Abstract
Two fermentation types exist in the Enterobacteriaceae family. Mixed-acid fermenters produce substantial amounts of lactate, formate, acetate, and succinate, resulting in lethal medium acidification. On the other hand, 2,3-butanediol fermenters switch to the production of the neutral compounds acetoin and 2,3-butanediol and even deacidify the environment after an initial acidification phase, thereby avoiding cell death. We equipped three mixed-acid fermenters (Salmonella Typhimurium, S. Enteritidis and Shigella flexneri) with the acetoin pathway from Serratia plymuthica to investigate the mechanisms of deacidification. Acetoin production caused attenuated acidification during exponential growth in all three bacteria, but stationary-phase deacidification was only observed in Escherichia coli and Salmonella, suggesting that it was not due to the consumption of protons accompanying acetoin production. To identify the mechanism, 34 transposon mutants of acetoin-producing E. coli that no longer deacidified the culture medium were isolated. The mutations mapped to 16 genes, all involved in formate metabolism. Formate is an end product of mixed-acid fermentation that can be converted to H2 and CO2 by the formate hydrogen lyase (FHL) complex, a reaction that consumes protons and thus can explain medium deacidification. When hycE, encoding the large subunit of hydrogenase 3 that is part of the FHL complex, was deleted in acetoin-producing E. coli, deacidification capacity was lost. Metabolite analysis in E. coli showed that introduction of the acetoin pathway reduced lactate and acetate production, but increased glucose consumption and formate and ethanol production. Analysis of a hycE mutant in S. plymuthica confirmed that medium deacidification in this organism is also mediated by FHL. These findings improve our understanding of the physiology and function of fermentation pathways in Enterobacteriaceae.
Keywords: mixed-acid fermentation, 2, 3-butanediol fermentation, acid stress, hydrogenase 3, formate hydrogen lyase
INTRODUCTION
Within the Enterobacteriaceae family, a distinction is made between mixed-acid (e.g., Escherichia, Salmonella, and Shigella) and 2,3-butanediol fermenters (e.g., Klebsiella, Serratia, and Enterobacter) based on their fermentation end products produced during sugar fermentation. Mixed-acid fermenters ferment sugars to ethanol and a range of organic acids, including lactate, succinate, acetate, and formate. Formate can be further converted to H2 and CO2 by the formate hydrogen lyase (FHL) complex (White, 2000). Mixed-acid fermentation generally leads to rapid and strong medium acidification and even cell death. On the other hand, 2,3-butanediol fermenters use the mixed-acids pathway only during the early growth phase, and switch in the late exponential phase to a different fermentation pathway, in which pyruvate is converted to the neutral end products acetoin or 2,3-butanediol, thereby preventing excessive acidification (Van Houdt et al., 2006; Xiao and Xu, 2007). Moreover, after the initial decline of medium pH, 2,3-butanediol fermenters typically deacidify the medium toward more neutral values during stationary phase (Johansen et al., 1975; Yoon and Mekalanos, 2006; Van Houdt et al., 2007; Moons et al., 2011). This is in contrast to mixed-acid fermenters or 2,3-butanediol fermenters with an inactivated 2,3-butanediol pathway, where a sustained pH decrease is usually observed during sugar fermentation (Yoon and Mekalanos, 2006; Moons et al., 2011). Thus, 2,3-butanediol fermentation is apparently associated with stationary-phase deacidification. Synthesis of 2,3-butanediol from pyruvate requires three steps. First, the conversion of two molecules of pyruvate to α-acetolactate is catalyzed by the α-acetolactate synthase (α-ALS). Next, α-acetolactate is decarboxylated to acetoin by the α-acetolactate decarboxylase (α-ALD). In a last step, acetoin is reduced to 2,3-butanediol by the 2,3-butanediol dehydrogenase (BDH), which can also catalyze the reversed reaction. Each of these three reactions consumes an intracellular proton, and this potentially explains the observed stationary-phase deacidification. In Serratia plymuthica RVH1, a strain previously isolated from a food processing environment (Van Houdt et al., 2005), α-ALS and α-ALD are encoded by the budB and budA genes, respectively, which are located on the budAB operon (Moons et al., 2011). We previously showed that transfer of the S. plymuthica RVH1 budAB operon conveys to Escherichia coli the capacity to produce acetoin, to prevent lethal medium acidification and to reverse acidification (Vivijs et al., 2014a). In the present study, we transferred the budAB operon to some additional mixed-acid fermenting enterobacteria, Salmonella Typhimurium, Salmonella Enteritidis, and Shigella flexneri, and show that these also acquire the capacity to produce acetoin. However, acetoin production was not associated with stationary-phase deacidification in S. flexneri. This observation is remarkable since Shigella and E. coli are considered as a single species based on DNA homology (Fukushima et al., 2002). Thus, our results suggested the involvement of a deacidification mechanism different from proton consumption during acetoin production. To identify this mechanism, we performed random transposon mutagenesis in budAB-containing E. coli searching for mutants that lost their stationary-phase deacidification capacity but still produced acetoin. This led us to identify the FHL complex as the primary deacidification mechanism in 2,3-butanediol-fermenting Enterobacteriaceae.
MATERIALS AND METHODS
BACTERIAL STRAINS, PLASMIDS, OLIGONUCLEOTIDES, AND GROWTH CONDITIONS
The bacterial strains and plasmids used in this study are listed in Table 1. All bacteria were cultured in lysogeny broth (LB; 10 g/l tryptone, 5 g/l yeast extract, 5 g/l NaCl) or on LB agar (15 g/l agar) at 37°C except Serratia plymuthica, which was grown at 30°C. Media were supplemented with the following chemicals (Applichem, Darmstadt, Germany) when appropriate: 5 g/l glucose; 100 μg/ml ampicillin (Ap); 200 μg/ml carbenicillin (Cb); 30 μg/ml chloramphenicol (Cm); 5 μg/ml gentamicin (Gm); 10 μg/ml tetracycline (Tc); 50 μg/ml kanamycin (Km); and 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG). Plasmids pTrc99A and pTrc99A-Ptrc-budAB were introduced into the mixed-acid fermenters by electroporation. All oligonucleotides used in this work are listed in Table 2, and were purchased from IDT (Haasrode, Belgium).
Table 1.
