Abstract
Background
Whether a coronary artery calcium (CAC) scan provides added value to coronary CT angiography (CCTA) in emergency department (ED) patients with acute chest pain (ACP) remains unsettled. We sought to determine the value of CAC scan in ACP patients undergoing CCTA.
Methods and Results
In the multicenter ROMICAT II trial, we enrolled low-intermediate risk ED patients with symptoms suggesting acute coronary syndrome (ACS). In this pre-specified sub-analysis of 473 patients (54±8years, 53%male) who underwent both CAC scanning and CCTA, the ACS rate was 8%. Overall, 53% of patients had CAC=0 of whom 2 (0.8%) developed ACS, while 7% had CAC>400 with 49% whom developed ACS. C-statistic of CAC>0 was 0.76, while that using the optimal cutpoint of CAC≥22 was 0.81. Continuous CAC score had lower discriminatory capacity than CCTA (c-statistic 0.86 vs. 0.92, p=0.03). Compared to CCTA alone, there was no benefit combining CAC score with CCTA (c-statistic 0.93, p=0.88) or with selective CCTA strategies after initial CAC>0 or optimal cutpoint CAC≥22 (p≥0.09). Mean radiation dose from CAC acquisition was 1.4±0.7mSv. Higher CAC scores resulted in more non-diagnostic CCTA studies though the majority remained interpretable.
Conclusions
In ED patients with ACP, CAC score does not provide incremental value beyond CCTA for ACS diagnosis. CAC=0 does not exclude ACS, nor a high CAC score preclude interpretation of CCTA in most patients. Thus, CAC results should not influence the decision to proceed with CCTA, and the decision to perform a CAC scan should be balanced with the additional radiation exposure required.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01084239.
Keywords: coronary artery calcium, acute coronary syndrome, coronary computed tomography angiography, emergency department
The use of computed tomography (CT) imaging is emerging as an acceptable alternative modality in the emergency department (ED) for early triage of patients with suspected acute coronary syndrome (ACS).1–3 Standard protocols routinely include both coronary artery calcium (CAC) scan and coronary CT angiography (CCTA). However, the utility of a CAC scan in this setting remains controversial. While a CAC study can be easily performed with low radiation exposure, whether it provides added diagnostic value in patients undergoing CCTA remains unsettled.
Previous studies evaluating the accuracy of CAC score to detect significant coronary artery disease (CAD) in a low-intermediate risk ED population have predominantly shown high sensitivity (97–100%) and negative predictive value (93–100%) with modest specificity in the range of 54–63%.4–7 Although these test characteristics would suggest utility of CAC score for ED triage of acute chest pain, CAC score as a stand-alone test for ED chest pain triage has not been recommended per current appropriate use criteria.8 Notable reasons for this recommendation are that evidence for benefit of CAC score was derived from relatively small cohort studies, the possibility of missing obstructive non-calcified plaque, and the rapidly emerging evidence base that CCTA is a safe, efficient tool in the ED for triage of low-intermediate risk acute chest pain (ACP).1–3, 9
In practice, the use of the CAC scan in the ED setting has evolved to become an adjunct to CCTA serving two primary functions. First, the CAC scan can be used as a “gatekeeper” to CCTA. A high calcium score could not only negatively impact the interpretability of CCTA10, but would also increase the likelihood of having obstructive CAD.11 Accordingly, some institutions may abort CCTA at a pre-specified CAC score threshold ranging from 400 to 1000. However, there is scant evidence to validate such an approach. Second, the CAC scan has been used to define the smallest scan length of the CCTA scan to minimize radiation exposure.12 However, in the era of dual source CT and prospective ECG triggering, which offer substantial reduction in radiation dose, the advantage of such a CAC scan-guided approach is less clear.
In this pre-specified analysis of the Rule Out Myocardial Infarction using Computer Assisted Tomography (ROMICAT) - II trial, we sought to assess the utility of a CAC scan prior to CCTA with respect to its diagnostic accuracy to exclude ACS, interpretability of CCTA, and radiation exposure. In an exploratory analysis, we also evaluate the diagnostic accuracy of selective CCTA strategies for diagnosing ACS, whereby CAC scan is performed initially, followed by selective use of CCTA.
Methods
Study population
We performed a sub-analysis of patients in the ROMICAT-II trial who underwent both CAC scanning and CCTA. The ROMICAT-II trial was a multi-centered randomized controlled clinical trial consisting of 1000 patients at 9 US centers who presented to the ED during weekday daytime hours with symptoms suggestive of ACS, but without ischemic ECG changes or elevated initial cardiac biomarker. The study design and primary results have been previously reported.1,13 Briefly, eligible patients were between the ages of 40 and 74 years with chest pain or anginal equivalent of at least five minutes duration within 24 hours of ED presentation, were in sinus rhythm, and warranted further risk stratification to rule out ACS. Major exclusion criteria were history of known CAD, new diagnostic ischemic changes on the initial ECG, initial troponin in excess of the 99th percentile of the local assay, impaired renal function (creatinine >1.5 mg/dL), hemodynamic or clinical instability, known allergy to iodinated contrast agent, body mass index >40 kg/m2, or currently symptomatic asthma. All patients were randomized to either CCTA as part of the initial evaluation or to the standard ED evaluation strategy, as dictated by local caregivers. Patients discharged within 24 hours of ED presentation were contacted by phone within 72 hours to evaluate for potential missed ACS. Patients were also evaluated 28 days after discharge from the ED or hospital by phone interview and queried regarding repeat ED visits or hospitalizations for recurrent chest pain, diagnostic testing/interventions, and major adverse cardiac events (MACE), with verification by medical records. The study was approved by the institutional review board at each participating site and all participants provided informed consent. In this secondary analysis, we included the 473 patients who underwent both CAC and CCTA, which represents 94% of the 501 subjects that were randomized to the CCTA arm.
