Abstract
Objective
Reference charts for classifying and monitoring pregnancy weight gain in severely obese women do not exist. Our goal was to construct pregnancy weight gain-for-gestational age z-score charts for overweight and obese mothers, stratified by severity of obesity.
Methods
We abstracted serial weight gain measurements from 1047, 1202, 1267, 730 overweight, class I, II, and III obese women, respectively, delivering uncomplicated term pregnancies at Magee-Womens Hospital in Pittsburgh, PA. Multi-level linear regression models were used to express serial weight gain measurements as a function of gestational age.
Results
There were a median [interquartile range] of 11 [9-12] and 11 [9-13] serial weight measurements for overweight and obese (class I, II, and III) women, respectively. The rate of weight gain was minimal until 15-20 weeks, then increased in a slow, linear manner until term. The slope of weight gain flattened as pre-pregnancy BMI increased. Charts were created describing the mean, standard deviation, and select percentiles of weight gain in class I, II, and III obese and overweight pregnancies.
Conclusions
These charts are an innovative tool for studying the association between gestational weight gain and adverse pregnancy outcomes.
Keywords: Gestational weight gain, reference values
Introduction
There is ongoing debate regarding the optimal amount of weight that severely obese mothers should gain during pregnancy. Some have advocated for weight restriction, and even weight loss, during pregnancy because higher weight gain increases the risk of post-partum weight retention and offspring obesity.1 Yet, there are concerns that lower maternal weight gain may increase the risk of stillbirth, infant death, and preterm birth.2 Public health policy makers need high-quality evidence on the safe lower limits for pregnancy weight gain among class II and III obese women to inform evidence-based weight gain guidelines, however, this evidence is currently unavailable.2
Filling these gaps in knowledge is methodologically complex. Stillbirth and infant death are highly correlated with early gestational age at delivery, and women who deliver early have less time to gain weight. Untangling the effects of low weight gain on stillbirth or infant death from the effects of prematurity is only possible using a gestational weight gain measure that is independent of gestational duration.3 We have recently developed an innovative tool that achieves this: a pregnancy weight-gain-for-gestational-age z-score chart.4 The charts’ means and standard deviations (SD) of weight gain at preterm ages were derived from the weight gains of women who subsequently delivered at term. As a result, converting women’s total weight gain to age-standardized z-scores produces a measure that is uncorrelated with gestational age at delivery. This, in turn, enables the effects of low weight gain on stillbirth or infant death to be isolated from the effects of prematurity.3 However, our chart was only developed for normal weight women due to the low number of overweight and obese women in our initial cohort.
Weight gain z-score charts for overweight and obese women are urgently needed. In this study, our goal was to create pregnancy weight-gain-for-gestational age z-score charts for overweight and class I, class II, and class III obese women.
Methods
Our study population was drawn from women delivering at Magee-Womens Hospital in Pittsburgh, PA, 1998-2010. The hospital’s obstetrical database was used to identify women with an uncomplicated pregnancy, defined as a non-anomalous, singleton, liveborn, term (37-41 weeks’) birth with no medical complications (diabetes or hypertension) and an ultrasound-confirmed estimate of gestational age. From this group of women, we randomly sampled overweight (pre-pregnancy body mass index (BMI) 25-29.9 kg/m2), class I (BMI 30-34.9 kg/m2), class II (BMI 35-39.9 kg/m2), class III obese women (BMI≥40 kg/m2) and abstracted their serial antenatal weights from the medical records. We calculated that 910, 1075, 1251, and 1345 women would be required to estimate the 16th and 84th percentiles (1 SD) to within 0.5 kg, based on means and SDs of total weight gain of term deliveries at Magee-Womens (15.0kg ±6.9, 12.5kg ±7.5, 10.0kg ±8.1, and 7.7kg ±8.5) for overweight, class I, class II, and class III obese women, respectively.5
Weight gain was calculated as the weight at the time of an antenatal visit less self-reported pre-pregnancy weight. Weight gain measurements ≥ 36.4kg (80 lbs) or < −18.2kg (−40 lbs) were considered outliers and excluded. Weight gain trajectories were then reviewed for implausible patterns through the use of conditional weight percentiles.6 Using this method, observations 4 SDs above or below the weight expected based on the previous weight measurement were flagged and excluded according to their clinical plausibility.
