibrils were only weakly correlated with elastic modulus (Pearson's correlation coefficient r = 0.13, P < 0.05), while glycosaminoglycans (GAGs) and cell nuclei, stained with Alcian blue (AB) and DAPI, respectively, had no significant correlation with elastic modulus (Figure 2B,C).
Based on these results, we decided to investigate collagen crosslinking as a possible contributor in the elaboration of embryonic tendon mechanical properties. Using mass spectrometry, we characterized changes in lysyl oxidase (LOX)-mediated crosslinks, hydroxylysyl pyridinoline (HP) and lysyl pyridinoline (LP), as a function of developmental stage, and demonstrated good correlation with elastic moduli (r2 = 0.8, p < 0.0001; (Marturano et al., 2014)). To inhibit LOX activity and thereby reduce collagen crosslinking during development, we treated chick embryos with β-aminopropionitrile (BAPN) in ovo. BAPN treatment resulted in a dose dependent reduction in both crosslink density and tendon elastic modulus at embryonic stages HH 40 and 43 (Figure 2A) but had no detectable effect on collagen content or organization (Marturano et al., 2013a). At the highest dosage, BAPN treatment completely ablated the developmental stage-dependent increases in tendon elastic modulus. This novel finding demonstrated that LOX-mediated collagen crosslinking is a critical contributor to embryonic tendon mechanical property elaboration, and that alterations in collagen crosslinking that affect mechanical properties may not be reflected in the biochemical concentration or organization of the ECM. Thus, it is critical to evaluate proper tissue formation with functional properties, rather than relying on ECM biochemical content to infer mechanical properties.
Having established good correlation between LOX crosslink density and mechanical properties of developing embryonic tendon, we pursued a potential method to non-destructively characterize LOX crosslinks as functional markers of tendon formation. Crosslink density is often measured via high performance liquid chromatography or mass spectrometry (Couppe et al., 2009). These evaluation methods require samples to be hydrolyzed prior to measurement, resulting in complete tissue destruction. Tissue destruction prohibits tracking changes in crosslink density over time within a single sample, and precludes acquisition of spatial information about the distribution of crosslinks. To investigate the potential to non-destructively quantify LOX-mediated crosslinks as a functional marker, we pursued the use of two-photon excitation fluorescence imaging to analyze tissue samples (Marturano et al., 2014). This microscopy-based approach takes advantage of the autofluorescence of HP and LP enzymatic crosslinks of collagen fibers. Collagen crosslinks were visualized in embryonic tendons by excitation with a two-photon laser using a 720 nm wavelength. When coupled with SHG imaging to observe collagen fibrils, the overlaid images (crosslinks and collagen) provided a visual representation of the location and density of crosslinks within the embryonic tendon. LOX-mediated crosslinks and collagen fibers were successfully visualized in embryonic chick tendons of various developmental stages, and shown via image analysis to have good correlation with mass spectrometry results. These results demonstrated the potential to use imaging to non-destructively quantify crosslink density without the need for an exogenous contrast agent or sample hydrolysis. Imaging of crosslinks may be a promising method to nondestructively, and perhaps non-invasively, characterize functional properties of tendon and other collagenous tissues during tissue formation. Studies are underway to further characterize this novel approach, which could potentially be used to evaluate engineered or embryonic tissue development, disease progression and even tissue healing over time.
5. Conclusions and Future Directions
As adult tendon healing results in aberrant tissue mechanics and scar formation rather than in regeneration, physical and chemical factors of adult tendon may not be effective for directing de novo tenogenesis of stem cells. In contrast, we propose that microenvironmental factors that regulate normal embryonic tendon development are potent tenogenic cues for stem cell differentiation. Our characterizations of the embryonic tendon microenvironment (e.g., tissue elastic modulus and biochemical composition) and its contributors (e.g., dynamic mechanical loads and growth factors) have provided a unique set of tissue engineering design parameters that may guide tenogenic differentiation of stem cells and assess new tissue formation. Our studies suggest that no single embryonic factor has been identified that will be sufficient to induce and maintain tenogenesis in vitro. Therefore, a combinatorial approach may be most effective to regulate stem cell tenogenesis, involving scaffold-dependent (e.g., elastic modulus) and exogenously applied (e.g., dynamic loading and chemical supplements) factors, including those we have identified (Figure 1). Identification of additional embryonic chemical and mechanical cues, as well as their optimal combinations, concentrations and parameters, and timing of delivery will advance embryonically inspired tissue engineering approaches.
An appropriate stem cell source will also be critical to the success of an embryonically informed strategy. Our studies suggest that MSCs may have limited differentiation potential compared to embryonic TPCs, even though both progenitor cell types originate from the mesenchyme. Characterization of molecular mechanisms responsible for differences in embryonic TPC and adult MSC response to factors may identify pathways that can be controlled to enhance the tenogenic capacity of MSCs. In addition to MSCs, a variety of other stem cells have been explored for tendon tissue engineering, including adipose-derived stem cells, embryonic stem cells, induced pluripotent stem cells, and even adult tendon-derived stem/progenitor cells (Bi et al., 2007; Cohen et al., 2010; James et al., 2011; Xu et al., 2013; Yin et al., 2013; Zhang and Wang, 2010). It is possible that specific stem cell types possess greater tenogenic potential.
