Abstract
The mammalian target of rapamycin (mTOR) is a central controller of cell growth and is currently being investigated as a potential target in breast cancer therapy. The essential amino acid leucine has been proposed to regulate mTOR signaling. The objective of this study was to determine whether leucine restriction would inhibit mTOR signaling in breast cancer cells. Leucine restriction did not decrease mTOR signaling in any of the eight breast cancer cell lines tested. In addition, in vivo administration of a leucine-free diet for up to four days did not result in a decrease in phosphorylation of mTOR target proteins in breast cancer xenografts. Further, in three different cell lines, an increase in Akt phosphorylation was observed after leucine restriction. This was observed without a decrease in S6K phosphorylation, suggesting a mechanism different from the feedback loop activation of Akt observed with rapamycin treatment. We conclude that leucine restriction is not sufficient to inhibit mTOR signaling in most breast cancer cell lines, but is associated with activation of survival molecule Akt, making leucine deprivation an undesirable approach for breast cancer therapy.
INTRODUCTION
Availability of amino acids (AA), particularly branched chain AA, such as leucine, has been reported to activate the mammalian target of rapamycin (mTOR)/ribosomal S6 kinase 1 (S6K1) signaling pathway (1–4). mTOR is a conserved serine/threonine kinase that integrates multiple signals to regulate diverse cell functions. mTOR’s activity is controlled by phosphatidylinositide 3-kinase (PI3K) and Akt, and by Ras/Raf/ERK pathways (5). The PTEN (phosphatase and tensin homolog deleted from chromosome 10) tumor suppressor negatively regulates PI3K signaling, and is mutated or decreased in several types of sporadic tumors, including breast cancer and in patients with Cowden’s breast cancer predisposition syndrome (6). Furthermore, overexpression of human epidermal growth factor receptor 2 (HER2/neu) (7) and activation of insulin-like growth factor receptor (8) and integrins (9) lead to activation of PI3K/Akt pathway. mTOR is activated in one half of breast cancer patients, and mTOR’s target S6K1 is overexpressed in one third of breast cancer patients; both alterations have been linked to a poor prognosis (10, 11). Thus mTOR/S6K1 pathway represents a promising target for breast cancer therapy, and mTOR inhibitor rapamycin and its analogues are currently in clinical trials (12–14).
mTOR exists in two distinct complexes (15). Rapamycin-sensitive mTOR Complex 1 (mTORC1) phosphorylates and activates the cell growth regulators S6K 1 and 2, and activates ribosomal protein S6 (16), and it phosphorylates and inactivates the translational repressors eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) (17). 4E-BP1 targets and prevents eIF4E-eIF4G interaction and thus inhibits translation initiation (17). mTOR inhibitor rapamycin dephosphorylates S6K1 and 4E-BP1, leads to G1 growth arrest, and inhibits ribosome biosynthesis and autophagy (18, 19). The antitumor activity of rapamycin as a single agent has been described in vitro and in vivo (20, 21). Although in some models rapamycin alone was shown to induce apoptosis in cells lacking p53 (22, 23), it is mostly reported acting as a cytostatic agent arresting cancer cells in the G1 phase. In preclinical models, rapamycin synergistically enhanced chemosensitivity to commonly utilized chemotherapy agents, such as paclitaxel, carboplatin and vinorelbine (24, 25). Rapamycin-insensitive mTOR Complex 2 (mTORC2) activates Akt and also regulates actin cytoskeleton (26, 27). Rapamycin dephosphorylates mTORC2 component rictor (rapamycin-insensitive companion of TOR) (28). Although rictor phosphorylation may effect Akt phosphorylation, the biologic consequence of its phosphorylation is relatively unknown (29). Both mTORC1 and mTORC2 respond to hormones and growth factors (15), however only mTORC1 has been proposed to be acutely regulated by nutrients, such as AA and glucose (30, 31).
Studies to date have suggested that AA activate mTORC1, increasing cell growth through increased ribosome biogenesis and protein biosynthesis, and suppression of autophagy (15, 32). Growth factor signals have little or no impact on mTORC1 signaling in the absence of AA (2). Leucine, acting through an mTORC1-dependent pathway stimulates the translation of specific mRNAs both by increasing availability of eIF4E and by stimulating phosphorylation of S6 (33). Leucine induces cell migration and Rac activation controlling cytoskeleton through mTORC2 (34). Leucine deprivation leads to a cellular response overlapping with, but distinct from mTOR inhibition (35). Leucine deprivation and rapamycin have been proposed to have synergistic effects on protein synthesis (36). This suggests that leucine modulation may hold promise as a novel approach to modulate mTOR signaling, or potentially enhance the efficacy of mTOR inhibitors.
Malignant cells require increased amounts of AA, especially leucine, to support DNA and protein synthesis and cell proliferation. Proliferation in human hepatoma cell lines has been shown to be dependent on the concentration of leucine in vitro, with a significant reduction in proliferation rates in 0.05 mM leucine-containing medium compared to 0.2 mM (37). Recent work suggests that leucine deprivation is associated with upregulation of insulin-like growth factor binding protein 1 through transcriptional activation and mRNA stabilization (38), which would be expected to decrease the mitogenic effects of insulin-like growth factors, and may contribute to the decreased cell proliferation seen with leucine deprivation. However, to date little work has been done to further exploit these finding therapeutically.
