Abstract
OBJECTIVE(S)
Clinical research characterizing the mechanisms responsible for sex based outcome differences post-injury remain conflicting. Currently lacking is an understanding of the early sex-hormone milieu of the injured patient and the effects these early hormone differences have on clinical outcomes and the innate immune response following injury.
METHODS
A prospective cohort study was performed over a 20-month period. Blunt injured patients requiring ICU admission were enrolled. Samples were collected within 6 hours and at 24 hours post-injury and were analyzed for total testosterone (TT) and estradiol (EST) concentrations. Outcomes of interest included Multiple Organ Failure (MOF, Marshall MODscore > 5), nosocomial infection (NI), mortality and serial cytokine/chemokine measurements. Multivariate logistic regression was utilized to determine the independent risks associated with early sex hormone measurements.
RESULTS
In 288 prospectively enrolled patients, 69% were male with a median ISS of 16 [IQR 10,21]. Elevated TT levels at 6 hours were associated with elevated IL-6 levels and cytokine/chemokine measurements (18 of 24 measured). Risng TT levels were significantly associated with over a 5-fold and 2-fold higher independent risk of MOF and NI, respectively (OR 5.2, p=0.02, 95%CI 1.2–22.3, OR 2.1, p= 0.03, 95%CI 1.02–4.2). At 24 hours TT levels were no longer associated with poor outcome while EST levels were significantly associated with nearly a 4-fold higher independent risk of MOF (OR 3.9, p=0.04, 95% CI 1.05–13).
CONCLUSIONS
Early elevations and increasing testosterone levels over initial 24 hours post-injury are associated with an exaggerated inflammatory response and a significantly greater risk of MOF and NI. High Estrogen levels at 24 hours are independently associated with an increased risk of MOF. The current analysis suggests an early evolving testosterone to estrogen hormonal environment is associated with a significantly higher independent risk of poor outcome following traumatic injury.
Level of Evidence
II, prospective observational cohort
Keywords: testosterone, estrogen, multiple organ failure, nosocomial infection, regression
Introduction
An important and persistent finding has been that males and females respond differently following traumatic injury and hemorrhagic shock, with a relative protection afforded to females.1,2 An increasing body of evidence from animal models has revealed that sex-hormones, and or their derivatives, play an intricate role in the pathological response to trauma-hemorrhage. Estrogen and testosterone in disparate ways have been shown to influence the hemodynamic, immunologic, organ system and cellular responses to traumatic insult in animals.1–10 The hormonal milieu of the proestrus female rodent has been shown to be protective following trauma and hemorrhage, while male sex steroids are associated with deleterious effects.11–13 The strength of these laboratory findings has even led some to consider estrogen based therapy as a possible therapeutic intervention following traumatic injury in human patients.12,14
Despite this mounting evidence, clinical studies have been unable to consistently reproduce these laboratory findings.15–22 Recent prospective evidence, where sex hormone levels were measured 48 hours following injury, provides compelling evidence for estrogen (17β-estradiol) levels being associated with a greater risk of mortality, a conclusion which contradicts the majority of the experimental animal literature.23 Similar findings for non-injured but critically ill patients have also been reported.24,25 It remains unknown whether elevated endogenous estrogens out from the time of injury (> 48 hours) are simply a marker or play a causal role for poor outcome.23–25 Currently lacking is an understanding of the early sex-hormone milieu of the injured patient (< 6 hours from injury thru 24 hours post-injury) and the effects early sex hormones have on clinical outcomes and the immune response trajectory soon after injury.
In the present study, we sought to characterize the early sex hormone environment and its independent association with important clinical outcomes and the early innate immune response post-injury. We hypothesized that estrogen would be associated with beneficial effects while testosterone moieties would be associated with poor outcome.
Methods
A prospective observational cohort study was performed over a 20 month time period (2/11–10/12) with the overarching goal of characterizing the mechanisms responsible for sex (male vs. female) based outcome differences following traumatic injury. Inclusion criteria for the overall cohort study included blunt injured patients ≥17 years of age requiring ICU admission who arrived within 6 hours of injury to obtain early blood samples. Patients > 90 years of age, with isolated traumatic brain injury (no other injury identified other than brain injury), pre-existing immune-suppression, those with an anticipated survival of < 24 hours or those patients where consent was unable to be obtained were excluded from enrollment. Blood samples were collected within 6 hours and again at 24 hours post-injury and were analyzed for sex hormones (total testosterone and estradiol) and serial cytokine concentrations by specifically trained staff.26 Clinical outcomes assessed included the development of multiple organ failure (MOF, Marshall MODscore > 5), nosocomial infection (NI), and in-hospital mortality.