Details of the statistical approach are provided elsewhere.4 Briefly, we used random effects models with unstructured covariance matrices to express the serial weight gain measurements as a function of gestational age, stratified by pre-pregnancy BMI category. To allow for a non-linear pattern of weight gain across gestation, we modelled gestational age using a restricted cubic spline.7 Weight gain was log transformed to ensure that assumptions of homoscedascity were not violated. The final models were chosen using the Akaike Information Criterion. A constant of the lowest observed value plus 1 was applied to eliminate negative values, which cannot be log transformed.
The goodness-of-fit of the estimated means and SDs to the raw data was assessed using a scatter diagram superimposing the fitted means and SDs on the raw data to ensure that no systematic over- or under-estimation was apparent. We also checked that the appropriate proportion of observations fell within the estimated thresholds for 1 and 2 SD. Estimates of the 3rd, 10th, 50th, 90th and 97th percentiles were exponentiated from the log scale for ease of interpretation.
Results
Our cohort contained 1047 overweight, 1202 class I, 1267 class II, and 730 class III obese women. We were unable to achieve our target sample size in obese class III women as only 730 of 1954 women delivering at the hospital met inclusion criteria. The mean maternal age was 29 years (± 5.7SD), 14.3% were smokers, 36.6% were nulliparous, and 72.1% were non-Hispanic white (with 26.0% non-Hispanic black, and 1.9% other race/ethnicity). Forty-one percent of women received Medicaid.
We removed 229 implausible weight gain observations. There were a median [interquartile range] of 11 [9-12], 11 [9-13], 11 [9-13], and 11 [9-13] weight gain measurements per woman in overweight, class II, II, and III obese, respectively. Weight measurements increased in frequency as gestation progressed. Total gestational weight gain was 15.3kg (± 6.8SD), 12.8kg (± 7.6SD), 9.9kg (± 7.7SD), and 7.7kg (± 8.6SD) in these respective BMI groups.
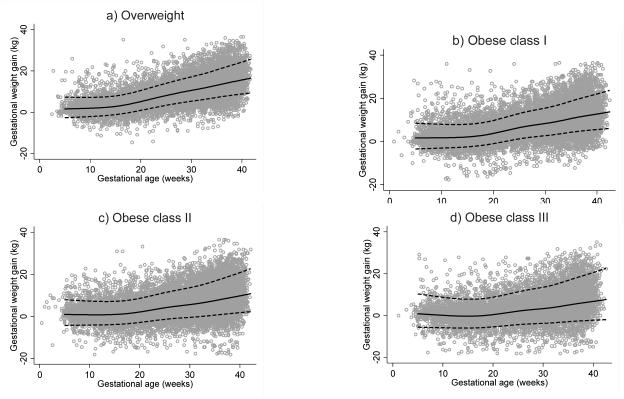
For all obesity classes, the best fitting model was one where gestational age was expressed as a restricted cubic spline with 6 knots in the default positions. For overweight women, the best fitting model had 7 knots. Constants of 15.5, 18.7, 19.2, and 19.2 kg were added to weight gain measurements for overweight, class I, II, and III obese women. Coefficients estimated in the final models are provided in Table 1. Figure 1a-d shows the estimated means and SDs superimposed on the crude data. For overweight, class I, II, and III obese women, 76%, 76%, 79%, and 83% of observations fell within 1 estimated SD (68% expected) and 96.1%, 96.2%, 96.7%, and 97.1% of observations fell within 2 estimated SD (95% expected), respectively. This demonstrated that the fit of the models was reasonable, although better at 2 SD than 1SD, and more accurate in lower BMI categories.
Table 1.