Strains and plasmids used in this study.
| Strain or plasmid | Relevant features | Reference |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| S17-1 λpir | pro thi recA hsdR- hsdM+ RP4: 2-Tc:Mu: Km Tn7 λpir | Simon et al. (1983) |
| DH5α | F- endA1 hsdR17 (rk-, mk+) supE44 thi-1λ- recA1 gyrA96 relA1 deoR Δ(lacZYA-argF)U169 Φ80d lacZΔM15 | Grant et al. (1990) |
| MG1655 | F- λ- rph-1 | Guyer et al. (1981) |
| MG1655 hycE | ΔhycE | This study |
| Salmonella enterica | ||
| Typhimurium LT2 | Wild-type | McClelland et al. (2001) |
| Enteritidis ATCC 13076 | Wild-type | Tindall et al. (2005) |
| Shigella flexneri | ||
| ATCC 12022 | Wild-type; serotype 2b | Daligault et al. (2014) |
| Serratia plymuthica | ||
| RVH1 | Wild-type; biofilm isolate from food processing plant | Van Houdt et al. (2005) |
| RVH1 budAB | ΔbudAB::cat, CmR | Vivijs et al. (2014b) |
| RVH1 hycE | ΔhycE | This study |
| Plasmids | ||
| pTrc99A | Cloning vector carrying IPTG-inducible trc promoter (Ptrc); ApR | Amann et al. (1988) |
| pTrc99A-Ptrc-budAB | pTrc99A carrying the S. plymuthica RVH1 budAB operon downstream of Ptrc; ApR | Moons et al. (2011) |
| pKD3 | Template plasmid containing cat gene flanked by FRT sites; CmR ApR | Datsenko and Wanner (2000) |
| pKD46 | Plasmid expressing γ, β, and exo recombination genes of phage λ under control of PBAD; temperature-sensitive replicon; ApR | Datsenko and Wanner (2000) |
| pCP20 | Plasmid expressing the FLP (flippase) gene, directing recombination of FRT sites; temperature-sensitive replicon; ApR CmR | Datsenko and Wanner (2000) |
| pUC18 | Cloning vector; ApR | Laboratory collection |
| pUCGmlox | pUC18-based vector containing the lox-flanked aacC1 gene; ApR GmR | Quénée et al. (2005) |
| pSF100 | pGP704 suicide plasmid; pir dependent; ApR KmR | Rubirés et al. (1997) |
| pCM157 | cre expression vector; TcR | Marx and Lidstrom (2002) |
Table 2.
Oligonucleotides used in this study.
| Primer | Sequence (5′-3′) |
|---|---|
| Linker 1 | TTTCTGCTCGAATTCAAGCTTCTAACGATGTACGGGGACACATG |
| Linker 2 | TGTCCCCGTACATCGTTAGAACTACTCGTACCATCCACAT |
| Y linker primer | CTGCTCGAATTCAAGCTTCT |
| NK_Cm_DWN | CCTCCCAGAGCCTGATAA |
| EC_HycE_pKD3_1 | GCCGTGCCGGTTTTGATGACTTTTTTGATAAAGGTAAACATGGCGATTCCATGGGAATTAGCCATGGTCC |
| EC_HycE_pKD3_2 | TTTTTAGCGTTCGTCTCCTTGCTGGCGGCGTGATTAAAGAGAGTTTGAGCGTGTAGGCTGGAGCTGCTTC |
| SP_HycE_1(XbaI) | GCAGTCTAGAATCAGCGTCTGGTTCATTGG |
| SP_HycE_2(XbaI) | ACTCTCTAGATTATCTGTTCGCCGTGGTGC |
| SP_HycE_3(XhoI) | GCGACTCGAGCATGATGTTCCTACTTGTGAATTAGC |
| SP_HycE_4(XhoI) | GCACTCGAGCGGAAAAACGCACCGTTTTAA |
| LoxP_Gm_1(XhoI) | AACTCGAGCTTCAGCTGTACAATTGGTAC |
| LoxP_Gm_2(XhoI) | AACTCGAGACCGGTTAACACGCG |
SCREENING FOR MUTANTS THAT HAVE LOST STATIONARY-PHASE DEACIDIFICATION CAPACITY
A random knockout library of E. coli MG1655 containing pTrc99A-Ptrc-budAB was constructed using λNK1324, which carries a mini-Tn10 transposon with a Cm resistance gene, according to the protocol described by Kleckner et al. (1991). The mutants were subsequently grown in 300 μl LB medium with glucose, IPTG, Ap, and Cm in a 96-well plate. The plates were sealed with an oxygen impermeable cover foil and incubated without shaking at 37°C. After 24 h, medium acidification was analyzed by adding 5 μl of a 0.06% w/v methyl red solution in 60% v/v ethanol to 200 μl culture (MR test). For mutants that no longer deacidified the medium, the remaining 100 μl culture was subjected to the Voges–Proskauer (VP) test by adding 30 μl of 5% w/v α-naphthol and 10 μl of 40% w/v KOH to 100 μl of culture. To quantify acetoin production, the mixture was stirred vigorously after 1 h and the optical density at 550 nm (OD550) was measured. Acetoin concentrations were determined using a standard curve relating the OD550 with the acetoin concentration in LB medium. From mutants that did not deacidify culture medium and still produced acetoin, transposon insertion sites were determined using the method described by Kwon and Ricke (2000). Briefly, genomic DNA of the mutants was isolated, digested with NlaIII and ligated with a Y-shaped linker, composed of oligonucleotides linker 1 and linker 2. Next, a PCR amplification was carried out using a transposon-specific primer (NK_Cm_DWN) and a primer specific to the Y-shaped linker (Y linker primer). The PCR product was subsequently sequenced using the transposon-specific primer and the insertion site was determined based on the known genome sequence of E. coli MG1655.
CONSTRUCTION OF hycE MUTANTS IN E. coli AND S. plymuthica
The deletion of hycE in E. coli MG1655 was achieved using the lambda red recombinase system described by Datsenko and Wanner (2000), followed by removal of the introduced antibiotic resistance cassette using the FRT/FLP recombination system. Briefly, 70-bp PCR primers were designed comprising a 50-bp 5′ part complementary to the region down- or upstream of hycE and a 20-bp 3′ part allowing amplification of the FRT-flanked Cm resistance cassette present in the plasmid pKD3. The purified PCR product was electrotransformed into E. coli MG1655 containing the pKD46 plasmid providing the lambda red recombinase. The resistance cassette was subsequently removed by expression of the flippase recombination enzyme (FLP) of the FRT/FLP recombination system on the temperature-sensitive pCP20 plasmid.
To delete the hycE gene in S. plymuthica RVH1, a fragment encompassing 643 bp upstream and 559 bp downstream of the gene was PCR-amplified using primers SP_HycE_1(XbaI) and SP_HycE_2(XbaI), cut with XbaI, ligated into a XbaI-digested pUC18 vector and transformed into E. coli DH5α. The resulting plasmid pUC18-hycE was used as a template for PCR using the outward-oriented primers SP_HycE_3(XhoI) and SP_HycE_4(XhoI). In a separate reaction, the loxP flanked Gm resistance cassette from plasmid pUCGmlox was amplified using primers LoxP_Gm_1(XhoI) and LoxP_Gm_2(XhoI). Both PCR products were then cleaved with XhoI and ligated together, generating pUC18-hycE::aacC1, which was transformed in E. coli DH5α. The hycE::aacC1 insert from this plasmid was then amplified using primers SP_HycE_1(XbaI) and SP_HycE_2(XbaI), cut with XbaI, ligated into a XbaI-digested pSF100 vector and transformed into E. coli S17-1 λpir. After conjugation of the resulting plasmid pSF100-hycE::aacC1 into S. plymuthica RVH1 (which does not support replication of this suicide plasmid), transconjugants were selected on LB agar with Gm at 15°C. This temperature allows good growth of S. plymuthica but prevents growth of E. coli S17-1 λpir. Loss of Km resistance (pSF100 marker) was assessed by replica plating on LB agar with Km. The Gm resistance cassette was then spliced out using the cre recombinase on plasmid pCM157, which catalyzes site specific recombination between loxP sites. Restriction endonucleases and T4 DNA ligase were purchased from Thermo Scientific (St. Leon Rot, Germany) and used according to the supplier’s instructions.