Strategies to inhibit signaling pathways have focused primarily on administering agents to inhibit growth of cancer cells and enhance efficacy of chemotherapy. Recently, Anthony et al. evaluated the effects of dietary leucine deprivation in mice (39). Leucine deprivation led to a dephosphorylation of both S6K1 and 4E-BP1 in the liver, after 1 hour as well as 6 days. As leucine-restricted diets already are commercially available for the nutritional support of children and adults with disorders of the leucine catabolism, it would be also possible to deliver patients a leucine-restricted diet. Therefore, we tested the hypothesis that short-term leucine restriction could downregulate mTOR/S6K1 signaling in breast cancer cell lines.
MATERIALS AND METHODS
Cell lines, culture and reagents
BT-474, MCF7, MDA-MB-231, MDA-MB-435, MDA-MB-436, MDA-MB-453, MDA-MB-468, and SKBr3 breast cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA) and cultured in Dulbecco’s modification of Eagle’s medium-Ham’s F12 50:50 mixture (DMEM/F-12) (Cellgro Mediatech, Inc., Herndon, VA) supplemented with 10% fetal bovine serum (SAFC Biosciences, Lenexa, KA) at 37° C and humidified in 5% CO2. Leucine-free DME/F12 basic medium, L-leucine, L-lysine monohydrochloride, L-glutamic acid potassium salt monohydrate, and L-methionine were obtained from Sigma Aldrich, Inc. (St Louis, MO). We purchased dimethyl sulphoxide (DMSO) from Sigma, rapamycin from LC Laboratories (Woburn, MA), and U0126, wortmannin and LY294002 from Cell Signaling Technology (Danvers, MA).
Western blot analysis
Cultured cell were washed with cold phosphate buffered saline twice and lysed in cell lysis buffer as described elsewhere (25). After determining the protein concentration with the Bradford protein assay (Bio-Rad Laboratories, Hercules, CA), cell lysates containing 50 μg of protein were separated by sodium dodecyl sulfate – polyacrylamide gel electrophoresis and transferred to 0.2 μM polyvinylidene fluoride membrane (Bio-Rad Laboratories, Hercules, CA). Membranes were blocked with 0.1% casein in tris-buffered saline. All antibodies were purchased from Cell Signaling Technology except β-actin (Sigma Aldrich, Inc.). Visualization of immunoblots and quantification of bands were done by Odyssey infrared imaging system and its application software (Li-Cor Biosciences, Lincoln, NE). A phospho-antibody/total-antibody ratio was calculated and normalized to control sample. All experiments were repeated at least three times.
Animal studies
All animal studies were approved by the M. D. Anderson Animal Care and Use Committee. The animal care program is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International. Four-week old female nude mice were purchased from National Cancer Institute (Frederick, MD) and housed in specific pathogen free conditions. An inoculum of 1x107 of MDA-MB-468 cells in 100 μl of phosphate-buffered saline was injected within the mammary fat pad. After 37 days, an average tumor size of 90 mm3 was achieved. Mice were separated into four groups (4 mice/group). One group of mice was fed with regular diet (control group) and other three groups of mice were fed with leucine-free diet (Research Diets Inc., New Brunswick, NJ) either for 1, 2 or 4 days as experimental groups. Mice were weighed at the initiation of the study and provided a measured amount of control diet or leucine-free diet daily. Final body weight and amount of food intake of mice were recorded. Mice were euthanized via CO2 narcosis and tumors were harvested, snap frozen in liquid nitrogen, and homogenized with an electric homogenizer in cell lysis buffer.
RESULTS
The effect of leucine on serum stimulation of mTOR signaling in vitro
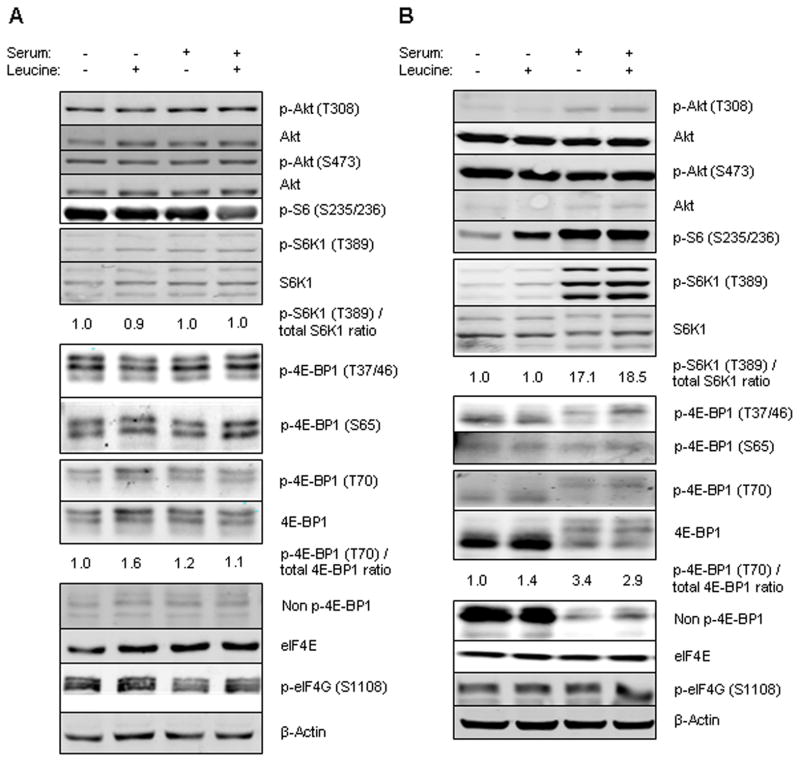
To determine the effect of leucine restriction on mTOR signaling, we performed immunoblotting to assess phosphorylation of mTOR’s targets. We evaluated mTOR signaling in two breast cancer cell lines: MDA-MB-468 cell line, a PTEN negative cell line with constitutive activation of the PI3K/mTOR pathway and MCF7, which has a PI3K mutation but does not show activation of downstream targets. Cells were leucine and serum starved for 16 h and then stimulated by leucine and/or serum for 2 h. In MDA-MB-468 cells, supplementing serum with or without leucine did not change the phosphorylation state of the target proteins (Fig. 1a). In the MCF7 cell line, as expected, supplementing serum phosphorylated these targets at both time points (Fig. 1b). Supplementing leucine in the absence of serum increased phosphorylation of S6. We also observed a minor increase in S6K1 phosphorylation in the MCF7 cell line, however, this was not a consistent finding. This experiment showed that in the absence of constitutive activation of PI3K pathway, supplementing leucine in the absence of serum increased phosphorylation of S6, but phosphorylation did not increase further in the presence of serum.
