Abstract
Background
Diabetic nephropathy is a clinical diagnosis where proteinuria is present in a patient with diabetes. Early intervention can significantly improve the prognosis. However, imprecision of the currently available biomarkers have impaired effective therapies in a timely manner. Urinary N-acetyl-β-D-glucosaminidase (NAG) is excreted in abnormally high amounts in many renal diseases. The aim of this study was to evaluate urinary NAG as an early biomarker in detection of diabetic nephropathy and whether it parallels the severity of kidney damage in different stages of diabetic nephropathy.
Methods
Fifty patients with type 2 DM were classified into 3 groups (normoalbuminurea, microalbuminurea and macroalbuminurea) and 10 healthy subjects served as a control group. Urinary NAG, albumin and creatinine were measured. Blood urea, serum creatinine, serum albumin, total proteins, serum cholesterol, alanine aminotransferase (ALT), aspartate aminotransferase (AST), fasting and postprandial blood glucose, HbA1c and creatinine clearance were measured for all subjects.
Results
All diabetic patients had a significantly higher level of urinary NAG compared to control. NAG value increased in parallel with the severity of renal involvement.
Conclusion
Urinary NAG expresses the degree of renal impairment in diabetic nephropathy.
Keywords: NAG, Diabetic nephropathy, Albuminurea
Background
Diabetic nephropathy occurs in 20–40% of patients with diabetes and is the leading cause of chronic kidney disease and end-stage renal disease [1,2]. The onset of elevated levels of urinary albumin excretion is an early sign of diabetic nephropathy. It has been shown in diabetic patients that, microalbuminuria predicts the occurrence of macroalbuminuria and renal function decline [3]. As a result, high albuminuria has become an established renal risk marker in these patients [4]. However, urinary albumin excretion can be affected by several factors including plasma concentrations of atrial natriuretic peptide, arginine vasopressin, angiotensin II, aldosterone and fasting blood glucose, glycated hemoglobin, and mean arterial blood pressure [5] and albumin can be degraded in a manner consistent with the activity of endogenous urinary proteases. Furthermore, the inter-individual variation is as high as 47%. Because of these problems, all results that are initially positive for albuminuria need to be confirmed with a second sample collected on a different day, and in cases of discrepancies between the first and second sample, a third sample is necessary [6].
Several tubular damage markers recently have been discovered. Increased levels of these markers are supposed to indicate proximal tubular damage in the case of kidney injury molecule (KIM)-1, neutrophil gelatinase–associated lipocalin (NGAL), N-acetyl-β-D glucosaminidase (NAG), and cystatin C and distal tubular damage in the case of heart fatty acid–binding protein (H-FABP). These tubular damage markers have been extensively investigated in the field of predicting the occurrence of acute kidney injury after various nephrotoxic insults, such as ischemia during cardiac surgery, sepsis, and administration of contrast medium [7,8]. Little research has been done in patients with chronic kidney disease. In this study, we aimed to investigate urinary level of the N-acetyl-β-Dglucosaminidase (NAG) as proximal tubular damage marker in diabetic patients and non-diabetic control subjects in order to evaluate the relation of this marker to the severity of kidney disease as assessed by albuminuria and the estimated glomerular filtration rate (eGFR).
Methods
This study was conducted in the Internal Medicine Department, Tanta University Hospital from April 2013 to October 2013. After approval of the Institutional Review Board of the College of Medicine, Tanta University, Egypt, fifty type 2 diabetic patients with initial diagnosis of diabetes at >30 years of age were recruited into the study. They were collected from inpatients and also from outpatients who were followed in the diabetic clinic. Hospitalized diabetic patients enrolled in our study were non-critically ill and their samples were not collected until they had fully recovered from their medical illness. Patients were stratified by the extent of albuminuria in the first morning urine void. Besides, we included 10 non diabetic volunteers as controls. Patients were divided into 4 groups: Group I: 10 non diabetic non hypertensive healthy persons as a control group, group II: 10 patients with normoalbuminuria, albumin creatinine ratio (ACR) ≤3 mg/mmol), group III: 20 had microalbuminuria ACR 3–30 mg/mmol, group IV: 20 have macroalbuminuria ACR >30 mg/mmol.
