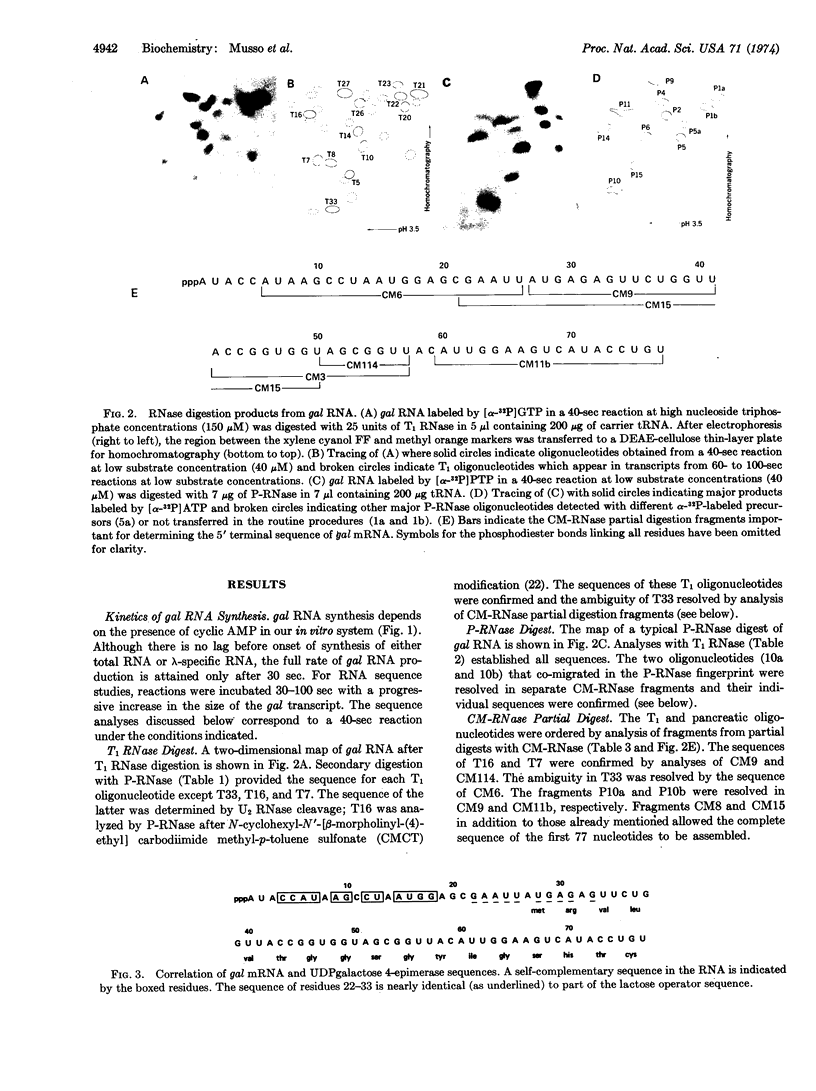
Abstract
The 5′-terminal sequence of mRNA from the galactose operon of E. coli has been determined. gal RNA is synthesized in vitro by means of a kinetically controlled purified transcription system and is isolated by a two-step RNA·DNA hybridization scheme. The following sequence of the first 77 nucleotides has been deduced by analysis of oligonucleotides produced by digestion with T1, pancreatic, and carboxymethylated pancreatic ribonucleases: ppA-U-A-C-C-A-U-A-A-G-C-C-U-A-A-U-G-G-A-G-C-G-A-A-U-U-A-U-G-A-G-A-G-U-U-C-U-G-G-U-U-A-C-C-G-G-U-G-G-U-A-G-C-G-G-U-U-A-C-A-U-U-G-G-A-A-G-U-C-A-U-A-C-C-U-G-U. Residues 27-77 correspond to the amino terminal 17 amino acids of UDPgalactose 4-epimerase (EC 5.1.3.2), the protein specified by the promoter-proximal structural gene of the operon. A self-complementary sequence occurs near the 5′ terminus; 12 of 15 nucleotides between residues 4 and 18 are symmetrically located about position 11. The sequence of residues 22-33 closely resembles part of the lactose operator sequence reported previously [Gilbert, W. & Maxam, A. (1973) Proc. Nat. Acad. Sci. USA 70, 3581-3584].