Under the auspices of a waiver of initial consent (up to 48 hours), blood was obtained from enrolled patients upon arrival or soon after within 6 hours from the time of injury and again at 24 hours post injury in most patients. A 48 hour window was approved to obtain consent for use of samples from the time of admission. All samples and data were destroyed if consent was unable to be obtained within the 48 hour widow. Plasma was separated from whole blood and stored at −70°C for batched analysis. Total Testosterone (TT) and 17β-estradiol (EST) levels were measured utilizing high sensitivity ELISA kits following the manufacturer’s directions (Testosterone ELISA kit, catalog #ADI-900-176, 17β-estradiol ELISA kit, catalog # ADI-900-174; ENZO Life Sciences, Inc., Farmingdale, NY). Human inflammatory MILLIPLEX ™ MAP Human Cytokine/Chemokine Panel-Premixed kits (catalog # HCYTOMAG-60K and # HCYTOMAG-60K; Millipore Corporation, Billerica, MA) and Luminex™ 100 IS (Luminex, Austin, TX) were used to measure plasma levels of cytokines and chemokines (IL-1β, IL-1RA, IL-2, sIL-2Rα, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-13, IL-15, IL-17, IFN-γ, IP-10, MIG, MIP-1α, MIP-1β, MCP-1, GM-CSF, Eotaxin, TNF-α, NO2/NO3 and IFN- α) per the manufacturer’s directions.
Sex hormone variables for TT and EST were first dichotomized at their median values into HIGH and LOW groups. Sex hormone levels were further categorized over time into a < 6 hour (<6HR) group, a 24 hour (24HR) group and additionally into a group where hormone measurements were increasing between 6HR and 24HR measurements (RISING). Finally EST/TT ratios were also characterized and utilized for the cytokine and outcome analyses.
Multiple organ failure was evaluated using the well-validated Marshall Multiple Organ Dysfunction Score (MODScore). 27–29 A MODScore > 5 beyond 48 hours from injury was classified as multiple organ failure (MOF). Primary nosocomial infectious outcomes of interest included ventilator associated pneumonia, blood stream infection (excluding those associated with an intra-abdominal abscess), and urinary tract infections.30 These were selected in attempts to use those infectious outcomes which can be used as a marker for the degree of relative immune dysregulation/suppression. The development of these nosocomial infections was based upon positive culture evidence. Diagnosis of a ventilator associated pneumonia required a quantitative culture threshold of 104 CFU/ml from broncho-alveolar lavage specimens. Diagnosis of catheter-related blood stream infections requires positive peripheral cultures with an identical organism obtained from either a positive semi-quantitative culture (>15 CFU/segment), or positive quantitative culture (>103 CFU/segment) from a catheter segment specimen. Urinary tract infections required > 105 organisms/ml of urine.
First, male and female patients underwent unadjusted comparison of demographics, injury characteristics, resuscitation and transfusion requirements, clinical outcomes and sex hormones. Correlation analysis was then performed between sex hormone levels and cytokine/chemokine measurements following variable log transformation. Finally, multivariable logistic regression analysis was then utilized to determine the independent odds of our clinical outcomes associated with sex hormone levels (HIGH vs. LOW) after adjusting for important confounders. Covariates adjusted for in the regression model included age (> or ≤ 50 yoa), sex, injury severity score (ISS), emergency department systolic blood pressure (SBP), emergency department Glasgow Coma Score (GCS, > or ≤ 8), intubation status (Yes/No), presenting coagulopathy (INR>1.3, Yes/No), 6 hour or 24 hour crystalloid and blood component transfusion requirements (PRBC, FFP, platelets), body mass index (BMI) and oral contraceptive use (Yes/No).
Mortality was used as the primary outcome to determine our sample size as this is the most stringent outcome to occur relative to the development of multiple organ failure and nosocomial infection. Based upon trauma admissions to the ICU at the University of Pittsburgh and using similar inclusion and exclusion criteria, for a similarly injured cohort as proposed, the mortality rate overall was 10%. Based upon these projections an allocation ratio of 0.10 (survivor vs. non-survivor) was used for sample size estimation. Based upon prior literature23,24 where serum levels of 17β-estradiol in both males and females were found to be significantly associated with mortality; survivors-[17β-estradiol] = 32.4±50 vs. non-survivors -[17β-estradiol] = 66.9±70 pg/ml, with an α = 0.05, and a β = 0.20, our projected sample size using a two-sided Mann-Whitney test was 320 patients.
All data were summarized as mean ± SD, median [IQR, inter-quartile range], or percentage (%). Student-t or Mann-Whitney statistical tests were used to compare continuous variables, while Chi-Square or Fischer’s Exact test was used for categorical variables. A p-value of ≤ 0.05 was considered statistically significant. The institutional review board at the University of Pittsburgh approved this study.
