Abstract
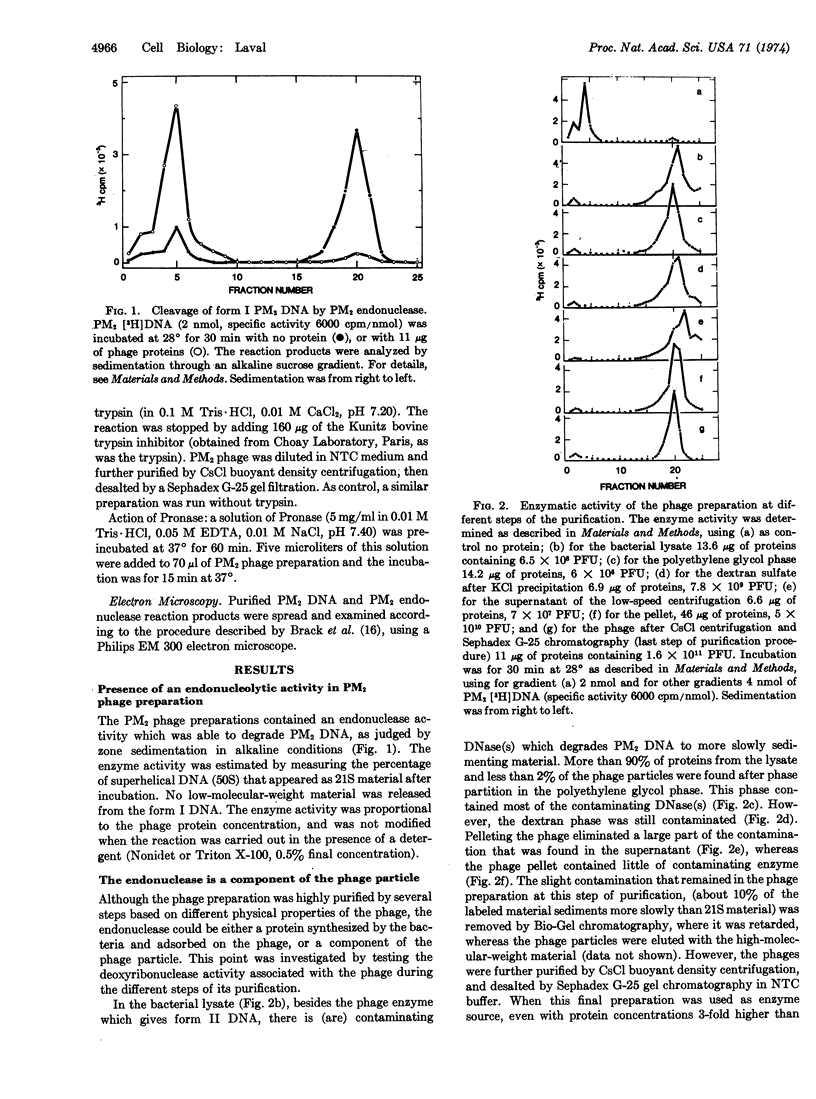
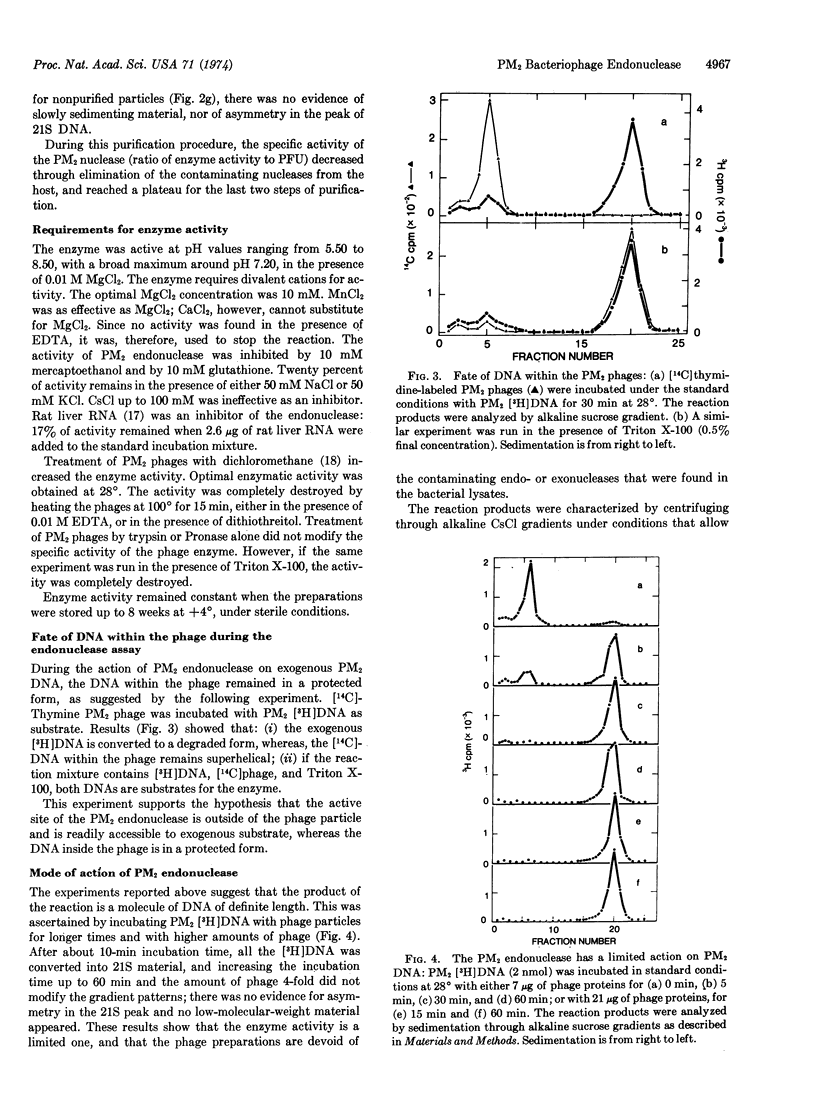
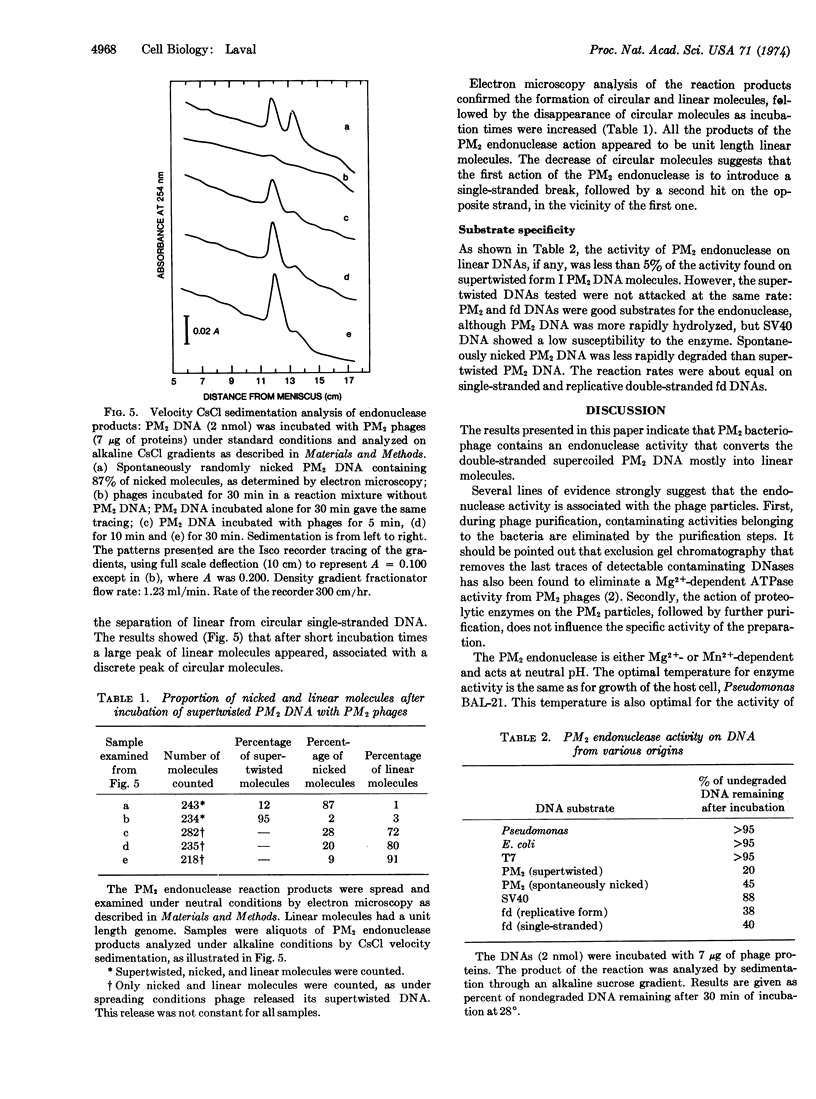
An endonucleolytic activity is associated with purified PM2 bacteriophages. It converts the double-stranded supercoiled PM2 DNA mostly into the linear form that sediments as a homogenous peak in alkaline sucrose gradients. The same activity is found in intact or detergent-lysed phages. Optimal activity is observed between pH 6.8 and 7.5 at 28°. Divalent cations, Mg2+ or Mn2+, are necessary for activity, and the enzyme is inhibited by RNA. The endonuclease has no appreciable activity on linear DNA molecules, but it attacks single-stranded circular DNA.