FIG. 1.
Cells were cultured in leucine-free and serum-free media for 16 h and then stimulated with 10% FBS and with or without leucine for 2 h. Western blotting was performed for p-Akt (T308), p-Akt (S473), Akt, p-S6 (S235/236), p-S6K1 (T389), S6K1, p-4E-BP1 (T70), p-4E-BP1 (T37/46), p-4E-BP1 (S65), 4E-BP1, Non p-4E-BP1, eIF4E, p-eIF4G (S1108) and β-actin. The relative p-S6K1 (T389)/total-S6K1 and p-4E-BP1/total 4E-BP1 ratios are given. A: MDA-MB-468 cell line, B: MCF7 cell line.
The effect of leucine on mTOR signaling in vitro
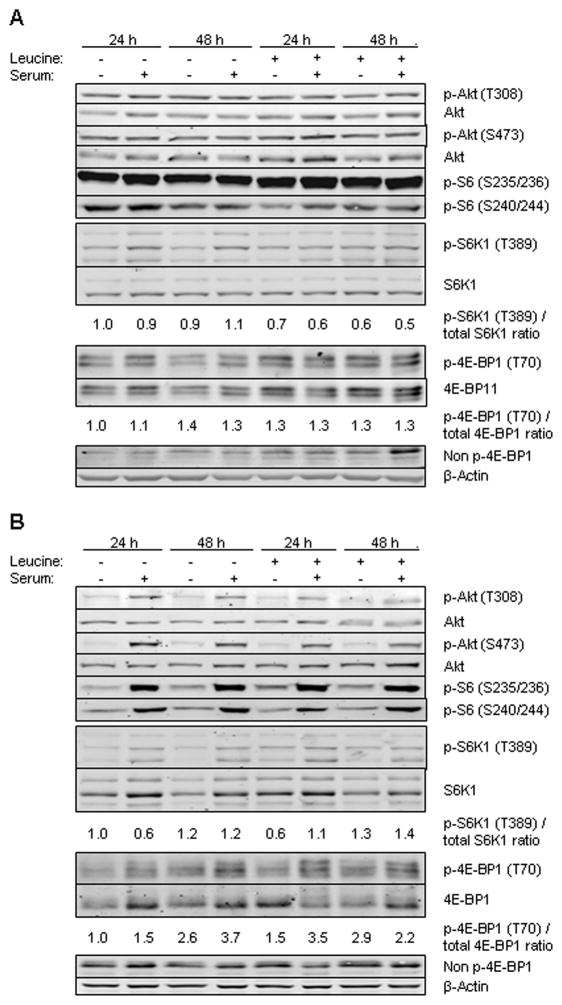
To determine whether leucine restriction results in inhibition of the mTOR pathway, we cultured the cells in media containing 0 or 0.5 mM leucine with or without 10% serum for 24 and 48 h. In MDA-MB-468 cell line, leucine or serum restriction alone or in combination did not affect phosphorylation of downstream targets 4E-BP1, S6K1, S6, and Akt (Fig. 2a). In the MCF7 cell line leucine restriction did not alter, but absence of serum decreased phosphorylation of mTORC1 targets at both time points. Combining serum deprivation with leucine restriction did not further decrease the level of phosphorylation of these targets (Fig. 2b). However, leucine restriction in the presence of serum minimally increased phosphorylation of Akt. This experiment demonstrates that leucine restriction by itself or in combination with serum starvation does not significantly decrease mTORC1 signaling, and constitutive activation of PI3K pathway in MDA-MB-468 cell line renders the cells insensitive to serum restriction. In MCF7 cells, Akt is activated by leucine restriction in the presence of serum.
FIG. 2.