Results
Over a 20 month period, over two thousand patients were screened with 288 patients being prospectively enrolled and consented who met all inclusion and exclusion criteria and underwent early (< 6HR) blood sampling from the time of injury. Figure 1. This cohort of patients was 69% male with an average age of 50±18 years and constituted a moderately injured study cohort with a median injury severity score (ISS) of 16 [IQR 10,21]. Over 31% of patients required blood transfusion in the first 24 hours with the prevalence of MOF, NI and in hospital mortality being 13.6%, 29.9% and 3.1%, respectively. Importantly, 24HR sample collection was attempted for all enrolled patients but were only able to be obtained in 237 patients representing an 82% patient sample follow up rate.
Figure 1.
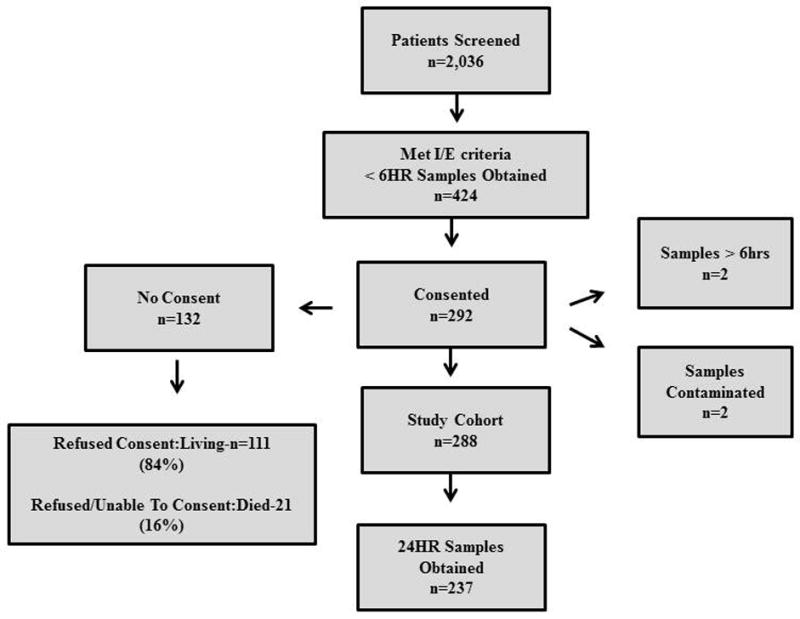
Study Cohort enrollment diagram.
Males and females were statistically similar in age, injury severity, presenting injury characteristics, transfusion and resuscitation requirements and important clinical outcomes. (Table 1.) Interestingly, there were no statistical differences in <6HR sex hormone measurements for either EST or TT as continuous variables across males or females. Males were, however, more likely to have pneumonia as a subtype of nosocomial infection despite nosocomial infections overall not being different across the groups.
Table 1.
Unadjusted comparison of Male and Female demographics, injury characteristics resuscitation needs and clinical outcomes.
| Males (n=197) | Females (n=91) | p-value | |
|---|---|---|---|
| Age (years) | 54±18 | 50±18 | 0.060 |
| ED SBP (mmHg) | 131±27 | 126±29 | 0.085 |
| % ED Hypotensive (SBP < 90 mmHg) | 8.1% | 10.0% | 0.601 |
| ED GCS | 15 [14,15] | 15 [15,15] | 0.446 |
| % ED GCS < 8 | 16.8% | 15.6% | 0.800 |
| Injury Severity Score (ISS) | 17[10,22] | 14[10,19] | 0.450 |
| ISS > 16 (%) | 51.3% | 45.6% | 0.386 |
| ED Intubation Status (%yes) | 13.7% | 11.2% | 0.573 |
| Presenting Coagulopathy (INR > 1.3) | 17.9% | 24.6% | 0.221 |
| Body Mass Index (BMI) | 29.1±7 | 28.3±7 | 0.153 |
| ICU Days | 6.1±6 | 5.0±6 | 0.183 |
| Length Of Stay | 11.7±9 | 11.2±9 | 0.685 |
| 24 hour Crystalloid (cc) | 3593±2527 | 3354±1963 | 0.428 |
| 24 hour Blood Transfusion (cc) | 509±1244 | 445±895 | 0.493 |
| 24 hour Plasma Tranfusion (cc) | 264±978 | 158±520 | 0.335 |
| 24 hour Platelet Transfusion (cc) | 92±284 | 41±187 | 0.109 |
| % Massive Transfusion (≥ 10 u PRBCs in 24hrs) | 5.6% | 2.2% | 0.204 |
| Nosocomial Infection | 30.5% | 27.8% | 0.645 |
| Pneumonia | 23.4% | 13.3% | 0.050* |
| MOF% | 14.7% | 11.1% | 0.408 |
| Mortality % | 4.1% | 1.1% | 0.183 |
| 6 hour Total Testosterone (pg/ml) | 38.4±44 | 33.6±16 | 0.315 |
| 6 hour 17β-Estradiol (pg/ml) | 44.2±38 | 41.2±22 | 0.849 |
When <6HR sex hormone levels were dichotomized into HIGH and LOW groups based upon the median of the measurement distribution, there was no statistical differences in early EST levels, early TT levels or the EST/TT ratio across males and females. (Table 2.) To verify EST and TT measurement were not concurrently elevated and collinear, we verified that over 36% of patients had either HIGH TT with LOW EST measurements or vice versa irrespective of male or female sex. (p< 0.001) We similarly found no significant differences in <6HR sex hormones (EST, TT) across age (≤50 y.o. vs. > 50 y.o., EST-p=0.444, TT-p=0.958) or when further stratified by male or female sex.