CHARACTERIZATION OF FERMENTATIVE GROWTH AND FERMENTATION END PRODUCTS
Strains were first grown overnight at the appropriate incubation temperature in 4 ml LB. For strains containing pTrc99A or pTrc99A-Ptrc-budAB, Ap was added to ensure plasmid maintenance. Since S. plymuthica RVH1 is somewhat Ap resistant, Cb was used instead of Ap. Next, the cultures were diluted 1:1000 in tubes containing 30 ml LB with glucose and, when appropriate, IPTG and Ap or Cb. Five ml of paraffin oil was layered on top of the cultures to create anaerobic conditions and the tubes were incubated at the appropriate incubation temperature for 48 h. The cultures were sampled at regular time points to determine cell concentrations, medium pH and acetoin concentration, and for analysis of fermentation end products. Plate counts were determined by spot-plating (5 μl) a decimal dilution series in potassium phosphate buffer (10 mM; pH 7.00) on LB agar. Gas production was evaluated qualitatively using Durham tubes. Fermentation end products were analyzed in 600 μl culture supernatants stored at –20°C. Succinic, lactic, formic, and acetic acid, ethanol, and glucose were determined via high-performance liquid chromatography (HPLC; Agilent 1200 series) using an ion exclusion column (Aminex® HPX-87H) maintained at 55°C, and with 5 mM H2SO4 as the mobile phase (0.6 ml/min). The system was equipped with a refractive index detector operating at 35°C and a diode array detector set at 210 nm.
STATISTICAL ANALYSIS
All experiments were carried out in triplicate using independent cultures, and results are presented as the mean values ± SD. Statistical significance between mean values were determined by Student’s t-test analysis using the Microsoft Excel statistical package. Results were reported as significant when a p-value of <0.05 was obtained, based on a two-sided t-test with unequal variance.
RESULTS AND DISCUSSION
INTRODUCTION OF ACETOIN SYNTHESIS PATHWAY IN MIXED-ACID FERMENTERS
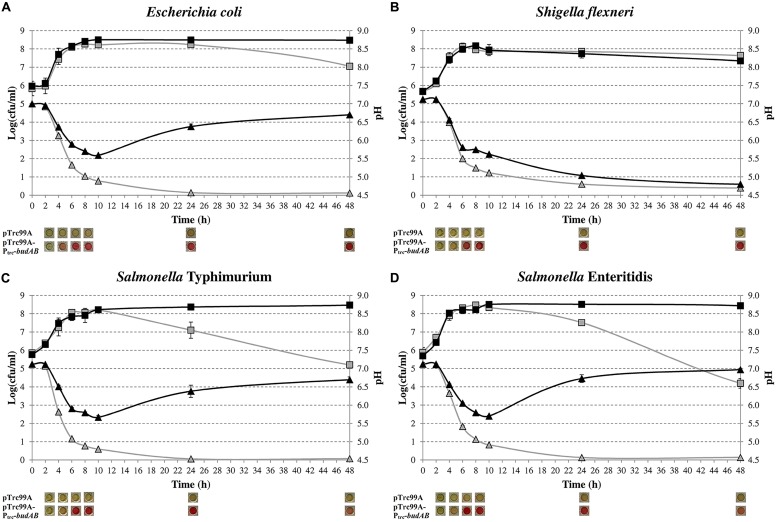
Previously, we introduced the budAB operon from S. plymuthica RVH1, encoding the α-ALS and α-ALD of the acetoin synthesis pathway, in E. coli MG1655 and observed that this attenuated lethal medium acidification during fermentative growth on glucose (Vivijs et al., 2014a). Here, we extended this experiment to S. Typhimurium, S. Enteritidis, and S. flexneri by introducing the pTrc99A-Ptrc-budAB plasmid into these organisms to see whether other mixed-acid fermenters would show a similar behavior. Figure 1 shows the growth curves and medium pH during fermentative growth in glucose-containing LB medium of these bacteria with and without the budAB genes. As expected, E. coli, both Salmonella strains and S. flexneri without budAB strongly acidified the medium (to pH 4.50–4.70 after 48 h) and this resulted in cell death during the stationary phase. Introduction of the budAB genes did not change growth of the bacteria until stationary phase was reached, but it changed the pH profile of the E. coli and Salmonella cultures in two aspects. Firstly, the acidification during the growth phase was less strong, reaching a minimum pH of about 5.60. Secondly, the pH increased again during stationary phase, up to 6.60–7.00 after 48 h. As a result, plate counts remained almost constant once they had reached their maximal stationary phase level (10–48 h).
FIGURE 1.
Growth of Escherichia coli MG1655 (A), S. flexneri ATCC 12022 (B), S. Typhimurium LT2 (C) and S. Enteritidis ATCC 13076 (D) in LB medium containing 5 g/l glucose, 1 mM IPTG and 100 μg/ml ampicillin (Ap), in sealed microtiter plates incubated at 37°C for 48 h. Cell numbers (squares) and medium pH (triangles) of strains harboring pTrc99A (gray) or pTrc99A-Ptrc-budAB (black) are shown. Pictures below the figures show the results of the VP test, with a red color indicating the presence of acetoin. Error bars represent SD.
Surprisingly, a different pattern was observed in S. flexneri. Introduction of the budAB genes also attenuated medium acidification during the growth phase (pH 5.60 after 10 h), but no deacidification occurred during stationary phase. As a result, this culture reached a final pH of 4.80 after 48 h and the plate counts decreased to a similar extent as those of the strain without budAB genes. The strain with the budAB genes produced acetoin in similar amounts as the E. coli and Salmonella strains carrying these genes, so that poor expression of the acetoin pathway could be ruled out to explain the different behavior of S. flexneri. Therefore, proton consumption in the acetoin production pathway cannot fully explain the deacidification during stationary phase in E. coli and Salmonella, and it can be concluded that other deacidification mechanisms must be involved.
SCREENING FOR LOSS OF DEACIDIFICATION CAPACITY IN E. coli CONTAINING A FUNCTIONAL ACETOIN PATHWAY
In order to identify additional mechanisms involved in stationary-phase deacidification, we performed random transposon mutagenesis in E. coli MG1655 containing the pTrc99A-Ptrc-budAB plasmid and searched for mutants that were unaffected in acetoin production (VP test), yet were no longer able to increase the pH of glucose-containing LB medium at 37°C after 24 h (MR test), thus having a MR+/VP+ phenotype. Although in most Enterobacteriaceae a positive VP test is usually associated with a negative MR test (MR–/VP+, e.g., Enterobacter aerogenes) and vice versa (MR+/VP–, e.g., E. coli), there are also some species in this family (e.g., Enterobacter intermedius, Klebsiella planticola, or Serratia liquefaciens) reported to be positive for both tests (MR+/VP+; Holt et al., 1994).