Cells were grown with (0.5 mM) and without leucine in the presence or absence of 10% FBS. Western blotting was performed for p-Akt (T308), p-Akt (S473), Akt, p-S6 (S235/236), p-S6 (S240/244), p-S6K1 (T389), S6K1, p-4E-BP1 (T70), 4E-BP1, Non p-4E-BP1 and β-actin. The relative p-S6K1 (T389)/total-S6K1 and p-4E-BP1/total 4E-BP1 ratios are given. A: MDA-MB-468 cell line, B: MCF7 cell line.
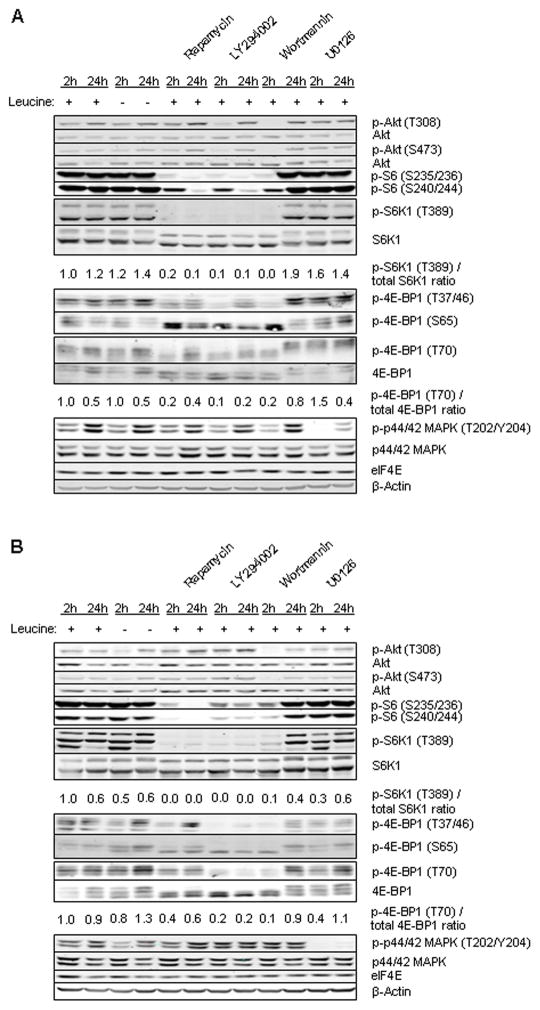
We next compared the effect of leucine deprivation with specific inhibitors of cell signaling. Cells were cultured in media containing 0 or 0.5 mM leucine with or without mTOR inhibitor rapamycin, PI3K inhibitors LY294002 and wortmannin, and ERK inhibitor U0126 for 2 and 24 h (Fig. 3a and b). In both cell lines, the results were similar. Leucine restriction did not affect phosphorylation of 4E-BP1, S6K1, and S6. In only the MCF7 cell line Akt phosphorylation showed a minor increase with leucine restriction at the 24 h time point. Rapamycin inhibited 4E-BP1, S6K1, and S6 phosphorylation, and did not alter ERK phosphorylation. In MCF7 cells, Akt phosphorylation increased with rapamycin treatment. LY294002 and wortmannin showed an inhibitory effect on Akt, 4E-BP1, S6K1, and S6 but not on ERK. U0126 inhibited only ERK phosphorylation. In this experiment, unlike inhibitors of PI3K/mTOR signaling which led to a robust inhibition of mTOR signaling, leucine restriction did not downregulate mTOR signaling.
FIG. 3.
Cells were grown with or without of leucine in the presence or absence of 10% serum and treated with different inhibitors: 100 nM rapamycin, 20 μM LY294002, 200 nM wortmannin or 10 μM U0126 for 2 and 24 h. Western blotting was performed for p-Akt (T308), p-Akt (S473), Akt, p-S6 (S235/236), p-S6 (S240/244), p-S6K1 (T389), S6K1, p-4E-BP1 (T70), 4E-BP1, Non p-4E-BP1, p-p44/42 MAPK (T202/Y204 ), p-p44/42 MAPK, eIF4E, and β-actin. The relative p-S6K1 (T389)/total-S6K1 and p-4E-BP1/total 4E-BP1 ratios are given. A: MDA-MB-468 cell line, (B) MCF7 cell line.
The effect of leucine on Akt phosphorylation
Because we found an unexpected increase in phosphorylation of Akt in MCF7 with leucine deprivation, we evaluated the effect of leucine deprivation on Akt phosphorylation in six additional breast cancer cell lines. A 48 h leucine restriction showed Akt activation in two additional cell lines, MDA-MB-435 and MDA-MB-453, (Fig. 4), whereas there was no effect on regulation of Akt in BT474, MDA-MB-231, MDA-MB-436, and SKBr3 cell lines (data not shown). In all six cell lines, there was no alteration in phosphorylation of S6K1 and 4E-BP1 with 48 h leucine deprivation (data not shown).
FIG. 4.