Written informed consent was obtained from all participants and the privacy of the data was considered. Exclusion criteria included patients with liver diseases, cardiovascular diseases, cancer, infections or inflammatory conditions, renal disease other than diabetic nephropathy and pregnancy. Patients and volunteers were fasted for at least 6 hours and were asked to abstain from unaccustomed physical activity for the preceding 24 hours. All subjects rested for 30 minutes in a supine position prior to initial blood sampling. All patients included in the study were subjected to full history taking, complete clinical examination (including blood pressure, fundus examination), ECG for exclusion of cardiovascular disease, laboratory investigations included: glucose, HbA1c, renal function tests, glomerular filtration rate by Cockcroft-Gault Equation Creatinine clearance = { [140 - age (years)] × weight (kg)} / [72 × serum creatinine (mg/dl)] × (0.85 if female), serum albumin, total proteins, Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST), cholesterol levels and urine analysis that included: albumin concentration, urine creatinine and urinary concentrations of NAG. The kit uses a double antibody sandwich enzyme Linked immunosorbent assay (ELISA). Catalog No. MB 5700742.
Statistical analysis of the data of the present study was conducted with SPSS Version 16 using the mean, standard deviation and Yates’ corrected chi-square test. One-way ANOVA was used to compare means from all groups. The relationship between urinary NAG and other parameters was determined using the Spearman correlation analysis and the linear regression method. P values 0.05% were considered to be statistically significant.
Results
Fifty diabetic patients were divided into three groups according to albumiurea and 10 control subjects were included in our study. No patient was excluded for any reason. Demographic data were comparable between groups while duration of the disease was significantly longer in groups III and IV compared to group II (Table 1). A total of 13 inpatients were enrolled in our study; 3 in group II and 5 in each of group III and VI. They were admitted because of uncontrolled hyperglycemia (7 patients), chest infection (6 patients).
Table 1.
Demographic characteristics
| Variable | Group I | Group II | Group III | Group VI | P |
|---|---|---|---|---|---|
| Age | 47.30 ± 4.63 | 51.36 ± 5.25 | 48.64 ± 5.74 | 52.84 ± 7.63 | 0.951 |
| Gender M/F | 6/4 | 8/2 | 10/10 | 8/12 | 0.635 |
| Disease duration | - | 8.63 ± 3.25 | 13.2 ± 4.63 | 12.4 ± 2.34 | 0.035* |
M = male, F = female, * = significant.
HbA1C, albumin/creatinine ratio, serum creatinine and serum urea showed statistically significant increase in group IV compared to group III and group III compared to group II and the all groups compared to group І (Table 2).
Table 2.
Laboratory and calculated data
| Variable | Group I | Group II | Group III | Group VI | P |
|---|---|---|---|---|---|
| HbA1C | 5.10 ± 1.24 | 6.40 ± 2.42 | 7.86 ± 2.85 | 8.60 ± 3.17 | 0.006* |
| Albumin/creatinine ratio | 1.53 ± 0.87 | 2.36 ± 0.95 | 21.69 ± 5.36 | 45.35 ± 13.25 | 0.001* |
| Serum creatinine mg/dl | 0.75 ± 0.11 | 0.86 ± 0.13 | 1.60 ± 0.23 | 2.08 ± 0.63 | 0.008* |
| Serum urea mg/dl | 32.14 ± 2.24 | 37.85 ± 5.64 | 55.96 ± 9.31 | 66.24 ± 10.24 | 0.002* |
| Estimated GFR ml/min | 110 ± 25.36 | 93 ± 19.52 | 70 ± 15.49 | 56 ± 12.69 | 0.001* |
| Serum cholesterol mg/dl | 180.6 ± 32.25 | 201.6 ± 29.60 | 214.9 ± 25.43 | 231.5 ± 29.6 | 0.020* |
| Urinary NAG ng/ml | 0.93 ± 0.15 | 1.15 ± 0.25 | 1.33 ± 0.36 | 1.59 ± 0.42 | 0.001* |
* = significant.
Estimated GFR, serum cholesterol, urinary NAG showed a statistical significant decrease in group IV compared to group III and in group III compared to group II and in the all groups compared to group І as showed (Table 2).
Significant positive correlation was observed between urinary NAG and albumin/creatinine ratio (P = 0.006), serum creatinine (P = 0.002) and HBA1C (P = 0.008) (Figure 1).