Table 2.
Dichotomized sex hormone levels (HIGH vs. LOW) for Early (< 6 hr) sex hormone level compared across males and females.
| Early (< 6hr) Sex Hormone Measurements (n=288) | Male (n=197) | Female (n=90) | p-value |
|---|---|---|---|
| HIGH 17β-Estradiol (EST) | 52.2% | 49.2% | 0.639 |
| HIGH Total Testosterone (TT) | 48.9% | 50.3% | 0.830 |
| HIGH EST/TT ratio | 48.7% | 53.3% | 0.469 |
When HIGH and LOW sex hormone and IL-6 cytokine levels were compared, both early (6hr) and 24 hour HIGH TT were significantly associated with elevated IL-6 levels (p=0.015, p= 0.004) while no significant relationship was found between IL-6 levels and EST. When correlation analysis of TT, EST and 24 cytokine/chemokine levels were performed following log transformation for normality considerations, no significant correlations were found for <6HR EST, RISING EST, or 24HR EST with any of the measured cytokine/chemokine levels. Similar results were found when correlation analysis was performed between cytokine/chemokine levels and EST/TT ratio at any time point. Interestingly, when <6HR TT levels were correlated with cytokine/chemokine levels, 3 out of 24 measurements demonstrated a significant correlation (IP10, MIP-1α and MIP-1β). When RISING TT levels were analyzed, the majority (16 out of 24) of the cytokine/chemokine panel were significantly correlated (positive correlation) with RISING TT levels. When 24HR TT levels underwent correlation analysis, the majority (16 out of 24) of cytokine/chemokine measurements again were significantly correlated (positive correlation) but with higher Pearson Correlation coefficients in all cases, consistent with a greater magnitude of correlation. (see supplemental digital content 1.)
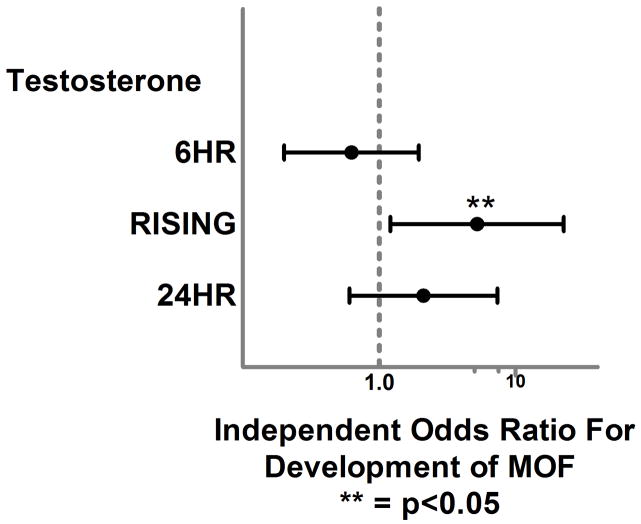
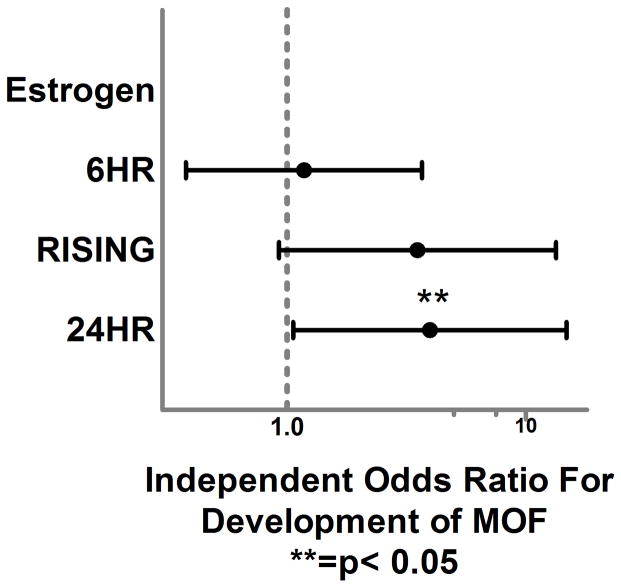
Our regression models were excellent predictors of our primary outcomes based upon area under the curve (AUC) from receiver operating characteristic curves and demonstrated adequate diagnostics. (Table 3.) After controlling for important confounders, logistic regression analysis demonstrated no significant independent relationship between <6HR TT levels or <6HR EST and the development of MOF, NI or in-hospital mortality. (Figures 2. and 3.) When the analysis focused on hormone levels which increased between the early and 24 hour time period, RISING TT levels were significantly associated with over a 5-fold and 2-fold higher independent odds of MOF and NI, respectively (OR 5.2, p=0.02, 95%CI 1.2–22.3, OR 2.1, p=0.03, 95%CI 1.02–4.2). RISING EST levels were associated with a 3-fold higher odds of MOF but this relationship failed to reach statistical significance (OR 3.0, p=0.089, 95% CI 0.85–10). Interestingly, at the 24HR time point TT levels were no longer significantly associated with the development of MOF or NI while EST levels were significantly associated with almost a 4-fold higher independent odds of MOF (OR 3.9, p=0.04, 95% CI 1.05–13) at this time point.
Table 3.
Logistic regression model diagnostics
| Logistic Regression Model Outcome | AUC via ROC curve analysis | Hosmer-Lemeshow |
|---|---|---|
| Mortality | 0.969 | 0.840 |
| MOF | 0.898 | 0.559 |
| NI | 0.760 | 0.463 |
Figure 2.
Forest plot depicting independent odds of MOF associated with early, increasing and 24 hour total testosterone levels.
Figure 3.
Forest plot depicting independent odds of MOF associated with early, increasing and 24 hour total 17β-estradiol levels.
Discussion