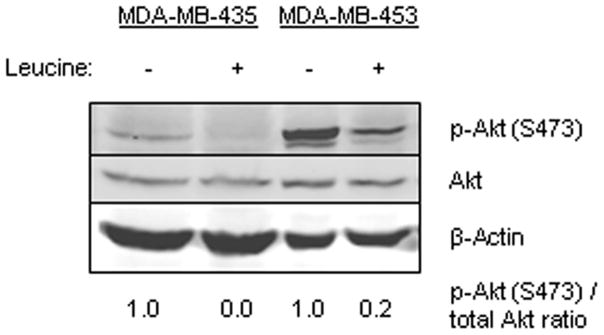
MDA-MB-435 and MDA-MB-453 cell lines were grown with or without leucine for 48 h. Western blotting was performed for p-Akt (T308), p-Akt (S473), Akt, and β-actin. The relative p-Akt (S473)/total-Akt ratio is given.
The effect of leucine restriction on mTOR signaling in vivo
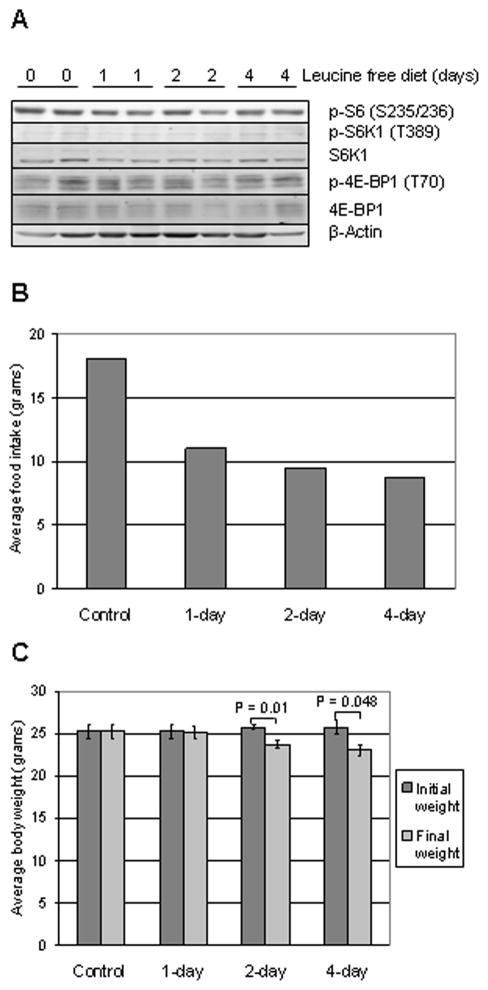
Next, we determined the effect of leucine deprivation in vivo, using the MDA-MB-468 breast cancer xenograft model. Mammary fat pads of female nude mice were injected with MDA-MB-468 cells. After the tumors reached an average of 90 mm3 in volume, mice were fed with leucine-free diet for 0 (control), 1, 2 or 4 days. At the end of the experiment, daily food intake was calculated, body weight was measured, tumors were harvested and protein was extracted from four xenografts per group.
Western blotting did not show a decrease in the phosphorylation levels of 4E-BP1, S6K and S6 in up to 4 days of leucine restriction in vivo (Fig. 5a). Average daily food intake was 18 gram/day in control group and decreased to 11, 9.5 and 8.75 gram/day in 1-day, 2-day, and 4-day leucine-free diet groups, respectively (Fig. 5b). The consequence of decreased food intake was observed in body weight: there was an average weight reduction of 0.1, 2 and 2.75 grams in 1-day, 2-day and 4-day leucine-free diet groups, respectively (Fig. 5c). On day-2 and -4, decrease in average body weight of mice in leucine restriction group was significant. Therefore, in this experiment leucine-free diet decreased food intake and body weight but did not alter phosphorylation status of mTOR effectors.
FIG. 5.
MDA-MB-468 cells injected to mammary fat pad after tumor formation, nude mice fed with leucine free diet for 0, 1, 2 or 4 days. A: Western blotting was performed on tumor lysates extracted from two xenografts at each time point for p-S6 (S235/236), p-S6K1 (T389), S6K1, p-4E-BP1 (T70), 4E-BP1 and β-actin. B: Average food intake of mice in control and leucine-free diet groups, C: Initial (before initiation of leucine restriction) and final average body weight of mice in control and leucine-free diet groups. Unpaired, two-tailed t test; error bars represent standard error of mean (SEM).
DISCUSSION
mTOR is being increasingly recognized as a critical mediator of cell growth. Several studies to date have reported that leucine activates mTOR signaling (1–4). We thus characterized the effect of leucine restriction on mTOR pathway signaling in breast cancer cells, with the long-term goal of determining whether breast cancer patients could benefit from restriction of leucine intake as a potential therapeutic strategy. We found that in all eight breast cancer cell lines tested, leucine restriction did not result in inhibition of mTOR pathway in vitro. This conclusion is supported by analysis of in vivo samples which also showed that leucine deprivation was insufficient to downregulate phosphorylation of mTOR downstream targets S6K1 and 4E-BP1.However, leucine deprivation was associated with Akt phosphorylation in MCF7, MDA-MB-435 and MDA-MB-453 cell lines.