All in all, embryonic chemical and physical factors appear to have the potential to regulate and enhance tenogenesis of post-natal stem cells. Continued identification and optimization of synergistic factor combinations and a deeper understanding of their effects on stem cell function may inform scaffold- and bioreactor-based strategies and accelerate efforts to regenerate tendon.
Acknowledgments
The authors thank Kaori Graybeal and Dr. Matteo Stoppato of Tufts University for reading the manuscript. We also acknowledge funding support by the NIH Training in Education and Critical Research Skills (K12GM074869) postdoctoral program (to N.R.S.), Research Grant 5-FY11-153 from the March of Dimes Foundation (to C.K.K.) and Award Number R03AR061036 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (to C.K.K.).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zachary A. Glass, Email: Zachary.Glass@tufts.edu.
Nathan R. Schiele, Email: Nathan.Schiele@tufts.edu.
References
- Ansorge HL, Adams S, Birk DE, Soslowsky LJ. Mechanical, compositional, and structural properties of the post-natal mouse Achilles tendon. Ann Biomed Eng. 2011;39:1904–1913. doi: 10.1007/s10439-011-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrom M, Rausing A. Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin Orthop Relat Res. 1995:151–164. [PubMed] [Google Scholar]
- Barsby T, Guest D. Transforming Growth Factor Beta3 Promotes Tendon Differentiation of Equine Embryo-Derived Stem Cells. Tissue Eng Part A. 2013;19:2156–2165. doi: 10.1089/ten.TEA.2012.0372. [DOI] [PubMed] [Google Scholar]
- Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- Brent AE, Braun T, Tabin CJ. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development. 2005;132:515–528. doi: 10.1242/dev.01605. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Brent AE, Tabin CJ. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development. 2004;131:3885–3896. doi: 10.1242/dev.01275. [DOI] [PubMed] [Google Scholar]
- Brown JP, Finley VG, Kuo CK. Embryonic mechanical and soluble cues regulate tendon progenitor cell gene expression as a function of developmental stage and anatomical origin. J Biomech. 2014 doi: 10.1016/j.jbiomech.2013.09.018. http://dx.doi.org/10.1016/j.jbiomech.2013.09.018. [DOI] [PMC free article] [PubMed]
- Cohen S, Leshansky L, Zussman E, Burman M, Srouji S, Livne E, Abramov N, Itskovitz-Eldor J. Repair of full-thickness tendon injury using connective tissue progenitors efficiently derived from human embryonic stem cells and fetal tissues. Tissue Eng Part A. 2010;16:3119–3137. doi: 10.1089/ten.TEA.2009.0716. [DOI] [PubMed] [Google Scholar]
- Couppe C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J App Physiol. 2009;107:880–886. doi: 10.1152/japplphysiol.00291.2009. [DOI] [PubMed] [Google Scholar]
- Del Buono A, Volpin A, Maffulli N. Minimally invasive versus open surgery for acute Achilles tendon rupture: a systematic review. Br Med Bull. 2013 doi: 10.1093/bmb/ldt029. [DOI] [PubMed] [Google Scholar]
- Doroski DM, Levenston ME, Temenoff JS. Cyclic tensile culture promotes fibroblastic differentiation of marrow stromal cells encapsulated in poly(ethylene glycol)-based hydrogels. Tissue Eng Part A. 2010;16:3457–3466. doi: 10.1089/ten.tea.2010.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835–841. doi: 10.1177/0363546509359679. [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F, Schuler B, Bonnin MA, Teillet MA, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev Biol. 2002;247:351–366. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Germiller JA, Goldstein SA. Structure and function of embryonic growth plate in the absence of functioning skeletal muscle. J Orthop Res. 1997;15:362–370. doi: 10.1002/jor.1100150308. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A Series of Normal Stages in the Development of the Chick Embryo. J Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- James R, Kumbar SG, Laurencin CT, Balian G, Chhabra AB. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomed Mater. 2011;6:025011. doi: 10.1088/1748-6041/6/2/025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalson NS, Holmes DF, Herchenhan A, Lu Y, Starborg T, Kadler KE. Slow stretching that mimics embryonic growth rate stimulates structural and mechanical development of tendon-like tissue in vitro. Dev Dyn. 2011;240:2520–2528. doi: 10.1002/dvdy.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125:4019–4032. doi: 10.1242/dev.125.20.4019. [DOI] [PubMed] [Google Scholar]
- Kieny M, Chevallier A. Autonomy of tendon development in the embryonic chick wing. J Embryol Exp Morphol. 1979;49:153–165. [PubMed] [Google Scholar]