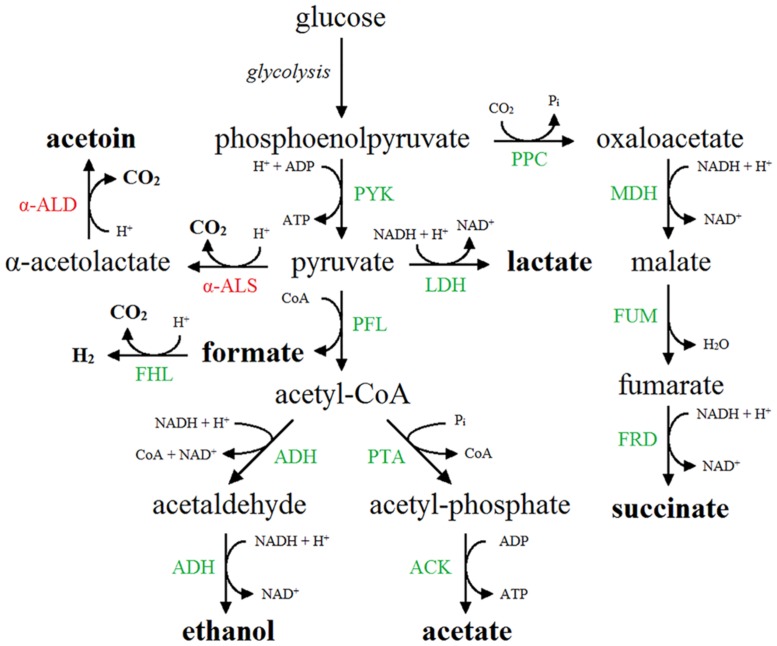
Out of 6.048 mutants screened, 34 MR+/VP+ mutants were identified and their phenotype was confirmed after transferring the mutation to a native MG1655 strain by P1-transduction, followed by transformation of pTrc99A-Ptrc-budAB. Identification of the transposon insertion sites of these 34 mutants led to 16 different genes (Table 3). Interestingly, all genes were related to the metabolism of formate, one of the acids formed by mixed-acid fermentation. Formate is produced by the pyruvate formate lyase (PFL) enzyme, which catalyzes the CoA-dependent cleavage of pyruvate to formate and acetyl-CoA (Sawers and Böck, 1988). An overview of the fermentation routes present in E. coli containing pTrc99A-Ptrc-budAB is shown in Figure 2. The formate that is produced and secreted can also be reimported in the cell through the FocA channel and become disproportionated to CO2 and H2 by the membrane-associated FHL complex (Sawers, 2005; Lü et al., 2012; Beyer et al., 2013). This complex consists of the formate dehydrogenase H (FDH-H), a selenoprotein carrying a molybdenum cofactor, and hydrogenase 3, a nickel-containing protein complex (Bagramyan and Trchounian, 2003). FDH-H catalyzes the oxidation of formate (HCOO-), generating CO2 and H+. The electrons from this reaction are transferred via several subunits of the FHL complex to hydrogenase 3, where they combine with two cytoplasmic protons to form dihydrogen. This pathway is thus a net consumer of protons and is used by E. coli to counteract acidification (Leonhartsberger et al., 2002). All gene products found in our screening could be linked to this particular pathway: FdhF (FDH-H), HycB, HycD, and HycE are part of the FHL complex (Bagramyan and Trchounian, 2003); HycI and HypE are both involved in maturation of the large subunit of hydrogenase 3 (Forzi and Sawers, 2007); SelA and SelD take part in the biosynthesis of selenocysteine, and mutants lacking these gene products fail to synthesize FDH-H (Leinfelder et al., 1988; Driscoll and Copeland, 2003); ModC is the ATP binding subunit of the molybdate ABC transporter and MoeA, MoeB, and Mog are other ancillary enzymes that participate in the biosynthesis of the molybdenum cofactor (Sawers, 1994; Grunden and Shanmugam, 1997; Leimkühler et al., 2001; Nichols and Rajagopalan, 2002); FdhD is an accessory protein functioning as a sulfurtransferase between IscS and FdhF and is required for FDH activity (Thomé et al., 2012); FocA and PflB are coexpressed from a single operon and form a bidirectional formate channel and the PFL enzyme, respectively (Lü et al., 2012); FhlA, finally, is a transcriptional activator of the FHL system (Leonhartsberger et al., 2002). In conclusion, the mutant screening approach provides a strong indication that the disproportionation of formate is responsible for the stationary-phase deacidification capacity in E. coli containing the budAB genes.
Table 3.
List of genes knocked out in transposon insertion mutants of E. coli MG1655 containing pTrc99A-Ptrc-budAB that had lost the stationary-phase deacidification capacity but still produced acetoin (MR+/VP+).
| Gene | Description |
|---|---|
| fdhD | Redox enzyme maturation protein (REMP) for FdnG/FdoG; required as a sulfurtransferase for FDH activity |
| fdhF | FDH-H |
| fhlA | FHL system activator |
| focA | Formate channel |
| hycB | FHL complex iron–sulfur protein |
| hycD | FHL complex inner membrane protein |
| hycE | Hydrogenase 3 large subunit |
| hycI | Maturation endoprotease for hydrogenase 3 large subunit HycE |
| hypE | Maturation protein required for the assembly of the CN ligand of the NiFe metal center of hydrogenase 1, 2, and 3. |
| modC | ATP binding subunit of the molybdate ABC transporter |
| moeA | Molybdopterin molybdenumtransferase |
| moeB | Molybdopterin-synthase adenylyltransferase |
| mog | Molybdochelatase incorporating molybdenum into molybdopterin |
| pflB | Pyruvate formate lyase |
| selA | Selenocysteine synthase |
| selD | Selenophosphate synthase |
FIGURE 2.
Mixed-acid fermentation pathway in E. coli expressing the acetoin synthesis operon (budAB operon). End products are shown in boldface. Native enzymes are shown in green. The enzymes of the additional acetoin synthesis pathway, in which pyruvate is converted to α-acetolactate by α-ALS (BudB) and further to acetoin by α-ALD (BudA), are shown in red. α-ALS, α-acetolactate synthase; α-ALD, α-acetolactate decarboxylase; ACK, acetate kinase; ADH, acetaldehyde dehydrogenase/alcohol dehydrogenase; FHL, formate hydrogen lyase complex; FRD, fumarate reductase; FUM, fumarase; LDH, lactate dehydrogenase; MDH, malate dehydrogenase; PFL, pyruvate formate lyase; PPC, phosphoenolpyruvate carboxylase; PTA, phosphate acetyltransferase; PYK, pyruvate kinase.
In addition to hydrogenase 3, E. coli also possesses three other hydrogenases catalyzing the reversible reaction 2H+ + 2e- ↔ H2. Hydrogenase 1 and 2 are H2-oxidizing enzymes which are maximally induced at low and alkaline pH, respectively (Trchounian et al., 2012). Hydrogenase 4 is not well characterized and its subunits have not been isolated and studied yet, but it may be part of a second FHL complex that may produce H2 at neutral and slightly alkaline pH (Self et al., 2004; Trchounian and Sawers, 2014). The contribution of hydrogenase 3 to acid resistance has been demonstrated previously since anaerobic cultures of E. coli W3110 ΔhycE showed a 20-fold loss in survival of an extreme acid stress (2 h at pH 2.0) when compared to the wild-type strain (Noguchi et al., 2010). This finding suggested that the FHL complex supports survival of extreme acid challenge by counteracting intracellular acidification. Our results now show that the complex can also accomplish an increase of the environmental pH during growth under moderate acid stress, thereby preventing stationary phase cell death during fermentative growth.