Significant advances in trauma care delivery and post-injury management practices have occurred over the last decade, yet patients who survive their initial injury continue to be plagued with the development of sepsis and multiple organ failure and their attributable morbidity and mortality.27,31–35 Despite a significant increase in our basic understanding of these detrimental outcomes, a dearth of effective interventions exist. An important and persistent literature finding, with possible therapeutic potential, has been that males and females respond differently following traumatic injury and hemorrhagic shock.1,17,18 A growing body of evidence from animal models suggests that this dimorphic response following trauma and hemorrhage is hormonally based (estrogen, testosterone, or their derivatives).4,6,36 Despite these advancements in our understanding, clinical studies have been unable to consistently reproduce these laboratory findings and have provided clinical evidence which contradicts the majority of animal literature.23–25 Lacking until this time has been a clear understanding of the early sex hormone environment which potentially has effects on clinical outcomes and the early immune response trajectory which follows traumatic injury. The results of the current analysis demonstrate that despite a paucity of sex specific differences in a moderate sized blunt injured cohort of patients, the evolving sex hormone environment post-injury is associated with both clinical outcome and innate immune response differences soon after injury. Despite sex hormones varying little across male and female sex early on (<6HR), these sex hormone specific associations were strong and independent of important confounders. Early and rising testosterone levels were found to be significantly associated with an exaggerated cytokine/chemokine response and detrimental clinical outcomes which diminished in strength over time up until 24 hours post-injury. Concurrently, estrogen levels were found to be strongly associated with detrimental clinical outcome at the delayed 24 hour time period alone.
These results correspond and add further understanding to prior literature which has demonstrated that estrogen is associated with mortality and poor outcome irrespective of male or female sex at 48 hours out from injury or sepsis.23–25 These results provide insight into the possible mechanisms by which the sex based outcome differences post-injury come about.2 Essential to understanding these associations is the fact that peripheral conversion of androgens to estrogens can occur via increased aromatase activity and may be stimulated by the early cytokine response which complicates traumatic injury.37–39 The current results suggest that early testosterone may be associated with an exaggerated innate immune response and an early evolving testosterone to estrogen hormonal environment is associated with a significantly higher independent risk of poor outcome following traumatic injury.
The potential implications of these results may bridge the current ‘bench to bedside divide’2 in our understanding of experimental animal evidence suggesting testosterone’s detrimental effects following hemorrhagic shock and the clinical evidence in humans demonstrating the negative associations of estrogen. Although the current results cannot imply causation nor was peripheral conversion of testosterone to estrogen measured, the strength of the independent findings in a relatively small, moderately injured cohort of patients does provide strength to the validity of these associations and provides the impetus to further study these relationships to determine if a therapeutic benefit can be derived from sex hormone therapy following injury.