CAC and CCTA Protocol
As part of the protocol, all patients undergoing CCTA had a CAC scan that was not used to guide the decision-making process. The results of the CCTA were used to triage patients under the discretion of the ED physician. CAC scanning and CCTA were performed with 64-slice or higher CT technology. Specific CT scanner types used among the sites included GE 64-Slice Lightspeed, GE Lightspeed VCT, Siemens 64-Slice Sensation, Siemens Dual Source 64-Slice Definition, Siemens Dual Source 128-Slice Flash, and Philips Brilliance 256-Slice iCT. For the CAC scan, a standard non-contrast prospective scan was performed. CAC scores were calculated using the Agatston method and expressed in Hounsfield units.14 For CCTA, either retrospective or prospectively gated acquisitions were allowed.
End points
The primary endpoint was ACS, defined as unstable angina and myocardial infarction (MI) during the index hospitalization. The safety endpoint, major cardiovascular events (MACE), was defined as death, MI, unstable angina, or urgent coronary revascularization that occurred within 28 days. Both ACS and MACE were adjudicated by an external, independent Clinical Events Committee. CAC score was dichotomized by 0 and its optimal cutpoint for ACS of 22. CCTA findings were categorized into no CAD, non-obstructive CAD, and obstructive CAD. Obstructive CAD was defined in two ways: (1) 50% stenosis threshold using a binary cutpoint of 50% luminal stenosis in any epicardial vessel, and (2) 70% stenosis threshold using ≥ 50% luminal diameter stenosis for left main, or≥ 70% stenosis for other major epicardial vessels as interpreted at the site level by Level III CT readers, who also determined the interpretability of the CCTA scans. We defined four selective CCTA strategies using the 50% and 70% stenosis thresholds. For these selective CCTA strategies, an initial use of CAC score was evaluated and if CAC score was “negative” (by cutpoints of 0 or 22), then CCTA would not be required; and if CAC score was “positive,” then selective CCTA findings would be utilized using either the 50% or 70% stenosis thresholds. Radiation exposure from testing was calculated in mSv for CAC scan and CCTA, using standard methods, including a conversion coefficient of 0.014 for the chest for CCTA scans.15
Statistical analysis
Descriptive statistics were expressed as mean ± standard deviation (SD) and medians with 25th and 75th percentile for continuous variables and as frequency and percentages for categorical variables. To test the association between categorical variables and our ordinal outcome of CAC score, we used the Mantel-Haenszel statistic with Row Mean Rank scores or linear trend, as appropriate. To measure statistical correlation between continuous variables and strata of CAC scores, we used the Spearman’s rank correlation coefficient. We determined the optimal cut-point for ACS detection of CAC score to be 22 using the greatest average of the sensitivity and specificity. To determine the diagnostic accuracy of the various strategies for the diagnosis of ACS (CAC>0, optimal CAC cutpoint ≥22, CCTA and selective CCTA using 50% and 70% stenosis thresholds with both CAC cutpoints), we calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy and computed the 95% binomial proportion confidence interval (CI). We used McNemar’s test to compare sensitivity and specificity between strategies. Logistic regression was used to determine the c-statistic, which is equivalent to the area under the receiver operator characteristic curve (AUC) to assess the discriminatory capacity of the various CAC, CCTA, and combinations of CAC score with CCTA strategies. C-statistics were compared using the ROC contrast test method of Delong and Delong.16 To assess for incremental value of CAC score when combined with CCTA, we performed a Likelihood ratio test. For comparison of CCTA and invasive coronary angiography (ICA) for stenosis, we used Cohen κ statistics with McNemar’s Chi-square test to determine the degree of agreement between the two modalities and report exact agreement by varying levels of CAC scores. All analyses were performed using SAS (Version 9.2, North Carolina). For all analyses, a two-tailed p value <0.05 denoted significance.
Results
Study population
Baseline characteristics of the 473 patients and as stratified by range of calcium score are summarized in Table 1. The ACS rate was 8% (n=38), identical to that in the overall trial. Patients with higher calcium scores were older, more likely to be male, and had more traditional risk factors. There were 253 (53%) patients with no coronary calcium (CAC=0), while 19 (7%) had CAC scores >400.
Table 1.