- Kuo CK, Petersen BC, Tuan RS. Spatiotemporal protein distribution of TGF-betas, their receptors, and extracellular matrix molecules during embryonic tendon development. Dev Dyn. 2008;237:1477–1489. doi: 10.1002/dvdy.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615–1627. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- Lee JY, Zhou Z, Taub PJ, Ramcharan M, Li Y, Akinbiyi T, Maharam ER, Leong DJ, Laudier DM, Ruike T, Torina PJ, Zaidi M, Majeska RJ, Schaffler MB, Flatow EL, Sun HB. BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PloS one. 2011;6:e17531. doi: 10.1371/journal.pone.0017531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Biomech. 2004;37:865–877. doi: 10.1016/j.jbiomech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, Ilic D, Baskaran H, Sakai T. Conversion of mechanical force into TGF-beta-mediated biochemical signals. Curr Biol. 2011;21:933–941. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marturano JE, Arena JD, Schiller ZA, Georgakoudi I, Kuo CK. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc Nat Acad Sci USA. 2013a;110:6370–6375. doi: 10.1073/pnas.1300135110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marturano JE, Schiele NR, Thibodeau JJ, Galassi TV, Kuo CK. Embryonic tendon cells respond differentially to mechanical environment in three-dimensional gels. Transactions of the Annual Meeting of the Orthopaedic Research Society. 2013b Abstract # 0583. [Google Scholar]
- Marturano JE, Xylas JF, Sridharan GV, Georgakoudi I, Kuo CK. Lysyl oxidase-mediated collagen crosslinks may be assessed as markers of functional properties of tendon tissue formation. Acta Biomater. 2014 doi: 10.1016/j.actbio.2013.11.024. http://dx.doi.org/10.1016/j.actbio.2013.11.024. [DOI] [PMC free article] [PubMed]
- Mikic B, Johnson TL, Chhabra AB, Schalet BJ, Wong M, Hunziker EB. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J Rehabil Res Dev. 2000a;37:127–133. [PubMed] [Google Scholar]
- Mikic B, Wong M, Chiquet M, Hunziker EB. Mechanical modulation of tenascin-C and collagen-XII expression during avian synovial joint formation. J Orthop Res. 2000b;18:406–415. doi: 10.1002/jor.1100180312. [DOI] [PubMed] [Google Scholar]
- Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGF-beta signaling are essential for tendon formation. Development. 2009;136:1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy KA, Prendergast PJ, Murphy P. Mechanical influences on morphogenesis of the knee joint revealed through morphological, molecular and computational analysis of immobilised embryos. PloS one. 2011;6:e17526. doi: 10.1371/journal.pone.0017526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiele NR, Marturano JE, Kuo CK. Mechanical factors in embryonic tendon development: potential cues for stem cell tenogenesis. Curr Opin Biotechnol. 2013;24:834–840. doi: 10.1016/j.copbio.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- Scott A, Danielson P, Abraham T, Fong G, Sampaio AV, Underhill TM. Mechanical force modulates scleraxis expression in bioartificial tendons. J Musculoskelet Neuronal Interact. 2011;11:124–132. [PubMed] [Google Scholar]
- Sharma RI, Snedeker JG. Biochemical and biomechanical gradients for directed bone marrow stromal cell differentiation toward tendon and bone. Biomaterials. 2010;31:7695–7704. doi: 10.1016/j.biomaterials.2010.06.046. [DOI] [PubMed] [Google Scholar]
- Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. J Biomech. 2003;36:1529–1553. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Stolz M, Raiteri R, Daniels AU, VanLandingham MR, Baschong W, Aebi U. Dynamic elastic modulus of porcine articular cartilage determined at two different levels of tissue organization by indentation-type atomic force microscopy. Biophys J. 2004;86:3269–3283. doi: 10.1016/S0006-3495(04)74375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Das R, Birman V, Smith L, Ku K, Elson EL, Pryse KM, Marquez JP, Genin GM. Fibrocartilage tissue engineering: the role of the stress environment on cell morphology and matrix expression. Tissue Eng Part A. 2011;17:1039–1053. doi: 10.1089/ten.tea.2009.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills CA, Washburn S, Caiozzo V, Prietto CA. Achilles tendon rupture. A review of the literature comparing surgical versus nonsurgical treatment. Clin Orthop Relat Res. 1986:156–163. [PubMed] [Google Scholar]
- Xu W, Wang Y, Liu E, Sun Y, Luo Z, Xu Z, Liu W, Zhong L, Lv Y, Wang A, Tang Z, Li S, Yang L. Human iPSC-Derived Neural Crest Stem Cells Promote Tendon Repair in a Rat Patellar Tendon Window Defect Model. Tissue Eng Part A. 2013;19:2439–2451. doi: 10.1089/ten.tea.2012.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Chen X, Zhu T, Hu JJ, Song HX, Shen WL, Jiang LY, Heng BC, Ji JF, Ouyang HW. The effect of decellularized matrices on human tendon stem/progenitor cell differentiation and tendon repair. Acta Biomater. 2013;9:9317–9329. doi: 10.1016/j.actbio.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Wang JHC. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11 doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]