The current analysis has several limitations that deserve discussion. First, the potential for selection and survivor bias exist, despite all attempts to minimize such difficulties, due to the non-randomized enrollment. Although the data collected for the prospective cohort analysis was extensive, potential unknown or unmeasured confounding variables may be responsible for the associations described and the conclusions formulated. Prehospital medicines that may interfere with sex hormone measurements were prospectively collected and controlled for in the analysis but unknown or undocumented medicines remain a potential confounder for the analysis. There was a lower than expected incidence of the selected pertinent outcomes of the study including MOF and mortality which can have an exaggeratory effect on the odds ratios presented in certain circumstances. Despite showing a robust association with MOF and NI, no relationship was found between sex hormone levels and mortality. Importantly, it has been previously demonstrated that a large portion of the most critically injured patients suffer mortality relatively early, commonly within the first 24–48 hours.40 Due to the requirement of informed consent the most critically ill patients had a lower consent rate significantly reducing the incidence of mortality for the study cohort. Although the < 6HR early cytokine expression measurements that were performed represents a relatively early time point compared to most other studies, this may still represent a delayed measurement for cytokine/chemokine expression which drives the development of MOF, NI and mortality. The time of sample obtainment in the 6 hour inclusion criteria window was not recorded and potentially may confound these early measurements and result in a time bias. There also existed a reduction or drop off in the number of samples collected from the enrolled 288 patients at <6HR period to 237 samples at the 24HR period. The potential exists that the 18% of measurements could alter the reported results and conclusions of the study. Interestingly, there existed no differences in early sex hormone measurements across males and females. Similarly, there were no differences found across age (< 50 or ≥ 50 y.o.) when compared. Despite this lack of hormone differences, there existed strong clinical associations for the sex hormone levels themselves. The study may be underpowered to see these sex and age based hormonal differences. The menstrual cycle status or the menopausal status was not obtained from females in the study cohort. Differences in these cycles and time periods in females may result in spurious modeling and alter the significance of these findings and limit the applicability to other studies. Finally, this study was performed at a single, level I trauma center and may not be generalizable or pertinent to other centers with differing admission demographics, injury characteristics or management practices.
In conclusion, early (< 6 hours) elevations and increasing testosterone levels over the initial 24hrs are associated with an exaggerated inflammatory response and a significantly greater independent odds of MOF and NI. By 24 hours post-injury, however, testosterone is no longer significantly associated poor outcome. Early elevations and increasing estrogen levels were not associated with differences in the early inflammatory response or a significant greater odds of poor outcome but estrogen levels at 24 hours post-injury are independently associated with a greater odds of MOF. These results suggest an early evolving testosterone to estrogen hormonal environment over the initial 24 hours post-injury has the potential to predict clinical outcome trajectory. These sex hormone changes may in part be responsible for sex based outcome differences following traumatic injury. Higher level studies are required to determine if these sex hormone changes play a causal role in these outcome differences and whether therapeutic potential exist via their actions.
Supplementary Material
Acknowledgments
Funding: This work was funded by NIH NIGMS K23GM093032 and Award # NTI-NTI-TRA-09-030 from the National Trauma Institute and sponsored by the Department of the Army, # W81XWH-10-1-0924. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense
Footnotes
Author Contributions: S. J. Z. and J.L.S. designed the study, performed the literature search, data collection, and data analysis. S.J.Z., Y.V. J.B.B., M.R.R., R.M.F and J.L.S. participated in initial manuscript preparation. All authors contributed to data interpretation and critical revision of the manuscript.
This paper was presented as an oral presentation at the annual meeting of the American Association for the Surgery of Trauma in Philadelphia PA, September 10–12, 2014.