Baseline Characteristics of All Patients Undergoing CAC Scan and CCTA, and as Stratified by CAC score
| All CAC | Coronary Artery Calcium Score | P-value | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1–9 | 10–100 | 101–400 | >400 | |||
| n (%) | 473 (100) | 253 (53) | 45 (10) | 91 (19) | 49 (10) | 35 (7) | |
|
| |||||||
| Demographics | |||||||
| Age, years (mean ± SD) | 53.9±8.0 | 51.2±7.1 | 54.2±7.7 | 56.5±7.9 | 58.0±7.3 | 60.4±7.8 | <0.0001 |
| Male sex (%) | 249(53) | 105 (42) | 28 (62) | 53 (58) | 36 (74) | 27 (77) | <0.0001 |
| Race | |||||||
| African American (%) | 129 (27) | 81 (32) | 11 (24) | 24 (26) | 10 (20) | 3 (9) | 0.004 |
| Caucasian (%) | 316 (67) | 157(62) | 30 (67) | 64 (70) | 36 (74) | 29 (83) | 0.007 |
| Asians (%) | 16 (3) | 9 (4) | 4 (9) | 0 | 2 (4) | 1 (3) | 0.60 |
| Others (%) | 12 (3) | 6 (2) | 0 | 3 (3) | 1 (2) | 2 (6) | 0.52 |
| Ethnicity, non-Hispanic n (%) | 409 (87) | 211(83) | 42 (93) | 79 (87) | 45 (92) | 32 (91) | 0.03 |
|
| |||||||
| Cardiovascular Risk Factors | |||||||
| Hypertension (%) | 252 (53) | 112 (44) | 22 (49) | 60 (66) | 30 (61) | 28 (80) | <0.0001 |
| Diabetes mellitus (%) | 79 (17) | 28 (11) | 7 (16) | 19 (21) | 12 (25) | 13 (37) | <0.0001 |
| Dyslipidemia (%) | 216 (46) | 93 (37) | 25 (56) | 49 (54) | 24 (49) | 25 (71) | <0.0001 |
| Former or current smoking (%) | 236 (50) | 113 (45) | 24 (53) | 49 (54) | 28 (57) | 22 (63) | 0.01 |
| Family history of premature CAD (%) | 131 (28) | 75 (30) | 9 (20) | 27 (30) | 7 (14) | 13 (37) | 0.45 |
| No. risk factors (% of 0–1 / 2–3 / ≥4) | |||||||
| 0–1 (%) | 172 (36) | 117 (46) | 14 (31) | 21 (23) | 17 (35) | 3 (9) | <0.0001 |
| 2–3 (%) | 256 (54) | 124 (49) | 29 (64) | 53 (58) | 29 (59) | 21 (60) | |
| ≥4 (%) | 45 (10) | 12 (5) | 2 (4) | 17 (19) | 3 (6) | 11 (31) | |
|
| |||||||
| Relevant Prior Medication | |||||||
| Aspirin (%) | 110 (23) | 51 (20) | 9 (20) | 23 (25) | 14 (29) | 13 (37) | 0.03 |
| Beta Blocker (%) | 82 (18) | 38 (15) | 7 (16) | 18 (20) | 9 (18) | 10 (29) | 0.08 |
| Statins (%) | 134 (28) | 60 (24) | 13 (29) | 29 (32) | 17 (35) | 15 (43) | 0.007 |
|
| |||||||
| Initial ED Presentation | |||||||
| Chief complaint | |||||||
| Chest pain with or without radiation (%) | 421 (89) | 231 (91) | 39 (87) | 77 (85) | 46 (94) | 28 (80) | 0.44 |
| Pain arm/jaw/shoulder/epigastric (%) | 20 (4) | 9 (4) | 3 (7) | 4 (4) | 1 (2) | 3 (9) | |
| Shortness of breath (%) | 6 (1) | 2 (1) | 1 (2) | 2 (2) | 1 (2) | 0 | |
| Other (%) | 26 (6) | 11 (4) | 2 (4) | 8 (9) | 1 (2) | 4 (11) | |
| Heart Rate (bpm) | 77±14 | 77±14 | 80±14 | 77±14 | 77±14 | 76±17 | 0.64 |
| Systolic blood pressure (mmHg) | 144±23 | 139±21 | 143±22 | 151±24 | 149±23 | 150±22 | <0.0001 |
| Diastolic blood pressure (mmHg) | 83±13 | 82±12 | 84±13 | 86±14 | 85±13 | 83±16 | 0.06 |
| Body mass index (kg/m2) | 29.4±5.2 | 29.0±5.2 | 30.4±4.7 | 29.4±5.0 | 29.2±7.0 | 30.3±3.9 | 0.07 |
|
| |||||||
| Discharge Diagnosis during Index | |||||||
| Non-cardiac CP (%) | 407 (86) | 245 (97) | 39 (87) | 77 (85) | 36 (74) | 10 (29) | <0.0001 |
| Non-coronary cardiac CP (%) | 6 (1) | 2 (1) | 0 | 1 (1) | 2 (4) | 1 (3) | |
| Coronary CP, not ACS (%) | 22 (5) | 4 (2) | 4 (9) | 3 (3) | 4 (8) | 7 (20) | |
| ACS (%) | 38 (8) | 2(1) | 2(4) | 10 (11) | 7(14) | 17 (49) | |
| Unstable angina pectoris (%) | 32 (7) | 1(0.4) | 2(4) | 10 (11) | 4(8) | 15 (43) | <0.0001 |
| Myocardial infarction (%) | 6 (1) | 1 (0.4) | 0 | 0 | 3 (6) | 2 (6) | <0.0001 |
CAC denotes coronary artery calcium; CCTA, cardiac computed tomography angiography; CAD, coronary artery disease; CP, chest pain; ED, emergency department; and ACS, acute coronary syndrome.
CAC of Zero and ACS
Patients with lower CAC scores were less likely to have a discharge diagnosis of ACS. Among 253 patients with CAC=0, there were 2 patients with ACS (0.8%; 95% CI 0.1–2.8%). One patient had unstable angina due to non-calcified severe stenosis in the left circumflex artery and the second patient had an anomalous right coronary artery arising from the main pulmonary artery (ARCAPA), resulting in a non-ST elevation myocardial infarction (NSTEMI), where the myocardial ischemia is a resultant of flow reversal via collateral and perfusion of the right coronary artery territory by relatively oxygen depleted blood and lower perfusion pressures.17 (Supplemental Figures 1 and 2)
Performance of Various CT Strategies for ACS
The sensitivity, specificity, PPV, NPV, diagnostic accuracy, and c-statistics of CAC and the various CCTA strategies for determination of ACS are shown in Table 2. The optimal cutpoint of CAC for ACS detection was 22 (c-statistic 0.81), with 318 patients (67%) having CAC <22. High sensitivity was observed with all CT strategies and not different when compared amongst strategies (all p=NS). Specificity increased markedly from 58% with CAC>0 to 89–96% when utilizing CCTA or selective CCTA strategies (all p<0.001). While specificity improved from 89% with the 50% CCTA threshold to 92% with optimal CAC-selective CCTA strategy (p=0.002), there were no differences between the other sensitivities or specificities when utilizing either CCTA or selective CCTA strategies with 50% or 70% stenosis thresholds (all p=NS).