Keywords: galactose operon, UDPgalactose 4-epimerase, regulation, transcription, RNA sequences
Full text
PDF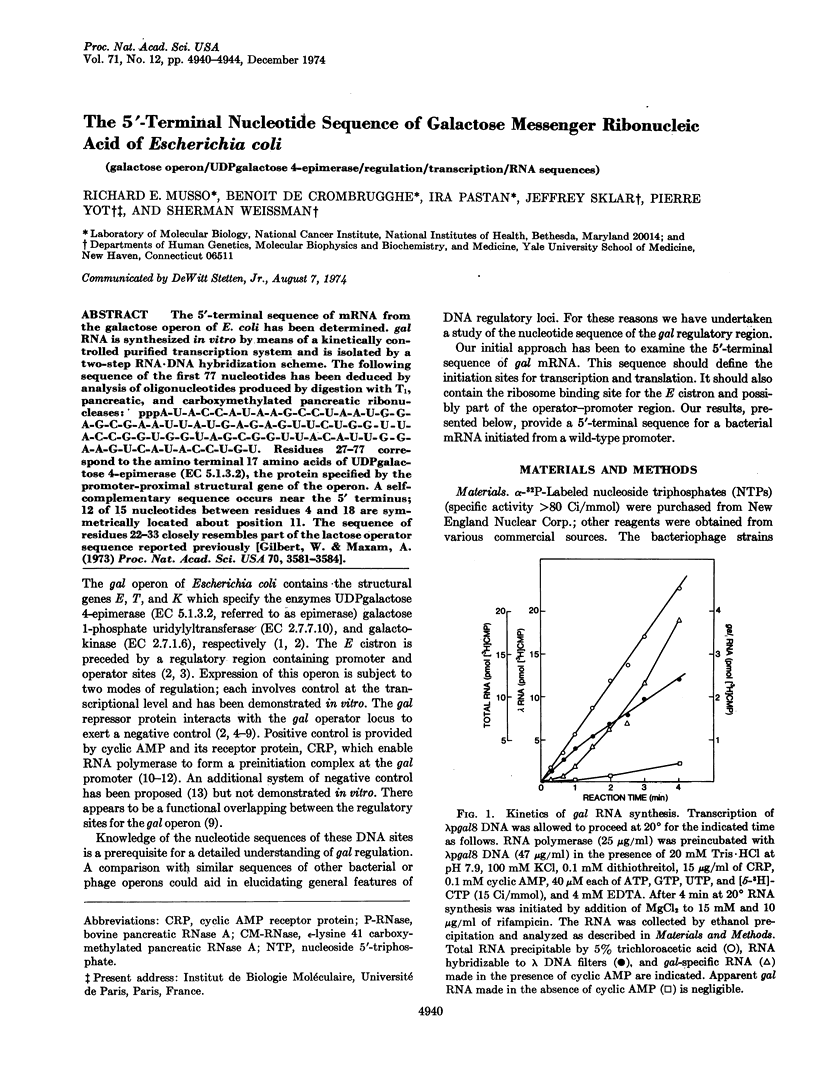



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Echols H. Glucose effect and the galactose enzymes of Escherichia coli: correlation between glucose inhibition of induction and inducer transport. J Bacteriol. 1966 Sep;92(3):601–608. doi: 10.1128/jb.92.3.601-608.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTIN G. M'ECANISMES R'EGULATEURS DANS LA BIOSYNTH'ESE DES ENZYMES DU M'ETABOLISME DU GALACTOSE CHEZ ESCHERICHIA COLI K12. I. LA BIOSYNTH'ESE INDUITE DE LA GALACTOKINASE ET L'INDUCTION SIMULTAN'EE DE LA S'EQUENCE ENZYMATIQUE. J Mol Biol. 1963 Aug;7:164–182. doi: 10.1016/s0022-2836(63)80044-3. [DOI] [PubMed] [Google Scholar]
- BUTTIN G. M'ECANISMES R'EGULATEURS DANS LA BIOSYNTH'ESE DES ENZYMES DU M'ETABOLISME DU GALACTOSE CHEZ ESCHERICHIA COLI K12. II. LE D'ETERMINISME G'EN'ETIQUE DE LA R'EGULATION. J Mol Biol. 1963 Aug;7:183–205. doi: 10.1016/s0022-2836(63)80045-5. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Dahlberg J. E. RNA synthesis startpoints in bacteriophage lambda: are the promoter and operator transcribed? Nat New Biol. 1972 Jun 21;237(77):227–232. doi: 10.1038/newbio237227a0. [DOI] [PubMed] [Google Scholar]
- Bronson M. J., Squires C., Yanofsky C. Nucleotide sequences from tryptophan messenger RNA of Escherichia coli: the sequence corresponding to the amino-terminal region of the first polypeptide specified by the operon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2335–2339. doi: 10.1073/pnas.70.8.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras R., Fiers W. A new method for partial digestion useful for sequence analysis of polynucleotides. FEBS Lett. 1971 Sep 1;16(4):281–283. doi: 10.1016/0014-5793(71)80370-8. [DOI] [PubMed] [Google Scholar]
- ECHOLS H., REZNICHEK J., ADHYA S. COMPLEMENTATION, RECOMBINATION, AND SUPPRESSION IN GALACTOSE NEGATIVE MUTANTS OF E. COLI. Proc Natl Acad Sci U S A. 1963 Aug;50:286–293. doi: 10.1073/pnas.50.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L. On the alkylation of amino acid residues at the active site of ribonuclease. J Biol Chem. 1966 Mar 25;241(6):1393–1405. [PubMed] [Google Scholar]
- Hua S. S., Markovitz A. Multiple regulation of the galactose operon-genetic evidence for a distinct site in the galactose operon that responds to capR gene regulation in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1974 Feb;71(2):507–511. doi: 10.1073/pnas.71.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalckar H. M., Kurahashi K., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, I. DETERMINATION OF ENZYME ACTIVITIES. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1776–1786. doi: 10.1073/pnas.45.12.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE J. C., HO N. W., GILHAM P. T. PREPARATION OF RIBOTRINUCLEOTIDES CONTAINING TERMINAL CYTIDINE. Biochim Biophys Acta. 1965 Mar 15;95:503–504. doi: 10.1016/0005-2787(65)90195-4. [DOI] [PubMed] [Google Scholar]
- Lebowitz P., Weissman S. M., Radding C. M. Nucleotide sequence of a ribonucleic acid transcribed in vitro from lambda phage deoxyribonucleic acid. J Biol Chem. 1971 Aug 25;246(16):5120–5139. [PubMed] [Google Scholar]