Figure 1.
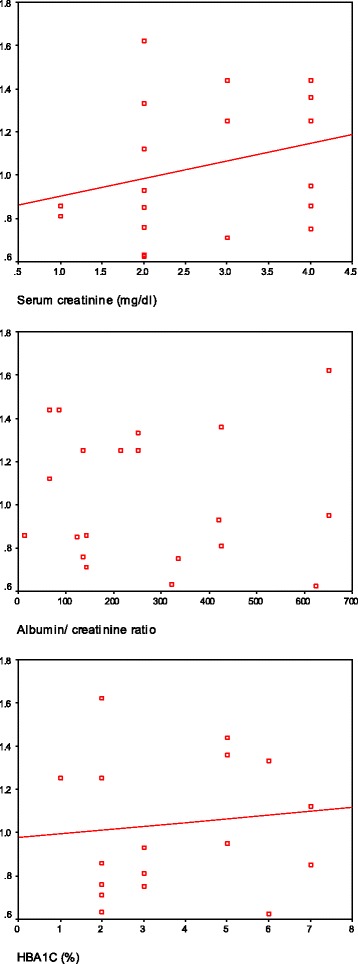
Correlation between NAG and serum creatinine, albumin/creatinine ratio and HBA1C.
Discussion
Our results revealed that there was significant increase in HbA1c in (macroalbuminuric and microalbuminuric) diabetic patients than (normoalbumiuric diabetic patients and healthy controls) and this coincides with other studies [9-13] that they recorded that Poor glycemic control is a well-known risk factor for most diabetic complications, not only diabetic nephropathy.
Comparison between all the studied groups as regard Albumin/creatinine ratio, serum creatinine and serum urea showed statistically significant increase in group IV than group III and group III than group II and the all groups than group І. This is agreement with the findings of Mitsnefes et al. [14] who reported that higher levels of blood urea and creatinine indicate a failing of GFR as a result of decreased capability of the kidney to excrete waste products. However, Devarajan [15] in chronic kidney disease, found that blood urea will only be elevated above the normal when more than 60% of kidney cells are no longer functioning and the usefulness of blood urea as an independent indicator or renal function is limited the variability of its blood levels as a result of non-renal factors such as gastro-intestinal hemorrhage, mild dehydration, high protein diet and decrease perfusion of the kidneys.
Our study revealed that eGFR was significantly lower in diabetic patients than that of the healthy control group, Similar findings were previously recorded by others [16-20]. Moreover Jamal et al. [21] in their retrospective study to assess the factors affecting the progression of diabetic nephropathy and its complications, they showed an average of 3.3 mL/year drop in GFR and even greater among patients who reached ESRD (5.9 mL/year) and the presence of persistent proteinuria was a strong risk factor for subsequent loss of GFR so they re-emphasizing earlier reports that established the importance of sustained increases in urine albumin excretion in the pathogenesis and diagnosis of diabetic kidney disease.
In this study, diabetic nephropathy was associated with elevated urinary NAG values compared to a control group. This increase in NAG was parallel to the severity of renal involvement with a characteristic increasing trend was observed among the three groups regarding albuminuria. These results are in agreement with other studies [22,23] that stated that changes in urinary NAG activity can reflect the activity of the disease as well as the residual functional capacity of the kidney. Our results showed that even in the absence of any clinical evidence of microvascular complications, urinary NAG excretion was invariably elevated indicating that subclinical renal tubular dysfunction may exist before the occurrence of glomerular damage. Kuzniar et al. [24] denoted that in proteinuric glomerular diseases the increased NAG excretion can occur even in absence of morphological evidence of tubular cell damage, probably reflecting increased lysosomal activity of these cells due to the increased uptake of filtered proteins. Also Navarro et al. [25] stated that NAG changes occur prior to microalbuminuria, probably because the tubular cells can reabsorb the increased albumin load that results from glomerular affection but the increased NAG will be lost from the damaged cells. Also, Nauta et al. [26] showed that urine NAG increases a surprising 9-fold in normoalbuminuric patients with diabetes compared to controls. It increases further with development and progress of microalbuminuria. Moreover, in another study of diabetic patients, Vaidya et al. [27] found, that urinary levels of tubular injury biomarkers KIM-1 and NAG were significantly elevated in patients with type 1 diabetes and macroalbuminuria in comparison with diabetics with normoalbuminuria and nondiabetic healthy controls. Low urinary KIM-1 and NAG at baseline estimation were strongly associated with regression of MA during a 2-year follow-up. It is generally agreed upon that there is some normally filtered albumin that is reabsorbed by the proximal tubule. If this reabsorption is impaired, we would expect to see macroalbuminuria; therefore, the