Table 2.
Performance of Various CT Strategies for Diagnosis of ACS: CAC>0, Optimal cutpoint CAC ≥22, CCTA and Selective CCTA using 50% and 70% Stenosis Thresholds
| Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) | |
|---|---|---|---|---|---|
| CAC Scan alone | |||||
| CAC score >0 | 36/38 95% (82–99%) | 251/435 58% (53–62%) | 36/220 16% (12–22%) | 251/253 99% (97–99.9%) | 287/473 61% (56–65%) |
| CAC score≥22 | 34/38 89% (75–97%) | 314/435 72% (68–76%) | 34/155 22% (16–29%) | 314/318 99% (97–99.7%) | 348/473 74% (69–77%) |
|
| |||||
| CCTA using 50% Stenosis Threshold | |||||
| CCTA | 36/38 95% (82–99%) | 389/435 89% (86–92%) | 36/82 44% (33–55%) | 389/391 99% (98–99.9%) | 425/473 90% (87–92%) |
| CAC > 0, then Selective CCTA | 35/38 92% (79–98%) | 391/435 90% (87–93%) | 35/79 44% (33–56%) | 391/394 99% (98–99.8%) | 426/473 90% (87–93%) |
| CAC ≥22, then Selective CCTA | 33/38 87% (72–96%) | 399/435 92% (89–94%) | 33/69 48% (36–60%) | 399/404 99% (97–99.6%) | 432/473 91% (88–94%) |
|
| |||||
| CCTA using 70% Stenosis Threshold | |||||
| CCTA | 33/38 87% (72–96%) | 416/435 96% (93–97%) | 33/52 63% (49–76%) | 416/421 99% (97–99.6%) | 449/473 95% (93–97%) |
| CAC >0, then Selective CCTA | 32/38 84% (69–94%) | 416/435 96% (93–97%) | 32/51 63% (48–76%) | 416/422 99% (97–99.5%) | 448/473 95% (92–97%) |
| CAC ≥22, then Selective CCTA | 30/38 79% (63–94%) | 418/435 96% (94–98%) | 30/47 64% (49–77%) | 418/426 98% (96–99%) | 448/473 95% (92–97%) |
Abbreviations as in Table 1. PPV denotes positive predictive value; NPV, negative predictive value; and CI, confidence interval. For “Selective CCTA” strategy, selective CCTA is performed after an initial CAC>0 as well as with optimal cutpoint CAC≥22.
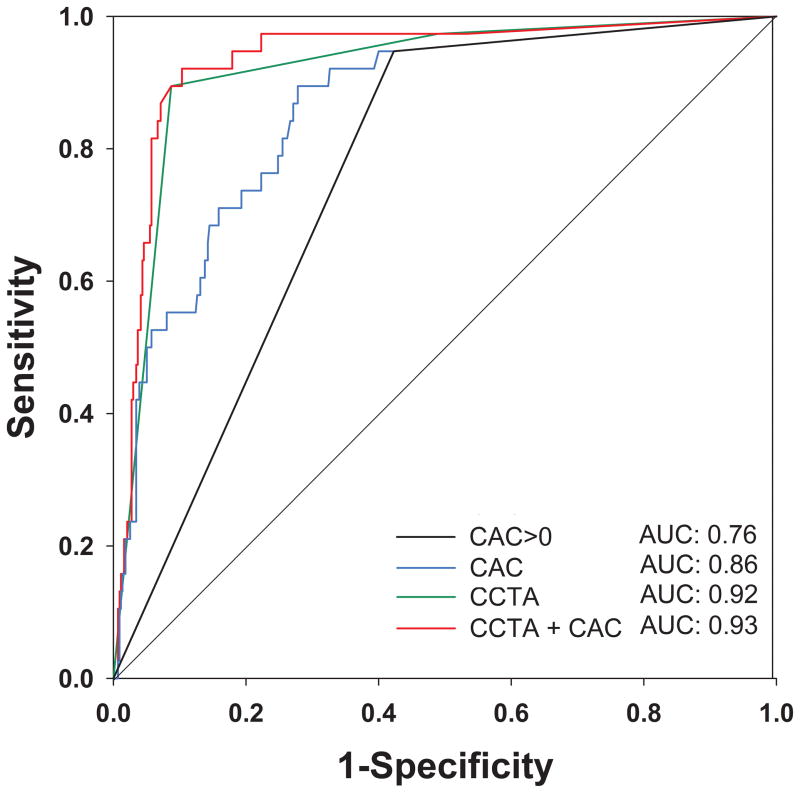
The Figure shows the area under the curve (AUC) and corresponding c-statistics of CAC>0 (0.76), continuous CAC (0.86), and CCTA (0.92) for predicting ACS. CAC was inferior to CCTA for predicting ACS (c-statistic 0.86 vs. 0.92, p=0.03). Combining CAC score with CCTA did not improve the detection of ACS (CAC+CCTA c-statistic 0.93 vs CCTA c-statistic 0.92, p=0.88).
Figure.
Receiver Operating Characteristic (ROC) curve for prediction of Acute Coronary Syndrome by CAC>0, Continuous CAC Score, CCTA, and Combined CCTA and CAC Score. Obstructive CAD on CCTA was defined using the 70% stenosis threshold.