Bilbiography
- 1.Choudhry MA, Bland KI, Chaudry IH. Gender and susceptibility to sepsis following trauma. Endocr Metab Immune Disord Drug Targets. 2006 Jun;6(2):127–135. doi: 10.2174/187153006777442422. [DOI] [PubMed] [Google Scholar]
- 2.Sperry JL, Minei JP. Gender dimorphism following injury: making the connection from bench to bedside. J Leukoc Biol. 2008 Mar;83(3):499–506. doi: 10.1189/jlb.0607360. [DOI] [PubMed] [Google Scholar]
- 3.Angele MK, Knoferl MW, Ayala A, Bland KI, Chaudry IH. Testosterone and estrogen differently effect Th1 and Th2 cytokine release following trauma-haemorrhage. Cytokine. 2001 Oct 7;16(1):22–30. doi: 10.1006/cyto.2001.0945. [DOI] [PubMed] [Google Scholar]
- 4.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000 Aug;14(2):81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 5.Angele MK, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Testosterone: the culprit for producing splenocyte immune depression after trauma hemorrhage. Am J Physiol. 1998 Jun;274(6 Pt 1):C1530–1536. doi: 10.1152/ajpcell.1998.274.6.C1530. [DOI] [PubMed] [Google Scholar]
- 6.Angele MK, Ayala A, Monfils BA, Cioffi WG, Bland KI, Chaudry IH. Testosterone and/or low estradiol: normally required but harmful immunologically for males after trauma-hemorrhage. J Trauma. 1998 Jan;44(1):78–85. doi: 10.1097/00005373-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Jarrar D, Wang P, Cioffi WG, Bland KI, Chaudry IH. The female reproductive cycle is an important variable in the response to trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2000 Sep;279(3):H1015–1021. doi: 10.1152/ajpheart.2000.279.3.H1015. [DOI] [PubMed] [Google Scholar]
- 8.Knoferl MW, Jarrar D, Angele MK, et al. 17 beta-Estradiol normalizes immune responses in ovariectomized females after trauma-hemorrhage. Am J Physiol Cell Physiol. 2001 Oct;281(4):C1131–1138. doi: 10.1152/ajpcell.2001.281.4.C1131. [DOI] [PubMed] [Google Scholar]
- 9.Choudhry MA, Schwacha MG, Hubbard WJ, et al. Gender differences in acute response to trauma-hemorrhage. Shock. 2005 Dec;24(Suppl 1):101–106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- 10.Yang S, Hu S, Chen J, et al. Mechanism of hepatoprotection in proestrus female rats following trauma-hemorrhage: heme oxygenase-1-derived normalization of hepatic inflammatory responses. Journal of leukocyte biology. 2009 Feb 24; doi: 10.1189/jlb.0508288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knoferl MW, Schwacha MG, Jarrar D, et al. Estrogen pretreatment protects males against hypoxia-induced immune depression. Am J Physiol Cell Physiol. 2002 May;282(5):C1087–1092. doi: 10.1152/ajpcell.00454.2001. [DOI] [PubMed] [Google Scholar]
- 12.Jarrar D, Wang P, Knoferl MW, et al. Insight into the mechanism by which estradiol improves organ functions after trauma-hemorrhage. Surgery. 2000 Aug;128(2):246–252. doi: 10.1067/msy.2000.107376. [DOI] [PubMed] [Google Scholar]
- 13.Angele MK, Wichmann MW, Ayala A, Cioffi WG, Chaudry IH. Testosterone receptor blockade after hemorrhage in males. Restoration of the depressed immune functions and improved survival following subsequent sepsis. Arch Surg. 1997 Nov;132(11):1207–1214. doi: 10.1001/archsurg.1997.01430350057010. [DOI] [PubMed] [Google Scholar]
- 14.Chaudry IH, Samy TS, Schwacha MG, Wang P, Rue LW, 3rd, Bland KI. Endocrine targets in experimental shock. The Journal of trauma. 2003 May;54(5 Suppl):S118–125. doi: 10.1097/01.TA.0000064511.14322.F1. [DOI] [PubMed] [Google Scholar]
- 15.Wichmann MW, Inthorn D, Andress HJ, Schildberg FW. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med. 2000 Feb;26(2):167–172. doi: 10.1007/s001340050041. [DOI] [PubMed] [Google Scholar]
- 16.Offner PJ, Moore EE, Biffl WL. Male gender is a risk factor for major infections after surgery. Arch Surg. 1999 Sep;134(9):935–938. doi: 10.1001/archsurg.134.9.935. discussion 938–940. [DOI] [PubMed] [Google Scholar]
- 17.George RL, McGwin G, Jr, Metzger J, Chaudry IH, Rue LW., 3rd The association between gender and mortality among trauma patients as modified by age. The Journal of trauma. 2003 Mar;54(3):464–471. doi: 10.1097/01.TA.0000051939.95039.E6. [DOI] [PubMed] [Google Scholar]
- 18.Gannon CJ, Pasquale M, Tracy JK, McCarter RJ, Napolitano LM. Male gender is associated with increased risk for postinjury pneumonia. Shock. 2004 May;21(5):410–414. doi: 10.1097/00024382-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Eachempati SR, Hydo L, Barie PS. Gender-based differences in outcome in patients with sepsis. Arch Surg. 1999 Dec;134(12):1342–1347. doi: 10.1001/archsurg.134.12.1342. [DOI] [PubMed] [Google Scholar]