The observation that acetoin-producing S. flexneri showed reduced acidification in the exponential growth phase, but did not deacidify the medium during the stationary phase (Figure 1), can also be linked to formate conversion. Although S. flexneri closely resembles E. coli at the genetic level, Shigella species (with the exception of a few strains) do not produce gas during carbohydrate fermentation (Brenner et al., 1982; Germani and Sansonetti, 2006). We confirmed that the S. flexneri strain used in this study did not produce gas from glucose and the absence of this mechanism may thus explain our observation. The reason why Shigella species do not produce gas in the presence of glucose is unclear. The genes encoding the FDH-H and the hydrogenase 3 are present in the Shigella genome, but apparently no functional FHL complex is formed.
EFFECT OF HYDROGENASE 3 INACTIVATION ON FERMENTATIVE GROWTH OF ACETOIN-PRODUCING E. coli
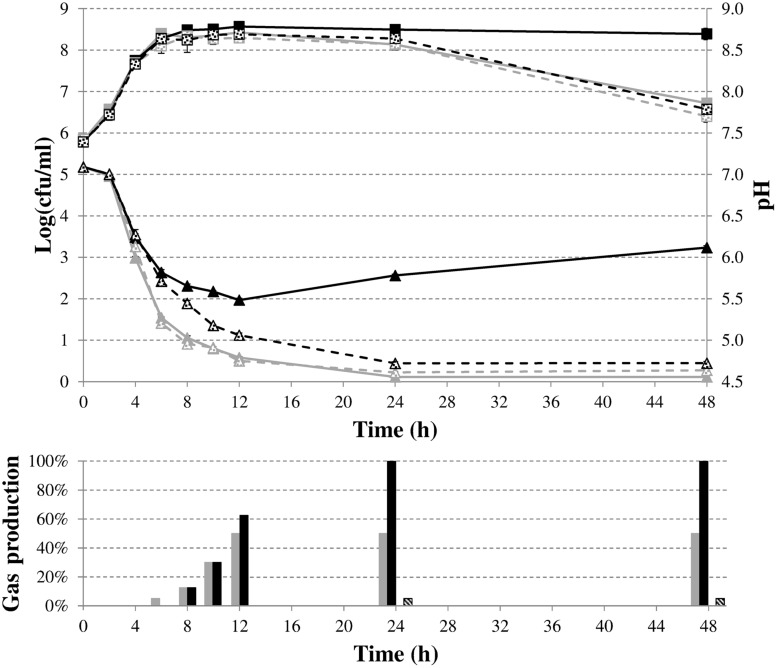
To characterize in more detail the role of formate disproportionation on the capacity of E. coli (with or without budAB genes) to attenuate medium acidification during fermentative growth, we constructed a clean deletion of the hycE gene. Since this gene encodes the large subunit of the hydrogenase 3 that contains the active site for proton reduction to dihydrogen (Trchounian et al., 2012), its deletion completely blocks the conversion of formate to CO2 and H2. Next, budAB-less and budAB-containing wild-type and ΔhycE strains of E. coli MG1655 were grown for 48 h in glucose-containing LB medium sealed from the air with a paraffin oil layer and with a Durham tube to observe gas production. Plate counts, medium pH, gas production and acetoin concentrations were determined at regular time points (Figure 3; Table 4). Knockout of hycE did not have any effect on the pH profile during fermentative growth of budAB-less E. coli over the entire 48 h growth period. In the budAB-containing strains, the effect of hycE deletion depended on the growth phase. During the exponential phase (first 6 h), hycE deletion had no effect on the acidification, but it can be seen that the acidification was slightly less compared to the two budAB-less strains, in line with the earlier observations shown in Figure 1. However, from the onset of stationary phase, the pH profile of both acetoin-producing strains diverged strongly. While acidification by the budAB-containing wild-type strain slowed down and reversed into deacidification after 12 h of growth (as already shown in Figure 1), acidification by the budAB-containing ΔhycE mutant was sustained until 24 h, after which the pH remained stable at a low value (pH = 4.72). Since both strains produced similar amounts of acetoin, and acetoin production stopped after 10 h (Table 4), it can be concluded that the stationary-phase deacidification by the budAB-containing wild-type E. coli MG1655 is not a direct consequence of proton consumption during acetoin production.
FIGURE 3.
Cell numbers (squares) and medium pH (triangles) during fermentative growth of E. coli MG1655 wild-type (solid lines) or ΔhycE (dashed lines) containing pTrc99A (gray) or pTrc99A-Ptrc-budAB (black) in LB medium with 5 g/l glucose, 1 mM IPTG and 100 μg/ml Ap at 37°C for 48 h. Error bars represent SD. Gas production (expressed as % of the volume of the Durham tube) is shown at the bottom.
Table 4.
Acetoin production (in mM) by E. coli MG1655 containing pTrc99A-Ptrc-budAB or E. coli MG1655 ΔhycE containing pTrc99A-Ptrc-budAB during fermentative growth in LB with 5 g/l glucose, 1 mM IPTG, and 100 μg/ml ampicillin (Ap) for 48 h.
| Time (h) | E. coli MG1655 pTrc99A-Ptrc-budAB | E. coli MG1655 ΔhycE pTrc99A-Ptrc-budAB |
|---|---|---|
| 4 | 0.7 ± 0.2 | 0.5 ± 0.2 |
| 6 | 5.3 ± 0.7 | 5.0 ± 0.4 |
| 8 | 9.8 ± 0.9 | 7.1 ± 1.3 |
| 10 | 21.8 ± 0.8 | 18.4 ± 0.5 |
| 12 | 19.4 ± 1.2 | 12.5 ± 1.1 |
| 24 | 18.7 ± 0.7 | 12.3 ± 0.5 |
| 48 | 20.5 ± 2.3 | 11.1 ± 2.3 |
More likely, deacidification is triggered by proton consumption in the reaction carried out by the FHL complex since deletion of hycE resulted in loss of deacidification. This explanation is also supported by the observed gas production. Since CO2 is very soluble in water, gas accumulation in a Durham tube can be mainly ascribed to H2 production, and is thus indicative of the action of the FHL complex (White, 2000). Both strains with an intact FHL complex produced more or less the same amount of gas at 12 h, filling approximately half of the Durham tube with gas (Figure 3). However, no additional gas production was seen in case of wild-type E. coli after 12 h, while the Durham tubes in case of acetoin-producing E. coli were completely filled with gas after 24 h, and additional gas bubbles were formed in the medium after 48 h. On the other hand, the ΔhycE mutant did not produce any gas, while only a small amount of gas was observed in the budAB-containing ΔhycE mutant, which might be the result of CO2 production during acetoin formation (Figure 3).