The c-statistics were similar between using CCTA alone versus a selective CCTA strategies with either 50% or 70% stenosis threshold, irrespective of using the CAC cutpoint of 0 or the optimal CAC cutpoint of 22 (both p≥0.09).
CCTA Findings and Study Endpoints in Patients With High CAC Scores
As shown in Table 3, across high strata of CAC score, the majority (64–86%) were identified as having obstructive CAD by CCTA, with 39–49% deemed to have ACS. About ¾ of patients with high CAC scores were admitted to the hospital with less than 18% being directly discharged from the ED. Subsequent non-invasive diagnostic testing was performed with nuclear imaging in 1 out of 5 of patients, with no patients undergoing subsequent ETT or stress Echo. About ½ of patients with high CAC score underwent invasive coronary angiography, and 1/3 requiring revascularization. Notably, while the proportion of interpretable CCTA studies decreased with increasing CAC score, the majority were deemed interpretable even among the highest CAC cutpoints (73% for CAC >1000).
Table 3.
CCTA Findings and Study Endpoints in All Patients and Those with High CAC Scores
| CAC score | All (n=473) | >400 (n=35) | >600 (n=23) | >800 (n=17) | >1000 (n=11) |
|---|---|---|---|---|---|
| CCTA Findings | |||||
| Interpretable CCTA (%) | 453 (96) | 32 (91) | 20 (87) | 14 (82) | 8 (73) |
| Obstructive CAD by 50% Threshold | 82 (17) | 30 (86) | 18 (78) | 14 (82) | 8 (73) |
| Obstructive CAD by 70% Threshold | 52 (11) | 28 (80) | 17 (74) | 13 (76) | 7 (64) |
| Primary and Secondary Endpoints | |||||
| Length of Stay, hours median [25th, 75th %ile] | 8.5 [6.3, 26.8] | 47.9 [29.2, 76.8] | 32.2 [26.7, 78.0] | 47.9 [29.2, 76.5] | 47.9 [26.7, 78.0] |
| Discharge status (%) | |||||
| Direct ED Discharge | 230 (49) | 4 (11) | 4 (17) | 2 (12) | 2 (18) |
| Admission to observational unit | 140 (30) | 5 (14) | 3 (13) | 2 (12) | 1 (9) |
| Admission to hospital | 98 (21) | 26 (74) | 16 (70) | 13 (76) | 8 (73) |
| Left against medical advice | 5 (1) | 0 | 0 | 0 | 0 |
|
| |||||
| Downstream Testing During Index Visit n (%) / At 28-days Follow-up n (%) | |||||
| Subsequent Stress Testing | |||||
| ETT | 10(2) / 20(4) | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
| Nuclear | 42(9) / 50(11) | 7(20) / 8(23) | 3(13) / 4(17) | 3(18) / 3(18) | 2(18) / 2(18) |
| Stress Echo | 13(3)/ 13(3) | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
|
| |||||
| Invasive Coronary Angiography (ICA) | 51(11) / 56(12) | 20(57) / 23(66) | 12(52) / 13(57) | 10(59) /11(65) | 5(45) / 6(55) |
|
| |||||
| ICA with >50% stenosis | 36(8) / 40(8) | 17(49) / 19(54) | 11(48) / 12(52) | 9(53) / 10(59) | 5(45) / 6(55) |
| Interventions During Index Visit, n (%)/ At 28-days Follow-up n (%) | |||||
|
| |||||
| PCI | 27 (6) / 25 (5) | 11 (31) / 12 (34) | 5 (22) / 5 (22) | 4 (24) / 4 (24) | 3 (27) / 3 (27) |
| PCI/CABG | 27 (6) / 30 (6) | 12 (34) / 11 (31) | 5 (22) / 5 (22) | 4 (24) / 4 (24) | 3 (27) / 3 (27) |
|
| |||||
| ACS (%) | 38 (8) | 17 (49) | 9 (39) | 8 (47) | 5 (45) |
| 28-day MACE (%) | 2 (0.4) | 1 (3) | 0 | 0 | 0 |
Abbreviations as in Table 1. ETT denotes exercise treadmill test; Echo, echocardiography; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; and MACE, major adverse cardiovascular event.
Agreement of CCTA and Invasive Coronary Angiography by CAC Score
In 51 patients who underwent ICA, there was moderate agreement between CCTA and ICA when using a stenosis threshold of 50% (κ 0.44, p=0.01) or 70% (κ 0.42, p=0.12). Table 4 depicts the agreement between CCTA and ICA in all patients with CAC scores and ICA, as well as stratified by high CAC score. The exact agreements were good but not perfect between CCTA and ICA, irrespective of CAC score.
Table 4.
Agreement of CCTA Findings with Invasive Coronary Angiography in All Subjects and as Stratified by CAC scores.
| Concordance | Discordance | Exact Agreement | |||
|---|---|---|---|---|---|
| CCTA + / ICA + | CCTA − / ICA − | CCTA + / ICA − | CCTA − / ICA + | ||
| All CAC Score with ICA (n=51) | |||||
| >50% Stenosis | 35 | 6 | 9 | 1 | 80% (41/51) |
| >70% Stenosis | 25 | 12 | 10 | 4 | 73% (37/51) |
|
| |||||
| CAC>400 and ICA (n=20) | |||||
| >50% Stenosis | 16 | 0 | 3 | 1 | 80% (16/20) |
| >70% Stenosis | 11 | 0 | 8 | 1 | 55% (11/20) |
|
| |||||
| CAC>600 and ICA (n=12) | |||||
| >50% Stenosis | 10 | 0 | 1 | 1 | 83% (10/12) |
| >70% Stenosis | 7 | 0 | 4 | 1 | 58% (7/12) |
|
| |||||
| CAC>800 and ICA (n=10) | |||||
| >50% Stenosis | 8 | 0 | 1 | 1 | 80% (8/10) |
| >70% Stenosis | 6 | 0 | 3 | 1 | 60% (6/10) |
|
| |||||
| CAC>1000 and ICA (n=5) | |||||
| >50% Stenosis | 4 | 0 | 0 | 1 | 80% (4/5) |
| >70% Stenosis | 4 | 0 | 0 | 1 | 80% (4/5) |
Radiation Dose from CAC Scan
Table 5 shows the mean effective radiation dose of CAC scan and CCTA by CT scanner type. Overall, the mean effective radiation dose from the CAC scan was an additional 1.4 mSv. With newer 128-dual source CT scanner technology, the mean effective radiation dose of CAC scan was 0.5 mSv. It is notable that the mean effective radiation dose of a CAC scan is 14% of that for CCTA; for the prospective ECG-triggered CCTA, the CAC scan represents 23% of the radiation dose of CCTA.