Regression equation describing patterns of pregnancy weight in a random sample of obese women with uncomplicated term pregnancies at Magee-Womens Hospital in Pittsburgh, PA.
| Parameter estimates |
Overweight | Obese class I |
Obese class II |
Obese Class III |
|
|---|---|---|---|---|---|
| Fixed effects | GA1* | 0.004 | 0.001 | −0.001 | −0.007 |
| GA2* | 0.082 | 0.061 | 0.053 | 0.062 | |
| GA3* | −0.210 | −0.182 | −0.153 | −0.174 | |
| GA4* | 0.133 | 0.251 | 0.199 | 0.246 | |
| GA5* | 0.100 | −0.292 | −0.174 | −0.287 | |
| GA6* | −0.244 | - | - | - | |
| Intercept | 2.822 | 3.010 | 3.005 | 3.032 | |
| Random effects |
Variance(GA1) | 0.000 | 0.000 | 0.000 | 0.000 |
| Variance (Intercept) | 0.095 | 0.096 | 0.102 | 0.167 | |
| Cov(Intercept,GA1) | −0.002 | −0.002 | −0.002 | −0.002 | |
| Variance(Residual) | 0.003 | 0.003 | 0.004 | 0.006 |
Spline basis terms for gestational age from a restricted cubic spline with 6 knots in default locations.
Figure 1.
Observed pregnancy weight gain measurements in a) overweight b) obese class I, c) obese class II, and d) obese class III with uncomplicated term pregnancies at Magee-Womens Hospital in Pittsburgh, PA, with predicted mean and standard deviation superimposed.
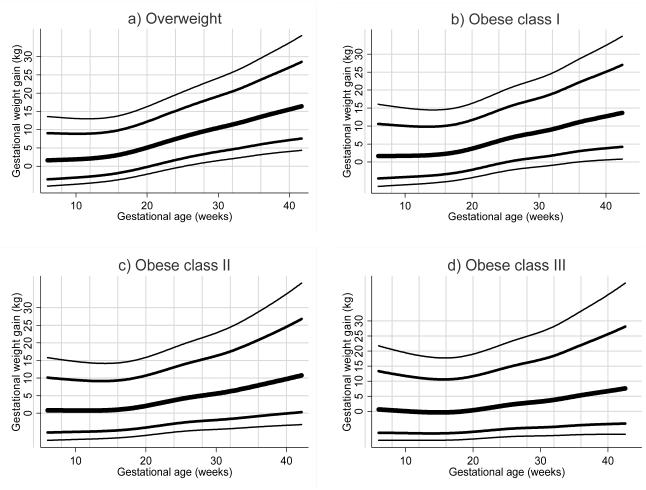
Figure 2a-d shows selected percentiles of pregnancy weight gain across gestation. Among overweight women, median weight gain was minimal until approximately 15 weeks, after which it increased in an approximately linear manner until term. As obesity became more severe, median weight gain remained minimal for longer (until ~20 weeks in class III obese women) and the rate weight gain decreased. Minimal weight gain and weight loss were common, with the 10th percentile corresponding to a weight loss at 40 weeks in obese class III women (−4.2 kg), no weight gain in obese class II women (0 kg), and low weight gain in class I women (3.9 kg). The Supplement shows the log mean, log SD, and selected percentiles of pregnancy weight gain by week of gestation.
Figure 2.
Selected percentiles of pregnancy weight gain for gestational age in a) overweight b) obese class I, c) obese class II, and d) obese class III women with uncomplicated term pregnancies at Magee-Womens Hospital in Pittsburgh, PA.
Discussion
In this study, we produced reference values for pregnancy weight gain in overweight and obese class I, II, and III women. For all obesity categories, the median (50th percentile) of gestational weight gain was close to zero (i.e., little weight gain) until mid-pregnancy. Weight gain during the second half of pregnancy increased in an approximately linear manner, with the rate of increase slowing as obesity increased. No weight gain, or weight loss, was more common as obesity became more severe.
