Abstract
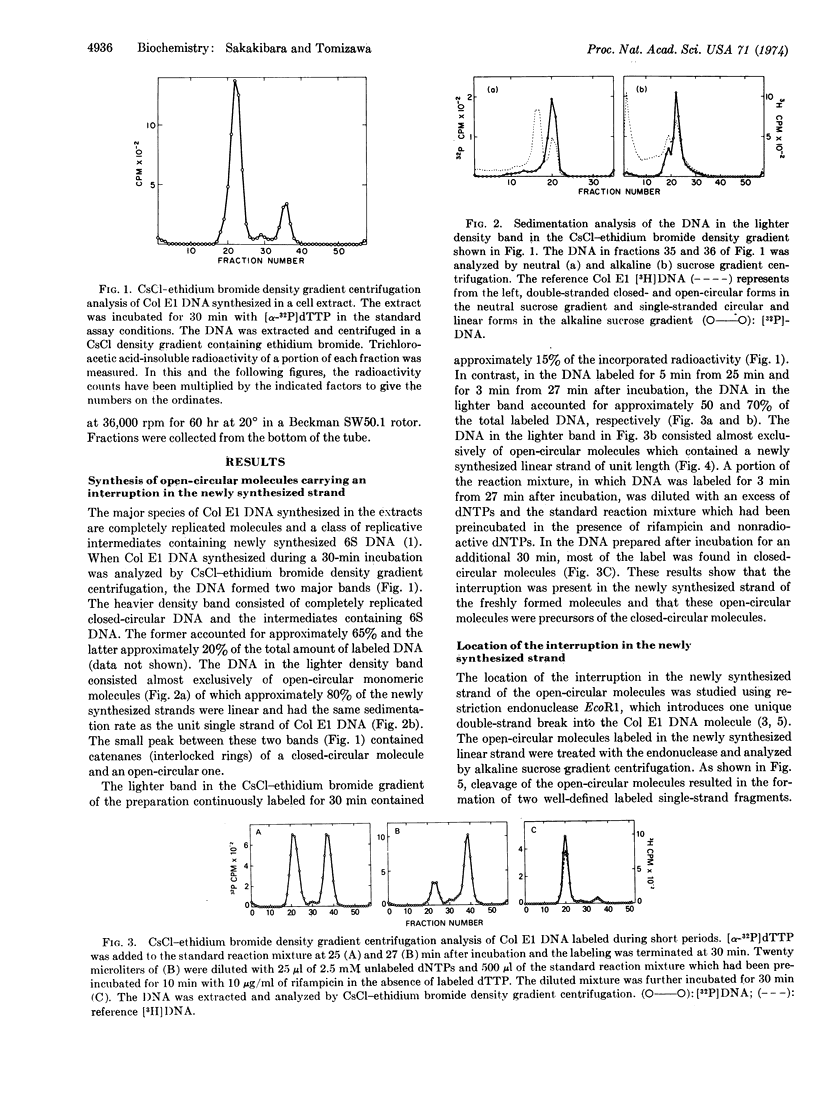
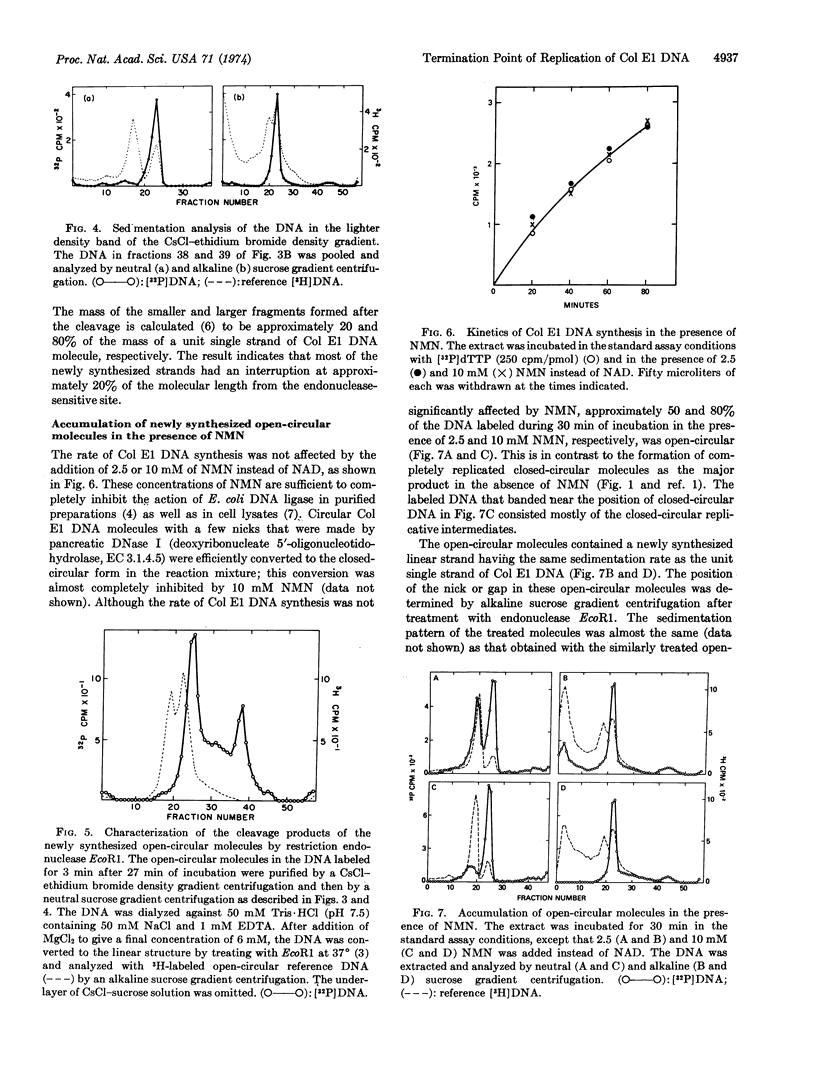
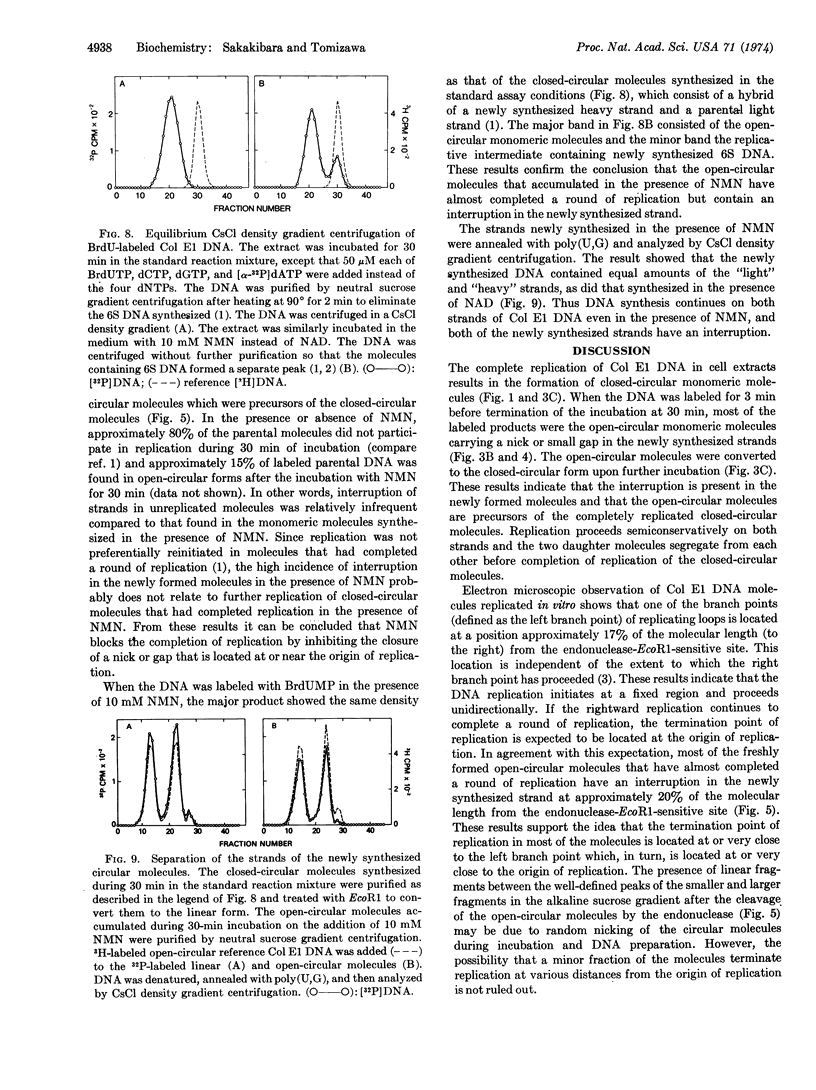
Closed-circular monomeric molecules were one of the major products of replication of colicin E1 plasmid DNA in cell extracts. However, when the plasmid DNA synthesized in the reaction mixture was labeled for 3 min after 27 min of incubation, most of the label was found in open-circular molecules. The open-circular molecules were converted to closed-circular molecules upon further incubation. The newly replicated open-circular molecules had a nick or small gap in their newly synthesized strand. The interruption was located at approximately 20% of the molecular length from the single site of cleavage by restriction endonuclease EcoR1 and at or very close to the origin of replication. The addition of 10 mM nicotinamide mononucleotide instead of nicotinamide adenine dinucleotide to extracts did not significantly affect the kinetics of the colicin E1 plasmid DNA synthesis. However, in the presence of nicotinamide mononucleotide the formation of completely replicated closed-circular molecules was suppressed and, instead, open-circular molecules accumulated with an interruption in the newly synthesized strand at the termination point of replication, which was located at or very close to the origin of replication.