Table 5.
Effective Mean Radiation Dose from CAC and CCTA Acquisitions
| CAC alone | CCTA alone | |||
|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | |
| All Protocols and Scanners | 472 | 1.4 ±0.7 | 473 | 9.9 ±4.9 |
|
| ||||
| Protocol | ||||
| Prospectively-gated CCTA | 63 | 1.5 ±0.5 | 63 | 6.5 ±3.8 |
| Retrospective-gated CCTA | 409 | 1.4 ±0.7 | 410 | 10.4 ±4.9 |
|
| ||||
| Scanner Type | ||||
| 128-Slice DSCT | 78 | 0.5 ±0.3 | 78 | 5.7 ±3.7 |
| CT scanners (excluding 128-slice DSCT) | 394 | 1.6 ±0.6 | 395 | 10.7 ±4.7 |
Abbreviations as in Table 1. DSCT denotes dual-source computed tomography.
Discussion
The main finding of this study is that CAC score does not provide incremental value beyond CCTA in ruling out ACS in low-intermediate risk patients presenting to the ED with acute chest pain. In fact, CAC score alone would have missed two patients with ACS in our cohort whose CAC scores were 0. However, higher CAC score was associated with an increased number of non-diagnostic exams even though the majority (73%) with CAC score >1000, were diagnostic. CAC scan resulted in 1.4 mSv of radiation exposure which was 14% of the dose associated with CCTA alone.
Our findings that a CAC scan alone offers inferior diagnostic accuracy and no incremental diagnostic value to CCTA for ACS prediction are both consistent with the fact that a CAC scan does not provide the direct visualization of a culprit coronary plaque. As many studies have shown, the development of coronary calcification occurs late in the atherosclerotic process, and does not necessarily identify the location of a vulnerable plaque that represents the culprit lesion in ACS.18 Vulnerable plaques typically are not heavily calcified and instead have been described as having lipid-rich cores separated from the vessel lumen by a thin fibrous cap. 19, 20
However, CAC scans do afford some advantages, including its potential to tailor the subsequent CCTA protocol, since the fraction of non-diagnostic exams and the fraction with obstructive CAD increased with higher calcium score. For example, given that 80% of subjects with a CAC score >400 have obstructive CAD, the temporal acquisition window can be widened during which the maximum tube current is applied to provide additional phases to be reconstructed during post-processing for image interpretation to evaluate for coronary stenosis. In addition, the CAC scan can optimize the scan length of the CCTA to potentially reduce overall radiation exposure.21 However, this potential advantage may ultimately be supplanted by many recent technological advances (independent of reducing scan length) that have been validated to decrease effective radiation dose of CCTA, including high-pitch helical prospective ECG-triggered scanning.22–24 In keeping with the “As Low As Reasonably Achievable” principle, some centers have newer CT scanners and software algorithms, where sub-mSv CCTA scans are already performed.25–27
Another benefit of CAC scans is that they do not require use of iodinated contrast. Similar to such improvements in radiation dose, modern CCTA protocols have resulted in significant reductions in potentially nephrogenic contrast dose, thus the risk of contrast-induced nephropathy is low, particularly in patients with preserved renal function.28, 29 However, the issue of contrast-induced nephropathy is valid, and patients with renal impairment should not undergo CCTA as there are other alternative noninvasive stress testing available (ETT, stress Echo, stress nuclear).
Moreover, the role of the CAC scan in the ED chest pain evaluation extends beyond its ability to predict near-term ACS. Numerous studies have established the longer term prognostic value of calcium scoring in intermediate risk asymptomatic individuals.30–33 Although these studies may not be applicable to our entire symptomatic cohort, most of the subjects in our cohort did not develop ACS, but in those with coronary atherosclerosis, the calcium score may arguably still provide an objective, quantitative measure of future cardiovascular risk.
With respect to using selective CCTA, although an argument can be made for performing selective CCTA following the CAC scan (for those with CAC>0 or optimal CAC cutpoint ≥22) given similar overall test characteristics for detection of ACS, we will still miss the few patients who develop ACS with “negative” CAC score with either 0 or 22. Furthermore, using a selective CCTA strategy, patients with “negative” CAC score would not undergo CCTA and may have unrecognized severity of non-calcified plaque that would be appropriate for risk factor modification and/or preventive medical therapy, particularly in those who has CAC score greater than 0 but less than 22.34 Additionally, alternative causes of acute chest pain, such as dangerous incidental findings of aortic dissection and pulmonary embolism even if not protocoled as a “triple rule out” scan,35 would not be detected on a non-contrast CT scan alone and would be missed in a patient with “negative” CAC if a selective CCTA strategy was employed. Lastly, selective CCTA in a real-world scenario may be less than ideal and hampered by workflow issues such as need for immediate CT interpretation at the scanner in a busy ED CT scanner where non-cardiac imaging such as strokes and trauma evaluations takes priority over low-intermediate risk acute chest pain patients awaiting triage decision.