- 20.Croce MA, Fabian TC, Malhotra AK, Bee TK, Miller PR. Does gender difference influence outcome? The Journal of trauma. 2002 Nov;53(5):889–894. doi: 10.1097/00005373-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Coimbra R, Hoyt DB, Potenza BM, Fortlage D, Hollingsworth-Fridlund P. Does sexual dimorphism influence outcome of traumatic brain injury patients? The answer is no! The Journal of trauma. 2003 Apr;54(4):689–700. doi: 10.1097/01.TA.0000058314.31655.5F. [DOI] [PubMed] [Google Scholar]
- 22.Rappold JF, Coimbra R, Hoyt DB, et al. Female gender does not protect blunt trauma patients from complications and mortality. The Journal of trauma. 2002 Sep;53(3):436–441. doi: 10.1097/00005373-200209000-00007. discussion 441. [DOI] [PubMed] [Google Scholar]
- 23.Dossett LA, Swenson BR, Heffernan D, et al. High levels of endogenous estrogens are associated with death in the critically injured adult. The Journal of trauma. 2008 Mar;64(3):580–585. doi: 10.1097/TA.0b013e31816543dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dossett LA, Swenson BR, Evans HL, Bonatti H, Sawyer RG, May AK. Serum estradiol concentration as a predictor of death in critically ill and injured adults. Surgical infections. 2008 Feb;9(1):41–48. doi: 10.1089/sur.2007.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May AK, Dossett LA, Norris PR, et al. Estradiol is associated with mortality in critically ill trauma and surgical patients. Critical care medicine. 2008 Jan;36(1):62–68. doi: 10.1097/01.CCM.0000292015.16171.6D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Early BJ, Huang DT, Callaway CW, et al. Multidisciplinary acute care research organization (MACRO): if you build it, they will come. J Trauma Acute Care Surg. 2013 Jul;75(1):106–109. doi: 10.1097/TA.0b013e318298779b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg. 1986 Feb;121(2):196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- 28.Marshall JC. Organ dysfunction as an outcome measure in clinical trials. Eur J Surg Suppl. 1999;(584):62–67. doi: 10.1080/11024159950188583. [DOI] [PubMed] [Google Scholar]
- 29.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995 Oct;23(10):1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: patient-oriented research core--standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. The Journal of trauma. 2006 May;60(5):1106–1113. doi: 10.1097/01.ta.0000220424.34835.f1. discussion 1113. [DOI] [PubMed] [Google Scholar]
- 31.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock. 1998 Aug;10(2):79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Manship L, McMillin RD, Brown JJ. The influence of sepsis and multisystem and organ failure on mortality in the surgical intensive care unit. Am Surg. 1984 Feb;50(2):94–101. [PubMed] [Google Scholar]
- 33.Nathens AB, Marshall JC. Sepsis, SIRS, and MODS: what’s in a name? World J Surg. 1996 May;20(4):386–391. doi: 10.1007/s002689900061. [DOI] [PubMed] [Google Scholar]
- 34.Roumen RM, Redl H, Schlag G, et al. Inflammatory mediators in relation to the development of multiple organ failure in patients after severe blunt trauma. Crit Care Med. 1995 Mar;23(3):474–480. doi: 10.1097/00003246-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995 Feb;38(2):185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Catania RA, Angele MK, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Dehydroepiandrosterone restores immune function following trauma-haemorrhage by a direct effect on T lymphocytes. Cytokine. 1999 Jun;11(6):443–450. doi: 10.1006/cyto.1998.0458. [DOI] [PubMed] [Google Scholar]
- 37.Spratt DI, Morton JR, Kramer RS, Mayo SW, Longcope C, Vary CP. Increases in serum estrogen levels during major illness are caused by increased peripheral aromatization. Am J Physiol Endocrinol Metab. 2006 Sep;291(3):E631–638. doi: 10.1152/ajpendo.00467.2005. [DOI] [PubMed] [Google Scholar]
- 38.Simpson ER, Merrill JC, Hollub AJ, Graham-Lorence S, Mendelson CR. Regulation of estrogen biosynthesis by human adipose cells. Endocr Rev. 1989 May;10(2):136–148. doi: 10.1210/edrv-10-2-136. [DOI] [PubMed] [Google Scholar]
- 39.Simpson ER. Aromatase: biologic relevance of tissue-specific expression. Semin Reprod Med. 2004 Feb;22(1):11–23. doi: 10.1055/s-2004-823023. [DOI] [PubMed] [Google Scholar]
- 40.Gunst M, Ghaemmaghami V, Gruszecki A, Urban J, Frankel H, Shafi S. Changing epidemiology of trauma deaths leads to a bimodal distribution. Proc (Bayl Univ Med Cent) 2010 Oct;23(4):349–354. doi: 10.1080/08998280.2010.11928649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.