- Maizels N. M. The nucleotide sequence of the lactose messenger ribonucleic acid transcribed from the UV5 promoter mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3585–3589. doi: 10.1073/pnas.70.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Z., Varmus H. E., Parks J. S., Perlman R. L., Pastan I. Regulation of gal messenger ribonucleic acid synthesis in Escherichia coli by 3',5'-cyclic adenosine monophosphate. J Biol Chem. 1971 May 10;246(9):2898–2903. [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M. E., Pastan I. In vitro repression of the transcription of gas operon by purified gal repressor. Proc Natl Acad Sci U S A. 1973 Feb;70(2):334–338. doi: 10.1073/pnas.70.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M., Pastan I. Studies on the mechanism of action of the gal repressor. J Biol Chem. 1973 Sep 10;248(17):5937–5942. [PubMed] [Google Scholar]
- Nisseley S. P., Anderson W. B., Gottesman M. E., Perlman R. L., Pastan I. In vitro transcription of the gal operon requires cyclic adenosine monophosphate and cyclic adenosine monophosphate receptor protein. J Biol Chem. 1971 Aug 10;246(15):4671–4678. [PubMed] [Google Scholar]
- Parks J. S., Gottesman M., Perlman R. L., Pastan I. Regulation of galactokinase synthesis by cyclic adenosine 3',5'-monophosphate in cell-free extracts of Escherichia coli. J Biol Chem. 1971 Apr 25;246(8):2419–2424. [PubMed] [Google Scholar]
- Robertson H. D., Barrell B. G., Weith H. L., Donelson J. E. Isolation and sequence analysis of a ribosome-protected fragment from bacteriophage phiX 174 DNA. Nat New Biol. 1973 Jan 10;241(106):38–40. doi: 10.1038/newbio241038a0. [DOI] [PubMed] [Google Scholar]
- Saedler H., Gullon A., Fiethen L., Starlinger P. Negative control of the galactose operon in E. coli. Mol Gen Genet. 1968;102(1):79–88. doi: 10.1007/BF00341872. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A., Adhya S. L. The galactose operon of E. coli K-12. II. A deletion analysis of operon structure and polarity. Genetics. 1969 Jun;62(2):249–264. doi: 10.1093/genetics/62.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogerson L., Roufa D., Leder P. Characterization of the initial peptide of Q-beta RNA polymerase and control of its synthesis. Proc Natl Acad Sci U S A. 1971 Feb;68(2):276–279. doi: 10.1073/pnas.68.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples D. H., Hindley J. Ribosome binding site of Q-beta RNA polymerase cistron. Nat New Biol. 1971 Sep 15;234(50):211–212. doi: 10.1038/newbio234211a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Oligonucleotide sequence of replicase initiation site in Q RNA. Nat New Biol. 1972 Mar 22;236(64):71–75. doi: 10.1038/newbio236071a0. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Weber K. Natural read-through at the UGA termination signal of Q-beta coat protein cistron. Nat New Biol. 1971 Sep 15;234(50):206–209. doi: 10.1038/newbio234206a0. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Billeter M. A., Goodman H. M., Hindley J., Weber H. Structure and function of phage RNA. Annu Rev Biochem. 1973;42:303–328. doi: 10.1146/annurev.bi.42.070173.001511. [DOI] [PubMed] [Google Scholar]