Limitations
The lack of follow-up beyond 28 days limits the ability to evaluate longer-term advantages of CAC scores for event prediction. However, there is evidence that CAC score provides useful prognostic information for both patients with and without chest pain. Despite 253 patients with CAC score of zero, we were not powered to detect a missed ACS rate of <1%. Thus, while the missed ACS rate may be lower than 1%, it can be as high as 2.8%. Even in our small cohort of 253 patients with CAC=0, we provide two examples in our study where the CCTA findings did indeed change the triage decision and influenced the overall care and management of the patients.
Conclusion
In ED patients with ACP, CAC score of zero does not exclude ACS, and CCTA is a superior test for the diagnosis of ACS. There were no incremental benefit of combining CAC score and CCTA nor were there differences with selective CCTA strategies over CCTA alone. The majority of CCTA scans with high CAC scores were interpretable. Thus, in ACP patients, CAC results should not influence the decision to proceed with CCTA, and its use for tailoring the subsequent CCTA scan and longer-term prognostic value should be balanced with the additional radiation exposure required.
Supplementary Material
Acknowledgments
We would like to thank all the investigators and ancillary support team from the ROMICAT II trial.
Sources of Funding
The study was supported by the NIH/NHLBI (U01HL092040 and U01HL092022). Dr. Truong was supported by the NIH (K23HL098370 and L30HL093896). Dr. Pursnani was supported by NIHT32 HL076136.
Footnotes
Disclaimer: The findings and opinions expressed here are the authors’ and do not necessarily represent the views of the National Institutes of Health or the Department of Health and Human Services.
Disclosures
Dr. Truong receives grant support from St. Jude Medical, American College of Radiology Imaging Network, and Duke Clinical Research Institute. Dr. Woodard receives grant support from Siemens Medical Systems, Astellas, and Bayer, and serves as consultant to GE Healthcare, Biotronik, Medtronic, and American College of Radiology Imaging Network.
References
- 1.Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG, Kalanjian S, Mullins ME, Mikati I, Peacock WF, Zakroysky P, Hayden D, Goehler A, Lee H, Gazelle GS, Wiviott SD, Fleg JL, Udelson JE, Investigators R-I. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, Leaming JM, Gavin LJ, Pacella CB, Hollander JE. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366:1393–1403. doi: 10.1056/NEJMoa1201163. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW, Hoffmann U, Lesser JR, Mikati IA, O’Neil BJ, Shaw LJ, Shen MY, Valeti US, Raff GL. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58:1414–1422. doi: 10.1016/j.jacc.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 4.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Cury RC, Abbara S, Brady TJ, Budoff MJ, Blumenthal RS, Nasir K. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–688. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Friera L, Garcia-Alvarez A, Bagheriannejad-Esfahani F, Malick W, Mirelis JG, Sawit ST, Fuster V, Sanz J, Garcia MJ, Hermann LK. Diagnostic value of coronary artery calcium scoring in low-intermediate risk patients evaluated in the emergency department for acute coronary syndrome. Am J Cardiol. 2011;107:17–23. doi: 10.1016/j.amjcard.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Georgiou D, Budoff MJ, Kaufer E, Kennedy JM, Lu B, Brundage BH. Screening patients with chest pain in the emergency department using electron beam tomography: a follow-up study. J Am Coll Cardiol. 2001;38:105–110. doi: 10.1016/s0735-1097(01)01364-x. [DOI] [PubMed] [Google Scholar]
- 7.Nabi F, Chang SM, Pratt CM, Paranilam J, Peterson LE, Frias ME, Mahmarian JJ. Coronary artery calcium scoring in the emergency department: identifying which patients with chest pain can be safely discharged home. Ann Emerg Med. 2010;56:220–229. doi: 10.1016/j.annemergmed.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P, Rubin GD. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525–555. doi: 10.1161/CIR.0b013e3181fcae66. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, Truong QA, Cury RC, Abbara S, Shapiro MD, Moloo J, Butler J, Ferencik M, Lee H, Jang IK, Parry BA, Brown DF, Udelson JE, Achenbach S, Brady TJ, Nagurney JT. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–1650. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pundziute G, Schuijf JD, Jukema JW, Lamb HJ, de Roos A, van der Wall EE, Bax JJ. Impact of coronary calcium score on diagnostic accuracy of multislice computed tomography coronary angiography for detection of coronary artery disease. J Nucl Cardiol. 2007;14:36–43. doi: 10.1016/j.nuclcard.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 12.Leschka S, Kim CH, Baumueller S, Stolzmann P, Scheffel H, Marincek B, Alkadhi H. Scan length adjustment of CT coronary angiography using the calcium scoring scan: effect on radiation dose. AJR Am J Roentgenol. 2010;194:W272–277. doi: 10.2214/AJR.09.2970. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann U, Truong QA, Fleg JL, Goehler A, Gazelle S, Wiviott S, Lee H, Udelson JE, Schoenfeld D, Romicat II. Design of the Rule Out Myocardial Ischemia/Infarction Using Computer Assisted Tomography: a multicenter randomized comparative effectiveness trial of cardiac computed tomography versus alternative triage strategies in patients with acute chest pain in the emergency department. Am Heart J. 2012;163:330–338. 338 e331. doi: 10.1016/j.ahj.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Gerber TC, Carr JJ, Arai AE, Dixon RL, Ferrari VA, Gomes AS, Heller GV, McCollough CH, McNitt-Gray MF, Mettler FA, Mieres JH, Morin RL, Yester MV. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009;119:1056–1065. doi: 10.1161/CIRCULATIONAHA.108.191650. [DOI] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 17.Mintz GS, Iskandrian AS, Bemis CE, Mundth ED, Owens JS. Myocardial ischemia in anomalous origin of the right coronary artery from the pulmonary trunk. Proof of a coronary steal. Am J Cardiol. 1983;51:610–612. doi: 10.1016/s0002-9149(83)80108-8. [DOI] [PubMed] [Google Scholar]