To test the hypothesis that leucine restriction would lead to a decrease in mTOR signaling, we studied the effect of leucine restriction in two cell lines representing different genetic backgrounds: MDA-MB-468 cell line is triple negative (ER, PR and HER2 negative) and PTEN null, whereas MCF7 cell line has PI3K E545K mutation and amplification of S6K1 expression, but does not show activation of PI3K/Akt/mTOR pathway. In our study, in vitro leucine starvation neither altered the phosphorylation states of mTORC1 targets nor augmented the effect of PI3K/mTOR pathway inhibitors tested. Further, leucine deprivation did not lead to downregulation of mTOR signaling in vivo in MDA-MB-468 cells. MDA-MB-468 is a commonly used breast cancer model, however, xenograft models have limitations, and often do not reflect efficacy of interventions in human trials. In our in vivo model we evaluated the effect of short term leucine deprivation as we felt that in the clinic, short-term dietary intervention would be associated with better compliance and quality of life. It is possible that longer leucine deprivation might have effects on signaling. Longer deprivation was not further pursued as we observed significant weight loss in the mice by four days. Dietary leucine deprivation has also been reported to lead to with weight loss by other investigators (39). In our study, leucine deprivation was also associated with a decrease in dietary intake. Whether this is simply due to a difference in taste of the dietary chow, or due to a specific role for leucine in appetite control is unclear.
Similar to our observations in breast cancer cell lines, in ATDC5, a chondrogenic cell line, S6 and 4E-BP1 phosphorylation was also shown to be unresponsive to leucine restriction (40). However, there are other studies where removal of leucine caused dephosphorylation of S6K1 and 4E-BP1 (41). These conflicting results among different studies may be attributable to differences in experimental design, or due to differences between cells of origin or baseline activity of cellular signaling. However, in our studies, mTORC1 was unresponsive to leucine deprivation in several breast cancer cell lines of differing molecular subtypes.
Although leucine restriction did not lead to a change in the phosphorylation status of mTORC1 downstream targets, it resulted in an increase in Akt phosphorylation. This is in contrast to work by Lee et al, in which leucine induced Akt phosphorylation in chicken hepatocytes in a time-dependent manner, and PI3K/mTOR inhibitor LY294002 inhibited leucine-induced Akt phosphorylation (42). We observed leucine restriction-induced Akt phosphorylation in three separate breast cancer cell lines, with differing baseline aberrations in PI3K/mTOR signaling. Interestingly, rapamycin treatment also regulates Akt phosphorylation (43). It is of note that mTORC2 directly phosphorylates Akt (44). Like leucine deprivation, effect of rapamycin is also cell line dependent, varying from no effect to inhibition or activation (45). Further, mTORC1 controls Akt activity through a negative feedback loop. S6K1 phosphorylates inhibitory serine sites of IRS-1 leading to its degradation (46–48), whereas rapamycin prevents inhibitory IRS-1 phosphorylation, stabilizing IRS-1 and induces Akt activity by augmenting IGF-IR signaling to PI3K/Akt. However, as our present study failed to confirm any change in phosphorylation of S6K1 in response to leucine deprivation, feedback loop activation of PI3K signaling through this mechanism is unlikely to be the mechanism of Akt phosphorylation. Another explanation may be a yet undefined pathway connecting leucine to Akt independent of mTORC1. Akt is a survival molecule involved in glucose homeostasis, transcription, apoptosis, cell motility, angiogenesis, proliferation, and cell growth (49), including activation of mTOR. Leucine-induced Akt activation would thus not be favorable in cancer patients and provides an additional reason why the use of leucine restriction as a complementary therapeutic strategy would be undesirable.
Several models describe how mTOR signaling is controlled by nutrients. Branched chain amino acids mediate mTOR activity (50) and their reduced concentrations can regulate apoptosis through mTOR (51–53). Another AA involved in regulation of mTOR pathway is glutamine, which leads to uptake of leucine and phosphorylation of S6K1 (54). Restriction of glutamine or inhibition of glutamine transporters decrease mTOR activity (54). Tumor cells are more sensitive to amino acid deprivation than normal cells (55, 56) and dietary restriction suppresses tumor growth in mice (57–59). Therefore, targeting transporters or regulation of amino acid provision by dietary restriction can be used to control tumor cell proliferation. PI3K pathway mediates effects of calorie restriction as cancer cell lines carrying constitutive activation of PI3K pathway are resistant to anti-growth effects of calorie restriction (60). In the same study, Kaalany and Sabatini also demonstrated that overexpression of FOXO1, a downstream effector of Akt, increases tumor growth; they speculate that mTORC1 signaling may also contribute to resistance to calorie restriction. A similar approach is short-term calorie restriction to induce resistance to stress of normal cells, which enables administering higher doses of chemotherapeutic agents. Calorie-restricted mice have better survival after high dose chemotherapy (61). This may increase the efficacy of established chemotherapies and offer new hope.
In conclusion, we describe that leucine restriction in the eight breast cancer cell lines we tested is not sufficient to inhibit mTOR signaling. It is possible that the effect of leucine restriction depends on the genetic background of a tumor, and certain tumor types may be more sensitive to leucine restriction. However, in breast cancers with constitutively activated Akt/mTOR signaling, leucine restriction alone is unlikely to modulate mTOR signaling. Thus, when mTOR is being pursued as a therapeutic target, rapamycin and its analogs, or new generation mTOR, Akt, PI3K, dual PI3K/mTOR kinase inhibitors are more likely to achieve target inhibition.