earliest kidney lesion in type 1 DM may be tubular injury, not glomerular injury. Also urinary NAG elevation would be expected to be enhanced with proximal tubule injury although it may also be increased because of enhanced lysosomal activity without injury. Also Kern et al. [28] showed the change in NAG independently predicted macroalbuminuria. Thus, the risk for macroalbuminuria raised more than two fold for every 50% increase in urine NAG at baseline, and additionally rose almost 9% for every unit increase in urine NAG across the time interval from baseline to the occurrence of macroalbuminuria. Moreover another study [29] denoted that urinary NAG might be elevated in other diseases other than diabetic nephropathy. They investigated 136 patients with primary glomerulonephritis [74 with idiopathic membranous nephropathy (IMN), 44 with primary focal segmental glomerulosclerosis (FSGS) and 18 with minimal change disease (MCD)] they found Urinary NAG excretion can be considered as a reliable marker of the tubulo-toxicity of proteinuria in the early stage of IMN, FSGS and MCD; the excretion values show a significant relationship with 24-h proteinuria, Also Orfeas et al. [30] found that patients in the highest urinary NAG had a significantly higher prevalence of sepsis, higher Acute Physiology and Chronic Health Evaluation (APACHE) II score, Multiple Organ Failure (MOF) score, a higher likelihood of mechanical ventilation, higher prevalence of oliguria, and fractional excretion of sodium 1% as compared with those with lower urinary NAG. Also Dialysis requirement and hospital death were significantly higher in the high urinary NAG level patient as compared with the low urinary NAG level patient.
Correlation between Urinary NAG level and Albumin/creatinine ratio, serum creatinine and HbA1c showed significant + ve Correlation between Urinary NAG level and all previous parameters , this coincides with Sato et al. [31] stated that NAG total activity and A2 isoenzyme were highly correlated with the level of proteinuria with A2/A1 ratio higher in glomerular diseases (e.g. glomerulonephritis and diabetic nephropathy) than tubulointerstitial pathologies (e.g. chronic pyelonephritis and polycystic kidney). Also Abdelshakoer et al. [23] showed that Urinary NAG was strongly affected by the blood glucose control over the 1–7 days before urine collection.
Our study is not without limitations. The number of patients might be not enough to determine the reliability and generalizability of urinary NAG levels as expressing the degree of renal impairment in diabetic nephropathy. Larger studies are needed to confirm or refute our results. Also, our results remain limited to our patient population in the fact that other factors than proteinuria may play a role in the increased urinary NAG excretion in diabetics like blood glucose control [32].
Conclusion
Based on the limitations above, it can be concluded that urinary NAG levels could be used as a useful biomarker reflecting the degree of renal impairment in diabetic nephropathy.
Acknowledgements
Authors wish to thank all staff of the outpatient diabetes clinic of Tanta University Hospital for their assistance during the data collection. We also wish to thank the participants who voluntarily agreed to participate in the research.
Footnotes
Competing interests
There are no any financial or non-financial competing interests to declare in relation to this manuscript.
Authors’ contributions
GS participated in the design of the study, analysis and interpretation of data and revised the manuscript. NN conceived of the study, carried out its designing, coordinated the implementation, helped to perform the statistical analysis and drafted the manuscript. AT participated in the conception and design of the study, collected the data and performed the statistical analyses. MAS participated in the conception and design of the study, participated in acquisition of data and revised the manuscript. Authors read and approved the final manuscript.
Contributor Information
Gehan Sheira, Email: gehansheria2009@hotmail.com.
Nashwa Noreldin, Email: nashwanor@hotmail.com.
Almokadem Tamer, Email: tamer_5632563@hotmail.com.
Mohamed Saad, Email: Mohamed.atya@med.tanta.edu.eg.