The evolution of plate counts during stationary phase in this experiment was generally in line with the observed pH changes, with cell death taking place in the strongly acidified cultures. In particular, lethal acidification could not be prevented by acetoin fermentation in a budAB-containing ΔhycE mutant since plate counts of this strain significantly decreased after the stationary phase, as was also the case for the two budAB-less strains performing a mixed-acid fermentation. Cell death can be explained by the combination of the low pH environment and the toxic accumulation of organic acids.
ANALYSIS OF METABOLITES PRODUCED DURING FERMENTATIVE GROWTH OF E. coli
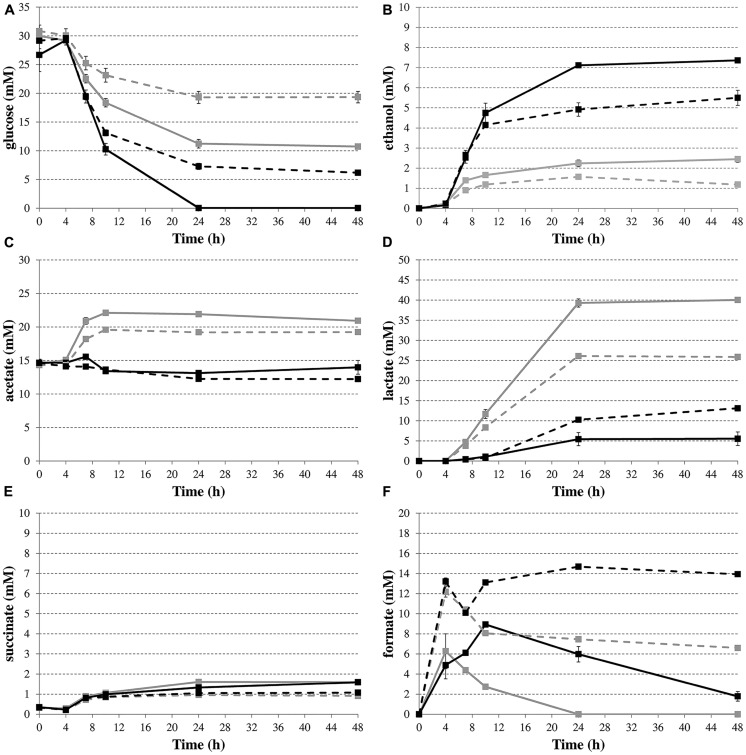
To provide more direct evidence for the involvement of formate disproportionation in the deacidification capacity of budAB-containing E. coli, glucose consumption and the production of metabolites were determined by HPLC during fermentative growth in LB with glucose (Figure 4). Succinate concentrations (Figure 4E) remained low for all strains during the course of the experiment. On the other hand, the budAB genes caused a marked shift in the production of two of the major acids of the mixed-acid fermentation pathway, especially in the late exponential and stationary growth phase, with no more acetate and much less lactate being produced (Figures 4C,D, respectively). With regard to formate (Figure 4F), the highest formate accumulation was seen in the ΔhycE mutants, probably because these have lost their major route to convert formate to H2 and CO2. During the stationary growth phase (up to 48 h), the formate concentrations remained almost constant in the hycE- strains, but strongly decreased in the hycE+ strains, indicating the reuptake and conversion of formate to CO2 and H2. Interestingly, a close look at the formate accumulation curves of the ΔhycE mutants reveals a transient decline in the late exponential growth phase (onset at 4 h of growth). Also in the hycE+ background a decline (budAB-less strain) or a diminished accumulation (budAB-containing strain) of formate was observed in this phase. A possible explanation for this is the activity of the FDH-N, which also catalyzes the oxidation of formate to CO2 (Sawers, 1994). However, FDH-N transfers the electrons to nitrate (via a nitrate reductase) instead of protons and has a much higher affinity for formate than the FDH-H (Leonhartsberger et al., 2002), which could explain why it is active in an earlier growth stage. The activity of FDH-N is limited, however, because LB medium contains only a small amount of nitrate. The disproportionation of formate (Figure 4F) by the hycE+ strains lasted longer when the budAB genes were present (48 h) than when they were absent (24 h), probably because a higher amount of formate was produced. This was also reflected by an increased gas production in the presence of the budAB genes during this phase, as reported above (Figure 3). Finally, ethanol was produced in higher quantities by the budAB-containing strains (Figure 4B).
FIGURE 4.
Time profiles of glucose consumption (A) and production of the metabolites ethanol (B), acetate (C), lactate (D), succinate (E), and formate (F) during fermentative growth of E. coli MG1655 wild-type (solid lines) or ΔhycE (dashed lines) containing pTrc99A (gray) or pTrc99A-Ptrc-budAB (black) in LB medium with 5 g/l glucose, 1 mM IPTG and 100 μg/ml Ap at 37°C for 48 h. Error bars represent SD.
As a final experiment to demonstrate that formate conversion causes medium deacidification during stationary phase, 5 or 10 mM formate from a 1 M solution (pH 5.50) was added to the medium after 10 h of fermentative growth of budAB-containing E. coli MG1655 and the pH was subsequently measured after 10, 24, and 48 h. As expected, the addition of formate in the medium resulted in a significantly stronger pH increase during stationary phase (Table 5).
Table 5.
Effect of exogenous formate addition on pH of fermentation medium.
| Concentration of formate added at 10 h | 10 h | 24 h | 48 h |
|---|---|---|---|
| 0 mM | 5.64 ± 0.03a | 5.81 ± 0.04a | 6.14 ± 0.02a |
| 5 mM | 5.66 ± 0.05a | 5.96 ± 0.04b | 6.35 ± 0.04b |
| 10 mM | 5.63 ± 0.08a | 6.05 ± 0.04c | 6.48 ± 0.05c |
E. coli MG1655 harboring pTrc99A-Ptrc-budAB was grown in LB medium with 5 g/l glucose, 1 mM IPTG, and 100 μg/ml Ap. Formate was added at 10 h and pH was measured at 10, 24, and 48 h. a-c pH values with different superscripts in the same column are significantly different (p < 0.05).
Taken together, the metabolite profiles lead us to propose the following model to explain the effect of introduction of the budAB genes in E. coli (see Figure 2). The introduction of these genes diverts part of the pyruvate generated from glycolysis to acetoin production. At the same time, possibly because of a reduced cellular pyruvate pool, the balance between the mixed-acid fermentation routes is shifted, with lactate production being almost shut down. Nevertheless, since the budAB-containing strain produced higher amounts of formate (see previous paragraph), it maintains a higher flux of pyruvate to acetyl-CoA, as also indicated by the higher glucose consumption. This can be explained by the reduced acid production and consequently the reduced metabolic inhibition. The fate of acetyl-CoA is also different in the budAB-containing strain. This is necessarily so, because the reduced production of lactic acid creates an excess of NADH that must be reoxidized by another route to maintain the cellular redox balance. As can be seen in Figure 2, this is only possible by increasing ethanol production at the expense of acetate production. This is indeed what happens, since the budAB-containing strain no longer produces acetate and has increased ethanol production. Since acetate production is coupled to the generation of an extra ATP, introduction of the acetoin pathway reduces the ATP yield per mole of glucose fermented. However, this does not result in reduced growth rate (Figures 1 and 3), because it is compensated by a higher glucose turnover. Thus, although the total biomass production (maximal cell density reached in early stationary phase) is approximately the same for all the strains, the budAB-containing strains require much more glucose to achieve this (Figure 4A).