- 18.Wexler L, Brundage B, Crouse J, Detrano R, Fuster V, Maddahi J, Rumberger J, Stanford W, White R, Taubert K. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation. 1996;94:1175–1192. doi: 10.1161/01.cir.94.5.1175. [DOI] [PubMed] [Google Scholar]
- 19.Ohtani T, Ueda Y, Mizote I, Oyabu J, Okada K, Hirayama A, Kodama K. Number of yellow plaques detected in a coronary artery is associated with future risk of acute coronary syndrome: detection of vulnerable patients by angioscopy. J Am Coll Cardiol. 2006;47:2194–2200. doi: 10.1016/j.jacc.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 20.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 21.Hausleiter J, Meyer T, Hermann F, Hadamitzky M, Krebs M, Gerber TC, McCollough C, Martinoff S, Kastrati A, Schomig A, Achenbach S. Estimated radiation dose associated with cardiac CT angiography. Jama. 2009;301:500–507. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 22.Bischoff B, Hein F, Meyer T, Hadamitzky M, Martinoff S, Schomig A, Hausleiter J. Impact of a reduced tube voltage on CT angiography and radiation dose: results of the PROTECTION I study. JACC Cardiovasc Imaging. 2009;2:940–946. doi: 10.1016/j.jcmg.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Hausleiter J, Martinoff S, Hadamitzky M, Martuscelli E, Pschierer I, Feuchtner GM, Catalan-Sanz P, Czermak B, Meyer TS, Hein F, Bischoff B, Kuse M, Schomig A, Achenbach S. Image quality and radiation exposure with a low tube voltage protocol for coronary CT angiography results of the PROTECTION II Trial. JACC Cardiovasc Imaging. 2010;3:1113–1123. doi: 10.1016/j.jcmg.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Hausleiter J, Meyer TS, Martuscelli E, Spagnolo P, Yamamoto H, Carrascosa P, Anger T, Lehmkuhl L, Alkadhi H, Martinoff S, Hadamitzky M, Hein F, Bischoff B, Kuse M, Schomig A, Achenbach S. Image quality and radiation exposure with prospectively ECG-triggered axial scanning for coronary CT angiography: the multicenter, multivendor, randomized PROTECTION-III study. JACC Cardiovasc Imaging. 2012;5:484–493. doi: 10.1016/j.jcmg.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Achenbach S, Marwan M, Ropers D, Schepis T, Pflederer T, Anders K, Kuettner A, Daniel WG, Uder M, Lell MM. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J. 2010;31:340–346. doi: 10.1093/eurheartj/ehp470. [DOI] [PubMed] [Google Scholar]
- 26.Chen MY, Shanbhag SM, Arai AE. Submillisievert median radiation dose for coronary angiography with a second-generation 320-detector row CT scanner in 107 consecutive patients. Radiology. 2013;267:76–85. doi: 10.1148/radiol.13122621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCollough CH, Chen GH, Kalender W, Leng S, Samei E, Taguchi K, Wang G, Yu L, Pettigrew RI. Achieving routine submillisievert CT scanning: report from the summit on management of radiation dose in CT. Radiology. 2012;264:567–580. doi: 10.1148/radiol.12112265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng M, Liu Y, Wei M, Wu Y, Zhao H, Li J. Low concentration contrast medium for dual-source computed tomography coronary angiography by a combination of iterative reconstruction and low-tube-voltage technique: feasibility study. Eur J Radiol. 2014;83:e92–99. doi: 10.1016/j.ejrad.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Cao JX, Wang YM, Lu JG, Zhang Y, Wang P, Yang C. Radiation and contrast agent doses reductions by using 80-kV tube voltage in coronary computed tomographic angiography: a comparative study. Eur J Radiol. 2014;83:309–314. doi: 10.1016/j.ejrad.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 30.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 32.Kondos GT, Hoff JA, Sevrukov A, Daviglus ML, Garside DB, Devries SS, Chomka EV, Liu K. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 33.Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, Witteman JC. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 34.Hulten E, Bittencourt MS, Singh A, O’Leary D, Christman MP, Osmani W, Abbara S, Steigner ML, Truong QA, Nasir K, Rybicki FF, Klein J, Hainer J, Brady TJ, Hoffmann U, Ghoshhajra BB, Hachamovitch R, Di Carli MF, Blankstein R. Coronary artery disease detected by coronary computed tomographic angiography is associated with intensification of preventive medical therapy and lower low-density lipoprotein cholesterol. Circ Cardiovasc Imaging. 2014;7:629–638. doi: 10.1161/CIRCIMAGING.113.001564. [DOI] [PubMed] [Google Scholar]
- 35.Rogers IS, Banerji D, Siegel EL, Truong QA, Ghoshhajra BB, Irlbeck T, Abbara S, Gupta R, Benenstein RJ, Choy G, Avery LL, Novelline RA, Bamberg F, Brady TJ, Nagurney JT, Hoffmann U. Usefulness of comprehensive cardiothoracic computed tomography in the evaluation of acute undifferentiated chest discomfort in the emergency department (CAPTURE) Am J Cardiol. 2011;107:643–650. doi: 10.1016/j.amjcard.2010.10.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.