References
- 1.Kdoqi K. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49:S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes. Diabetes Care. 2007;30(Suppl. 1):S4–41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 3.O’Hare AM, Hailpern SM, Pavkov ME, Rios-Burrows N, Gupta I, Maynard C, et al. Prognostic implications of the urinary albumin to creatinine ratio in veterans of different ages with diabetes. Arch Intern Med. 2010;170:930–6. doi: 10.1001/archinternmed.2010.129. [DOI] [PubMed] [Google Scholar]
- 4.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin dependent patients. N Engl J Med. 1984;311:89–93. doi: 10.1056/NEJM198407123110204. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen MM, Christiansen JS, Pedersen EB, Mogensen CE. Determinants of intra-individual variation in kidney function in normoalbuminuric insulin-dependent diabetic patients: importance of atrial natriuretic peptide and glycaemic control. Clin Sci. 1992;83:445–51. doi: 10.1042/cs0830445. [DOI] [PubMed] [Google Scholar]
- 6.Kania K, Byrnes EA, Beilby JP, Webb SA, Strong KJ. Urinary proteases degrade albumin: implications for measurement of albuminuria in stored samples. Ann Clin Biochem. 2010;47(Pt 2):151–7. doi: 10.1258/acb.2009.009247. [DOI] [PubMed] [Google Scholar]
- 7.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury aftercardiacsurgery. Clin J Am Soc Nephrol. 2009;4:873–82. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagshaw SM, Bellomo R. Early diagnosis of acute kidney injury. Curr Opin Crit Care. 2007;13:638–44. doi: 10.1097/MCC.0b013e3282f07570. [DOI] [PubMed] [Google Scholar]
- 9.Prashant P, Sulaiman KJ, Kadaha G, Bazarjani N, Bakir S, El Jabri K, et al. Prevalence and risk factors for albuminuria among type 2 diabetes mellitus patients: a Middle- East perspective. Diabet Res Clin Pract. 2010;88:24–7. doi: 10.1016/j.diabres.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Gall M-A, Hougaard P, Borch-Johnsen K, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ. 1997;314:783–8. doi: 10.1136/bmj.314.7083.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allawi J, Rao PV, Gilbert R, Scott G, Jarrett RJ, Keen H, et al. Microalbuminuria in non-insulin dependent diabetes: its prevalence in Indian compared with Europid patients. Br Med J. 1988;296:462–4. doi: 10.1136/bmj.296.6620.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanakamani J, Ammini AC, Gupta N, Dwivedi SN. Prevalence of microalbuminuria among patients with type 2 diabetes mellitus–a hospital-based study from north India. Diabetes Technol Ther. 2010;12:161–6. doi: 10.1089/dia.2009.0133. [DOI] [PubMed] [Google Scholar]
- 13.Alrawahi AH, Rizvi SGA, Al-Riyami D, Al-Anqoodi Z. Prevalence and risk factors of diabetic nephropathy in omani type 2 diabetics in Al-Dakhiliyah Region. Oman Med J. 2012;27:212–6. doi: 10.5001/omj.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitsnefes M, Kathman T, Mishra J, Kartal J, Khoury PR, Nickolas TL, et al. Serum NGAL as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol. 2007;22:1018. doi: 10.1007/s00467-006-0244-x. [DOI] [PubMed] [Google Scholar]
- 15.Devarajan P. Neutrophil gelatinase-associated lipocalin –an emerging troponin for kidney injury. Nephrol Dial Transpln. 2008;23:3737–43. doi: 10.1093/ndt/gfn531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malyszko J, Bachorzewska G, Sitniewska E, Malyszko JS, Poniatowski B, Dobrzycki S. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in non-diabetic patients with stage 2–4 chronic kidney disease. Ren Fail. 2008;30:1–4. doi: 10.1080/08860220802134607. [DOI] [PubMed] [Google Scholar]
- 17.Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH. Progression of diabetic nephropathy. Kidney Int. 2001;59:702–9. doi: 10.1046/j.1523-1755.2001.059002702.x. [DOI] [PubMed] [Google Scholar]