Acknowledgments
Supported by grants from NIH1 R01 CA112199 and American Institute of Cancer Research (to F. M.-B.), NIH 5 T32 CA09599 (F. M.-B.; D. L.) and the U. T. M.D. Anderson Cancer Center Support Core Grant (CA 16672). We thank Judy Roehm for assistance with manuscript preparation.
References
- 1.Blommaart EF, Luiken JJ, Blommaart PJ, van Woerkom GM, Meijer AJ. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem. 1995;270:2320–6. doi: 10.1074/jbc.270.5.2320. [DOI] [PubMed] [Google Scholar]
- 2.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–94. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 3.Um SH, D’Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Campbell LE, Miller CM, Proud CG. Amino acid availability regulates p70 S6 kinase and multiple translation factors. Biochem J. 1998;334( Pt 1):261–7. doi: 10.1042/bj3340261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–60. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- 6.Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–7. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 7.Zhou BP, Hu MC, Miller SA, Yu Z, Xia W, Lin SY, Hung MC. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kappaB pathway. J Biol Chem. 2000;275:8027–31. doi: 10.1074/jbc.275.11.8027. [DOI] [PubMed] [Google Scholar]
- 8.Cui X, Zhang P, Deng W, Oesterreich S, Lu Y, Mills GB, Lee AV. Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol. 2003;17:575–88. doi: 10.1210/me.2002-0318. [DOI] [PubMed] [Google Scholar]
- 9.Mills GB, Lu Y, Fang X, Wang H, Eder A, Mao M, Swaby R, Cheng KW, Stokoe D, et al. The role of genetic abnormalities of PTEN and the phosphatidylinositol 3-kinase pathway in breast and ovarian tumorigenesis, prognosis, and therapy. Semin Oncol. 2001;28:125–41. doi: 10.1016/s0093-7754(01)90290-8. [DOI] [PubMed] [Google Scholar]
- 10.Barlund M, Forozan F, Kononen J, Bubendorf L, Chen Y, Bittner ML, Torhorst J, Haas P, Bucher C, et al. Detecting activation of ribosomal protein S6 kinase by complementary DNA and tissue microarray analysis. J Natl Cancer Inst. 2000;92:1252–9. doi: 10.1093/jnci/92.15.1252. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Tan M, Stone Hawthorne V, Klos KS, Lan KH, Yang Y, Yang W, Smith TL, Shi D, Yu D. Activation of the Akt/mammalian target of rapamycin/4E-BP1 pathway by ErbB2 overexpression predicts tumor progression in breast cancers. Clin Cancer Res. 2004;10:6779–88. doi: 10.1158/1078-0432.CCR-04-0112. [DOI] [PubMed] [Google Scholar]
- 12.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–87. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, Eiermann W, Hess D, Morant R, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–22. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 14.Ellard SL, Clemons M, Gelmon KA, Norris B, Kennecke H, Chia S, Pritchard K, Eisen A, Vandenberg T, et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol. 2009;27:4536–41. doi: 10.1200/JCO.2008.21.3033. [DOI] [PubMed] [Google Scholar]
- 15.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–80. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–80. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 18.Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006;25:6436–46. doi: 10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- 19.Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev. 2005;69:79–100. doi: 10.1128/MMBR.69.1.79-100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dilling MB, Dias P, Shapiro DN, Germain GS, Johnson RK, Houghton PJ. Rapamycin selectively inhibits the growth of childhood rhabdomyosarcoma cells through inhibition of signaling via the type I insulin-like growth factor receptor. Cancer Res. 1994;54:903–7. [PubMed] [Google Scholar]
- 21.Noh WC, Mondesire WH, Peng J, Jian W, Zhang H, Dong J, Mills GB, Hung MC, Meric-Bernstam F. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res. 2004;10:1013–23. doi: 10.1158/1078-0432.ccr-03-0043. [DOI] [PubMed] [Google Scholar]
- 22.Hosoi H, Dilling MB, Shikata T, Liu LN, Shu L, Ashmun RA, Germain GS, Abraham RT, Houghton PJ. Rapamycin causes poorly reversible inhibition of mTOR and induces p53-independent apoptosis in human rhabdomyosarcoma cells. Cancer Res. 1999;59:886–94. [PubMed] [Google Scholar]
- 23.Huang S, Liu LN, Hosoi H, Dilling MB, Shikata T, Houghton PJ. p53/p21(CIP1) cooperate in enforcing rapamycin-induced G(1) arrest and determine the cellular response to rapamycin. Cancer Res. 2001;61:3373–81. [PubMed] [Google Scholar]
- 24.Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Ruetz S, O’Reilly T, Natt F, Hall J, Lane HA, Thomas G. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–59. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 25.Mondesire WH, Jian W, Zhang H, Ensor J, Hung MC, Mills GB, Meric-Bernstam F. Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin Cancer Res. 2004;10:7031–42. doi: 10.1158/1078-0432.CCR-04-0361. [DOI] [PubMed] [Google Scholar]
- 26.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 27.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 28.Akcakanat A, Singh G, Hung MC, Meric-Bernstam F. Rapamycin regulates the phosphorylation of rictor. Biochem Biophys Res Commun. 2007;362:330–3. doi: 10.1016/j.bbrc.2007.07.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009 doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–9. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–15. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr. 2006;83:500S–7S. doi: 10.1093/ajcn/83.2.500S. [DOI] [PubMed] [Google Scholar]