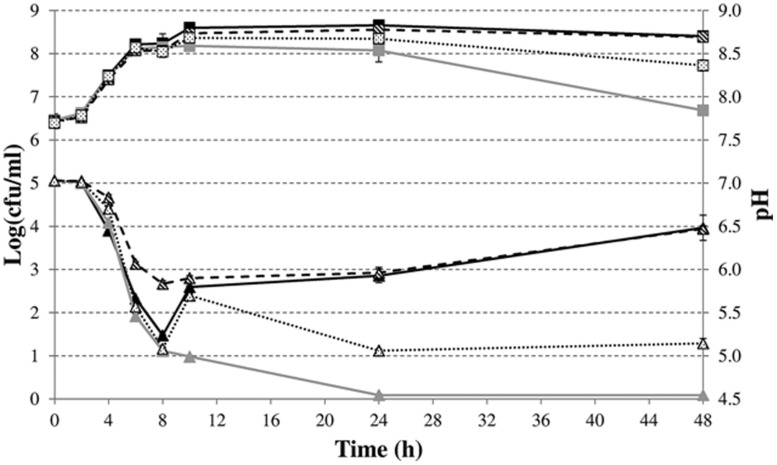
ROLE OF HYDROGENASE 3 IN FERMENTATIVE GROWTH OF S. plymuthica RVH1
Finally, we investigated whether the FHL complex also attenuates acid formation and drives deacidification during fermentative growth of a natural 2,3-butanediol fermenter, using S. plymuthica RVH1 as a model. To this end, we constructed a ΔhycE mutant in this strain. The evolution of medium pH for S. plymuthica RVH1 wild-type shows three phases (Figure 5). There was a decrease during the first 8 h, followed by a rapid increase between 8 and 10 h, and then a slower increase until 48 h. The initial pH increase is probably due to the switch to 2,3-butanediol production in the late exponential phase since it was lost upon knockout of the 2,3-butanediol pathway (budAB::cat) but not by knockout of hydrogenase 3 (ΔhycE). In contrast, the deacidification during stationary phase required both an active 2,3-butanediol pathway and an active hydrogenase 3. Genetic complementation of the budAB mutant restored its pH profile to that of the wild-type strain. However, since this complemented strain produces acetoin under the control of the plasmid Ptrc promoter right from the start of the experiment, its acidification is more attenuated than in the wild-type strain. Cell numbers declined after 48 h in the mutant strains that had lost or reduced deacidification capacity and, as a result, there was a clear correlation between cell numbers and medium pH at the end of the experiment.
FIGURE 5.
Cell numbers (squares) and medium pH (triangles) during fermentative growth of S. plymuthica RVH1 wild-type (black solid lines), budAB::cat (gray solid lines), budAB::cat containing pTrc99A-Ptrc-budAB (dashed line), or ΔhycE (dotted line) in LB medium with 5 g/l glucose at 30°C for 48 h. For the strain containing pTrc99A-Ptrc-budAB, 1 mM IPTG and 200 μg/ml carbenicillin (Cb) were added to the medium. Error bars represent SD.
Previously, the pH profile during glucose fermentation in the 2,3-butanediol fermenter Enterobacter aerogenes was divided into three phases (Johansen et al., 1975). The first phase was characterized by a rapid drop to about pH 5.8, in the second phase the pH remained almost constant at pH 5.6 and in the third phase the pH increased again to about 6.5. However, during the last phase, the total amount of acetoin and 2,3-butanediol remained constant and 2,3-butanediol was reoxidized to acetoin, indicating that the 2,3-butanediol pathway is not involved in this deacidification (Johansen et al., 1975). Our results demonstrate that –at least in S. plymuthica- the FHL complex is responsible for stationary-phase deacidification since the final pH was about 1.3 pH units lower in a S. plymuthica RVH1 ΔhycE mutant compared to the wild-type.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a doctoral fellowship to BV from the Agency for Innovation by Science and Technology (IWT), by a research grant from the Brazilian program Science without Borders (process number 5511/10-0), by research grants from the Research Foundation – Flanders (FWO; G.A061.11), and from the KU Leuven Research Fund (METH/07/03). Kenneth Simoens is acknowledged for the HPLC metabolite analyses.
REFERENCES
- Amann E., Ochs B., Abel K. (1988). Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69 301–315 10.1016/0378-1119(88)90440-4 [DOI] [PubMed] [Google Scholar]
- Bagramyan K., Trchounian A. (2003). Structural and functional features of formate hydrogen lyase, an enzyme of mixed-acid fermentation from Escherichia coli. Biochemistry (Mosc.) 68 1159–1170 10.1023/B:BIRY.0000009129.18714.a4 [DOI] [PubMed] [Google Scholar]
- Beyer L., Doberenz C., Falke D., Hunger D., Suppmann B., Sawers R. G. (2013). Coordination of FocA and pyruvate formate-lyase synthesis in Escherichia coli demonstrates preferential translocation of formate over other mixed-acid fermentation products. J. Bacteriol. 195 1428–1435 10.1128/JB.02166-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J., Steigerwalt A. G., Wathen H. G., Gross R. J., Rowe B. (1982). Confirmation of aerogenic strains of Shigella boydii 13 and further study of Shigella serotypes by DNA relatedness. J. Clin. Microbiol. 16 432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daligault H. E., Davenport K. W., Minogue T. D., Bishop-Lilly K. A., Broomall S. M., Bruce D. C., et al. (2014). Genome assembly of Shigella flexneri ATCC 12022, a quality control reference strain. Genome Announc. 2:e01052-14. 10.1128/genomeA.01052-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97 6640–6645 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll D. M., Copeland P. R. (2003). Mechanism and regulation of selenoprotein synthesis. Annu. Rev. Nutr. 23 17–40 10.1146/annurev.nutr.23.011702.073318 [DOI] [PubMed] [Google Scholar]
- Forzi L., Sawers R. G. (2007). Maturation of [NiFe]-hydrogenases in Escherichia coli. Biometals 20 565–578 10.1007/s10534-006-9048-5 [DOI] [PubMed] [Google Scholar]
- Fukushima M., Kakinuma K., Kawaguchi R. (2002). Phylogenetic analysis of Salmonella, Shigella, and Escherichia coli strains on the basis of the gyrB gene sequence. J. Clin. Microbiol. 40 2779–2785 10.1128/JCM.40.8.2779-2785.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germani Y., Sansonetti P. J. (2006). “The genus Shigella,” in The Prokaryotes: A Handbook on the Biology of Bacteria, eds Dworkin M., Falkow S., Rosenberg E., Schleifer K. H., Stackebrandt E. (New York, NY: Springer; ), 99–122 10.1007/0-387-30746-X_6 [DOI] [Google Scholar]
- Grant S. G. N., Jessee J., Bloom F. R., Hanahan D. (1990). Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. U.S.A. 87 4645–4649 10.1073/pnas.87.12.4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunden A. M., Shanmugam K. T. (1997). Molybdate transport and regulation in bacteria. Arch. Microbiol. 168 345–354 10.1007/s002030050508 [DOI] [PubMed] [Google Scholar]