- 18.Rossing P. Risk factors in the progression of diabetic nephropathies. Ugeskr Laeger. 2000;162:5057–61. [PubMed] [Google Scholar]
- 19.Murussi M, Gross JL, Silveiro SP. Glomerular filtration rate changes in normoalbuminuric and microalbuminuric type 2 diabetic patients and normal individuals. A 10-year follow-up. J Diabetes Complications. 2006;20:210–5. doi: 10.1016/j.jdiacomp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Keane W, Brenner B, Zeeuw D, Grunfeld JP, McGill J, Mitch WE, et al. The risk of developing endstage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003;63:1499–507. doi: 10.1046/j.1523-1755.2003.00885.x. [DOI] [PubMed] [Google Scholar]
- 21.Jamal S, Alwakeel A, Arthur C, Isnani A, Abdulkareem A, AlHarbi AB, et al. Factors affecting the progression of diabetic nephro-pathy and its complications: a single-center experience in Saudi Arabia. Ann Saudi Med. 2011;31:236–42. doi: 10.4103/0256-4947.81528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko K, Chiba M, Hashizume M, Kunii O, Sasaki S, Shimoda T, et al. Renal tubular dysfunction in children living in the Aral Sea Region. Arch Dis Cild. 2003;88:966–8. doi: 10.1136/adc.88.11.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdel Shakour S, el-Hefnawy H, el-Yamani M, Azmi Y. Urinary N-acetyl-beta-D-glucosaminidase in children with diabetes as an early marker of diabetic nephropathy. East Mediterr Health J. 2002;8:24–30. [PubMed] [Google Scholar]
- 24.Kuźniar J, Marchewka Z, Lembas-Bogaczyk J, Kuźniar TJ, Klinger M. Etiology of increased enzymuria in different morphological forms of glomerulonephritis. Nephron Physiol. 2004;98:8–14. doi: 10.1159/000079932. [DOI] [PubMed] [Google Scholar]
- 25.Navarro JF, Mora C, Muros M, Maca M, Garca J. Effects of pentoxifylline administration on urinary N-acetyl- beta-glucosaminidase excretion in type 2 diabetic patients: a short-term, prospective, randomized study. Am J Kidney Dis. 2003;422:264–70. doi: 10.1016/S0272-6386(03)00651-6. [DOI] [PubMed] [Google Scholar]
- 26.Nauta F, Bakker S, Oeveren W, Navis G, van der Heide JJ, van Goor H, et al. Albuminuria, proteinuria, and novel urine biomarkers as predictors of long-term allograft outcomes in kidney transplant recipients. Am J Kidney Dis. 2011;57:733–43. doi: 10.1053/j.ajkd.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Vaidya VS, Niewczas MA, Ficociello LH, Johnson AC, Collings FB, Warram JH, et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-β-D-glucosaminidase. Kidney Int. 2011;79:464–70. doi: 10.1038/ki.2010.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kern E, Erhard P, Sun W, Genuth S, Weiss MF. Early urinary markers of diabetic kidney disease: a nested case–control study from the Diabetes Control And Complications Trial (DCCT) Am J Kidney Dis. 2010;55:824–34. doi: 10.1053/j.ajkd.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bazzi C, Pctrini C, Rizza V, Arrigo G, Napodano P, Paparella M, et al. Urinary N-acetyl-beta-Dglucosaminidase excretion is a marker of tubular cell dysfunction and a predictor of outcome in primary glomerulonephritis. Nephrol Dial Transplant. 2002;17:1890–6. doi: 10.1093/ndt/17.11.1890. [DOI] [PubMed] [Google Scholar]
- 30.Orfeas L, Mary C, Vishal S, et al. (Urinary N-Acetyl-_-(D)-Glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:904–12. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- 31.Sato R, Soeta S, Syuto B, Yamagishi N, Sato J, Naito Y. Urinary excretion of N-acetyl-beta-D-glucosaminidase and its isoenzymes in urinary disease. J Vet Med Sci. 2002;64:367–71. doi: 10.1292/jvms.64.367. [DOI] [PubMed] [Google Scholar]
- 32.Nikolov G, Boncheva M, Gruev T, Biljali S, Stojceva-Taneva O, Masim-Spasovska E. Urinary biomarkers in the early diagnosis of renal damage in diabetes mellitus patients. Scripta Scientifica Medica. 2013;45:58–64. doi: 10.14748/ssm.v45i3.307. [DOI] [Google Scholar]