Pregnancy weight gain z-score charts have previously been created for normal weight women4 and a cohort of women in Malawi,8 but this study provides the first values for obese women, stratified by severity of obesity. Although our study compiled one of the largest datasets on pattern of weight gain for severely obese mothers, our chart for obese class III women would have benefited from a larger cohort. For class III obese women, the values of selected percentiles in our chart (particularly the more extreme percentiles) may differ from the true values in the underlying population. Further, exploration of other modelling strategies to describe weight gain patterns in a larger sample of class III obese women is worthwhile. We only sampled deliveries from a single tertiary care center, and although weight gain at our institution is comparable to that in other contemporary US cohorts,3 future work should develop pregnancy weight gain charts using nationally-representative samples. Finally, as with most research on gestational weight gain,2 our reliance on self-reported rather than measured pre-pregnancy weights is a source of error. However, measured pre-pregnancy weight is rarely available in routine clinical practice, so our reference values reflect the information used to assess weight gain in a real world setting.
The creation of these charts is a critical step in obtaining high-quality evidence on the relationship between gestational weight gain and stillbirth, infant death, and preterm term in severely obese women. Further, once studies have identified the gestational weight gain z-scores associated with optimal pregnancy outcomes, women and their healthcare providers can use these charts in antenatal care to monitor the progress of weight gain and to prompt discussion on the need to intervene.
Supplementary Material
What is already known about this subject?
A pregnancy weight gain-for-gestational age z-score chart for monitoring and classifying gestational weight gain in normal weight women has recently been published, but charts for overweight and obese class I, II, and III mothers are unavailable
What does this study add?
This study produced pregnancy weight gain charts establishing the mean, standard deviation, and select percentiles of gestational weight gain in uncomplicated class I, II, and III obese and overweight term pregnancies
The charts provide a novel tool for establishing the link between gestational weight gain and adverse pregnancy outcomes
After establishing the z-scores associated with optimal pregnancy outcomes, the charts will be useful for monitoring weight gain in pregnancy in clinical practice in overweight and obese women.
Acknowledgements
This work was supported by the National Institute of Child Health & Human Development (R01 HD072008 to LMB. and JAH). JAH holds a Canadian Institutes of Health Research New Investigator Award and is a Career Scholar of the Michael Smith Foundation for Health Research. RWP holds a Chercheur-National from the Fonds de la Recherche en Santé du Quebec (FRSQ). The Montreal Children’s Hospital Research Institute receives core operating funds from the FRSQ.
Footnotes
Conflicts of interest: All authors report no conflicts of interests
References
- 1.Artal R1, Lockwood CJ, Brown HL. Weight gain recommendations in pregnancy and the obesity epidemic. Obstet Gynecol. 2010;115:152–5. doi: 10.1097/AOG.0b013e3181c51908. [DOI] [PubMed] [Google Scholar]
- 2.IOM . Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- 3.Hutcheon JA, Bodnar LM, Joseph KS, Abrams B, Simhan HN, Platt RW. The bias in current measures of gestational weight gain. Ped Perinatal Epidem. 2012;26:109–16. doi: 10.1111/j.1365-3016.2011.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr. 2013;97:1062–7. doi: 10.3945/ajcn.112.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altman DG, Chitty LS. Charts of fetal size: 1 Methodology. Br J Obstet Gynaecol. 1994;101:29–34. doi: 10.1111/j.1471-0528.1994.tb13006.x. [DOI] [PubMed] [Google Scholar]
- 6.Royston P. Calculation of unconditional and conditional reference intervals for foetal size and growth from longitudinal measurements. Stat Med. 1995;14:1417–36. doi: 10.1002/sim.4780141303. [DOI] [PubMed] [Google Scholar]
- 7.Harrell FE. Regression modeling strategies with applications to linear models, logistic regression and survival analysis. USA: Springer-Verlag; New York: 2001. [Google Scholar]
- 8.Xu J, Luntamo M, Kulmala T, Ashorn P, Cheung YB. A longitudinal study of weight gain in pregnancy in Malawi: unconditional and conditional standards. Am J Clin Nutr. 2014;99:296–301. doi: 10.3945/ajcn.113.074120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.