Keywords: precursor, segregation, restriction endonuclease EcoR1, NMN
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., McKerlie M. L., Salzman N. P. Characterization of Simian virus 40 DNA component II during viral DNA replication. J Mol Biol. 1973 Feb 25;74(2):95–111. doi: 10.1016/0022-2836(73)90101-0. [DOI] [PubMed] [Google Scholar]
- Gellert M., Little J. W., Oshinsky C. K., Zimmerman S. B. Joining of DNA strands by DNA ligase of E. coli. Cold Spring Harb Symp Quant Biol. 1968;33:21–26. doi: 10.1101/sqb.1968.033.01.007. [DOI] [PubMed] [Google Scholar]
- Inselburg J. Replication of colicin E1 plasmid DNA in minicells from a unique replication initiation site. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2256–2259. doi: 10.1073/pnas.71.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Bonhoeffer F. Replication of Phi-X174 DNA by Escherichia coli polA- in vitro (Phi-X174 DNA-DNA replication-E. coli polA-). Proc Natl Acad Sci U S A. 1972 Jan;69(1):25–29. doi: 10.1073/pnas.69.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y., Tomizawa J. I. Replication of colicin E1 plasmid DNA in cell extracts. Proc Natl Acad Sci U S A. 1974 Mar;71(3):802–806. doi: 10.1073/pnas.71.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Tomizawa J. Replication of colicin E1 plasmid DNA in cell extracts. II. Selective synthesis of early replicative intermediates. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1403–1407. doi: 10.1073/pnas.71.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak H. F., Griffith J., Geider K., Schaller H., Kornberg A. Initiation of deoxyribonucleic acid synthesis. VII. A unique location of the gap in the M13 replicative duplex synthesized in vitro. J Biol Chem. 1974 May 25;249(10):3049–3054. [PubMed] [Google Scholar]
- Tomizawa J., Sakakibara Y., Kakefuda T. Replication of colicin E1 plasmid DNA in cell extracts. Origin and direction of replication. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2260–2264. doi: 10.1073/pnas.71.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard O., Brutlag D., Kornberg A. Initiation of deoxyribonucleic acid synthesis. IV. Incorporation of the ribonucleic acid primer into the phage replicative form. J Biol Chem. 1973 Feb 25;248(4):1361–1364. [PubMed] [Google Scholar]