- 33.Kimball SR, Shantz LM, Horetsky RL, Jefferson LS. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem. 1999;274:11647–52. doi: 10.1074/jbc.274.17.11647. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez-Negrete I, Carretero-Ortega J, Rosenfeldt H, Hernandez-Garcia R, Calderon-Salinas JV, Reyes-Cruz G, Gutkind JS, Vazquez-Prado J. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J Biol Chem. 2007;282:23708–15. doi: 10.1074/jbc.M703771200. [DOI] [PubMed] [Google Scholar]
- 35.Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol. 2002;22:5575–84. doi: 10.1128/MCB.22.15.5575-5584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mordier S, Deval C, Bechet D, Tassa A, Ferrara M. Leucine limitation induces autophagy and activation of lysosome-dependent proteolysis in C2C12 myotubes through a mammalian target of rapamycin-independent signaling pathway. J Biol Chem. 2000;275:29900–6. doi: 10.1074/jbc.M003633200. [DOI] [PubMed] [Google Scholar]
- 37.Wasa M, Bode BP, Souba WW. Adaptive regulation of amino acid transport in nutrient-deprived human hepatomas. Am J Surg. 1996;171:163–9. doi: 10.1016/S0002-9610(99)80093-2. [DOI] [PubMed] [Google Scholar]
- 38.Averous J, Maurin AC, Bruhat A, Jousse C, Arliguie C, Fafournoux P. Induction of IGFBP-1 expression by amino acid deprivation of HepG2 human hepatoma cells involves both a transcriptional activation and an mRNA stabilization due to its 3′UTR. FEBS Lett. 2005;579:2609–14. doi: 10.1016/j.febslet.2005.03.077. [DOI] [PubMed] [Google Scholar]
- 39.Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem. 2004;279:36553–61. doi: 10.1074/jbc.M404559200. [DOI] [PubMed] [Google Scholar]
- 40.Kim MS, Wu KY, Auyeung V, Chen Q, Gruppuso PA, Phornphutkul C. Leucine restriction inhibits chondrocyte proliferation and differentiation through mechanisms both dependent and independent of mTOR signaling. Am J Physiol Endocrinol Metab. 2009;296:E1374–82. doi: 10.1152/ajpendo.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab. 2009;296:E592–602. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee MY, Jo SD, Lee JH, Han HJ. L-leucine increases [3H]-thymidine incorporation in chicken hepatocytes: involvement of the PKC, PI3K/Akt, ERK1/2, and mTOR signaling pathways. J Cell Biochem. 2008;105:1410–9. doi: 10.1002/jcb.21959. [DOI] [PubMed] [Google Scholar]
- 43.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 45.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 46.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–23. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–62. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 48.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–5. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 49.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–64. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 50.Kimball SR, Jefferson LS. Molecular mechanisms through which amino acids mediate signaling through the mammalian target of rapamycin. Curr Opin Clin Nutr Metab Care. 2004;7:39–44. doi: 10.1097/00075197-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakajo T, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, Naomoto Y. Arginine and Leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med. 2004;13:537–43. [PubMed] [Google Scholar]
- 52.Dhar R, Basu A. Constitutive activation of p70 S6 kinase is associated with intrinsic resistance to cisplatin. Int J Oncol. 2008;32:1133–7. [PubMed] [Google Scholar]
- 53.Peyrollier K, Hajduch E, Blair AS, Hyde R, Hundal HS. L-leucine availability regulates phosphatidylinositol 3-kinase, p70 S6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the L-leucine-induced up-regulation of system A amino acid transport. Biochem J. 2000;350(Pt 2):361–8. [PMC free article] [PubMed] [Google Scholar]
- 54.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–34. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kokkinakis DM, Brickner AG, Kirkwood JM, Liu X, Goldwasser JE, Kastrama A, Sander C, Bocangel D, Chada S. Mitotic arrest, apoptosis, and sensitization to chemotherapy of melanomas by methionine deprivation stress. Mol Cancer Res. 2006;4:575–89. doi: 10.1158/1541-7786.MCR-05-0240. [DOI] [PubMed] [Google Scholar]
- 56.Scott L, Lamb J, Smith S, Wheatley DN. Single amino acid (arginine) deprivation: rapid and selective death of cultured transformed and malignant cells. Br J Cancer. 2000;83:800–10. doi: 10.1054/bjoc.2000.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tannenbaum A, Silverstone H. Effect of limited food intake on survival of mice bearing spontaneous mammary carcinoma and on the incidence of lung metastases. Cancer Res. 1953;13:532–6. [PubMed] [Google Scholar]
- 58.Thompson HJ, Zhu Z, Jiang W. Dietary energy restriction in breast cancer prevention. J Mammary Gland Biol Neoplasia. 2003;8:133–42. doi: 10.1023/a:1025743607445. [DOI] [PubMed] [Google Scholar]
- 59.Zhu Z, Haegele AD, Thompson HJ. Effect of caloric restriction on pre-malignant and malignant stages of mammary carcinogenesis. Carcinogenesis. 1997;18:1007–12. doi: 10.1093/carcin/18.5.1007. [DOI] [PubMed] [Google Scholar]
- 60.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–31. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215–20. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]