Keywords: DNase; zone sedimentation; electron microscopy; PM2, simian virus 40, and fd supercoiled DNA
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brack C., Delain E., Riou G. Replicating, convalently closed, circular DNA from kinetoplasts of Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1642–1646. doi: 10.1073/pnas.69.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzin F., Blangy D., Rouget P. Activité endonucléasique de préparations purifiées du virus du Polyome. C R Acad Sci Hebd Seances Acad Sci D. 1971 Dec 20;273(25):2650–2653. [PubMed] [Google Scholar]
- Datta A., Camerini-Otero R. D., Braunstein S. N., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. VII. Structural proteins of bacteriophage PM2. Virology. 1971 Jul;45(1):232–239. doi: 10.1016/0042-6822(71)90130-9. [DOI] [PubMed] [Google Scholar]
- Datta A., Franklin R. M. DNA-dependent RNA polymerase associated with bacteriophage PM2. Nat New Biol. 1972 Apr 5;236(66):131–passim. doi: 10.1038/newbio236131a0. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. Replication of bacteriophage PM2 deoxyribonucleic acid: a closed circular double-stranded molecule. J Mol Biol. 1971 Mar 28;56(3):597–621. doi: 10.1016/0022-2836(71)90404-9. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Vogt V. M., Hirt B. Characterization of the single-strand-specific nuclease S1 activity on double-stranded supercoiled polyoma DNA. Eur J Biochem. 1974 Apr 16;43(3):591–600. doi: 10.1111/j.1432-1033.1974.tb03446.x. [DOI] [PubMed] [Google Scholar]
- HIATT H. H. A rapidly labeled RNA in rat liver nuclei. J Mol Biol. 1962 Aug;5:217–229. doi: 10.1016/s0022-2836(62)80085-0. [DOI] [PubMed] [Google Scholar]
- Henry T. J., Knippers R. Isolation and function of the gene A initiator of bacteriophage phi-chi 174, a highly specific DNA endonuclease. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1549–1553. doi: 10.1073/pnas.71.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. C., Wilbert S. M., Black P. H. Endonuclease activity associated with purified simian virus 40 virions. J Virol. 1972 May;9(5):800–803. doi: 10.1128/jvi.9.5.800-803.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell W. R., Saral R., Martin R. G., Ozer H. L. Characterization of an endonuclease associated with simian virus 40 virions. J Virol. 1972 Sep;10(3):410–416. doi: 10.1128/jvi.10.3.410-416.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laval F., Malaise E., Laval J. Heterologous DNA nuclear uptake by mouse DNA fibroblasts in vitro and its early fate. Exp Cell Res. 1970 Nov;63(1):69–77. doi: 10.1016/0014-4827(70)90332-0. [DOI] [PubMed] [Google Scholar]
- Laval J., Paoletti C. Mechanism of deoxyribonucleic acid degradation by an acid deoxyribonuclease from the snail Helix aspersa Müll. Biochemistry. 1972 Sep 12;11(19):3604–3610. doi: 10.1021/bi00769a017. [DOI] [PubMed] [Google Scholar]
- Laval J., Paoletti C. Purification and properties of an acid deoxyribonuclease from the snail Helix aspersa Müll. Biochemistry. 1972 Sep 12;11(19):3596–3603. doi: 10.1021/bi00769a016. [DOI] [PubMed] [Google Scholar]
- Le Pecq J. B. Use of ethidium bromide for separation and determination of nucleic acids of various conformational forms and measurement of their associated enzymes. Methods Biochem Anal. 1971;20:41–86. doi: 10.1002/9780470110393.ch2. [DOI] [PubMed] [Google Scholar]
- Linn S., Arber W. Host specificity of DNA produced by Escherichia coli, X. In vitro restriction of phage fd replicative form. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1300–1306. doi: 10.1073/pnas.59.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méchali M., de Recondo A. M., Girard M. Action of the S1 endonuclease from Aspergillus oryzae on simian virus 40 supercoiled component I DNA. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1306–1320. doi: 10.1016/0006-291x(73)91130-3. [DOI] [PubMed] [Google Scholar]
- Parodi A., Rouget P., Croissant O., Blangy D., Cuzin F. Endonucleolytic cleavage of polyoma virus DNA: general properties and site specificity of the virion-associated endonuclease. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):247–254. doi: 10.1101/sqb.1974.039.01.032. [DOI] [PubMed] [Google Scholar]
- Salditt M., Braunstein S. N., Camerini-Otero R. D., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. X. Improved techniques for the purification of bacteriophage PM2. Virology. 1972 Apr;48(1):259–262. doi: 10.1016/0042-6822(72)90133-x. [DOI] [PubMed] [Google Scholar]
- Silbert J. A., Salditt M., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. 3. Purification of bacteriophage PM2 and some structural studies on the virion. Virology. 1969 Dec;39(4):666–681. doi: 10.1016/0042-6822(69)90005-1. [DOI] [PubMed] [Google Scholar]