- Guyer M. S., Reed R. R., Steitz J. A., Low K. B. (1981). Identification of a sex-factor-affinity site in E. coli as γδ. Cold Spring Harb. Symp. Quant. Biol. 45 135–140 10.1101/SQB.1981.045.01.022 [DOI] [PubMed] [Google Scholar]
- Holt J. G., Krieg N. R., Sneath P. H. A., Staley J. T., Williams S. T. (1994). Bergey’s Manual of Determinative Bacteriology. Baltimore: Williams & Wilkins. [Google Scholar]
- Johansen L., Bryn K., Stormer F. C. (1975). Physiological and biochemical role of the butanediol pathway in Aerobacter (Enterobacter) aerogenes. J. Bacteriol. 123 1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Bender J., Gottesman S. (1991). Uses of transposons with emphasis on Tn10. Methods Enzymol. 204 139–180 10.1016/0076-6879(91)04009-D [DOI] [PubMed] [Google Scholar]
- Kwon Y. M., Ricke S. C. (2000). Efficient amplification of multiple transposon-flanking sequences. J. Microbiol. Methods 41 195–199 10.1016/S0167-7012(00)00159-7 [DOI] [PubMed] [Google Scholar]
- Leimkühler S., Wuebbens M. M., Rajagopalan K. V. (2001). Characterization of Escherichia coli MoeB and its involvement in the activation of molybdopterin synthase for the biosynthesis of the molybdenum cofactor. J. Biol. Chem. 276 34695–34701 10.1074/jbc.M102787200 [DOI] [PubMed] [Google Scholar]
- Leinfelder W., Forchhammer K., Zinoni F., Sawers G., Mandrand-Berthelot M. A., Böck A. (1988). Escherichia coli genes whose products are involved in selenium metabolism. J. Bacteriol. 170 540–546 10.1002/jtra.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhartsberger S., Korsa I., Böck A. (2002). The molecular biology of formate metabolism in enterobacteria. J. Mol. Microbiol. Biotechnol. 4 269–276. [PubMed] [Google Scholar]
- Lü W., Du J., Schwarzer N. J., Gerbig-Smentek E., Einsle O., Andrade S. L. A. (2012). The formate channel FocA exports the products of mixed-acid fermentation. Proc. Natl. Acad. Sci. U.S.A. 109 13254–13259 10.1073/pnas.1204201109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx C. J., Lidstrom M. E. (2002). Broad-host-range cre-lox system for antibiotic marker recycling in Gram-negative bacteria. BioTechniques 33 1062–1067. [DOI] [PubMed] [Google Scholar]
- McClelland M., Sanderson K. E., Spieth J., Clifton S. W., Latreille P., Courtney L., et al. (2001). Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413 852–856 10.1038/35101614 [DOI] [PubMed] [Google Scholar]
- Moons P., Van Houdt R., Vivijs B., Michiels C. W., Aertsen A. (2011). Integrated regulation of acetoin fermentation by quorum sensing and pH in Serratia plymuthica RVH1. Appl. Environ. Microbiol. 77 3422–3427 10.1128/AEM.02763-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J., Rajagopalan K. V. (2002). Escherichia coli MoeA and MogA function in metal incorporation step of molybdenum cofactor biosynthesis. J. Biol. Chem. 277 24995–25000 10.1074/jbc.M203238200 [DOI] [PubMed] [Google Scholar]
- Noguchi K., Riggins D. P., Eldahan K. C., Kitko R. D., Slonczewski J. L. (2010). Hydrogenase-3 contributes to anaerobic acid resistance of Escherichia coli. PLoS ONE 5:e101321 10.1371/journal.pone.0010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quénée L., Lamotte D., Polack B. (2005). Combined sacB-based negative selection and cre-lox antibiotic marker recycling for efficient gene deletion in Pseudomonas aeruginosa. BioTechniques 38 63–67 10.2144/05381ST01 [DOI] [PubMed] [Google Scholar]
- Rubirés X., Saigi F., Piqué N., Climent N., Merino S., Albertí S., et al. (1997). A gene (wbbL) from Serratia marcescens N28b (O4) complements the rfb-50 mutation of Escherichia coli K-12 derivatives. J. Bacteriol. 179 7581–7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers G. (1994). The hydrogenases and formate dehydrogenases of Escherichia coli. Antonie Van Leeuwenhoek 66 57–88 10.1007/BF00871633 [DOI] [PubMed] [Google Scholar]
- Sawers G., Böck A. (1988). Anaerobic regulation of pyruvate formate-lyase from Escherichia coli K-12. J. Bacteriol. 170 5330–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers R. G. (2005). Formate and its role in hydrogen production in Escherichia coli. Biochem. Soc. Trans. 33 42–46 10.1042/BST0330042 [DOI] [PubMed] [Google Scholar]
- Self W. T., Hasona A., Shanmugam K. T. (2004). Expression and regulation of a silent operon, hyf, coding for hydrogenase 4 isoenzyme in Escherichia coli. J. Bacteriol. 186 580–587 10.1128/JB.186.2.580-587.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R., Priefer U., Pühler A. (1983). A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat. Biotechnol. 1 784–791 10.1038/nbt1183-784 [DOI] [Google Scholar]
- Thomé R., Gust A., Toci R., Mendel R., Bittner F., Magalon A., et al. (2012). A sulfurtransferase is essential for activity of formate dehydrogenases in Escherichia coli. J. Biol. Chem. 287 4671–4678 10.1074/jbc.M111.327122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall B. J., Grimont P. A. D., Garrity G. M., Euzéby J. P. (2005). Nomenclature and taxonomy of the genus Salmonella. Int. J. Syst. Evol. Microbiol. 55 521–524 10.1099/ijs.0.63580-0 [DOI] [PubMed] [Google Scholar]
- Trchounian A., Sawers R. G. (2014). Novel insights into the bioenergetics of mixed-acid fermentation: can hydrogen and proton cycles combine to help maintain a proton motive force? IUBMB Life 66 1–7 10.1002/iub.1236 [DOI] [PubMed] [Google Scholar]
- Trchounian K., Poladyan A., Vassilian A., Trchounian A. (2012). Multiple and reversible hydrogenases for hydrogen production by Escherichia coli: dependence on fermentation substrate, pH and the F0F1-ATPase. Crit. Rev. Biochem. Mol. Biol. 47 236–249 10.3109/10409238.2012.655375 [DOI] [PubMed] [Google Scholar]
- Van Houdt R., Aertsen A., Michiels C. W. (2007). Quorum-sensing-dependent switch to butanediol fermentation prevents lethal medium acidification in Aeromonas hydrophila AH-1N. Res. Microbiol. 158 379–385 10.1016/j.resmic.2006.11.015 [DOI] [PubMed] [Google Scholar]
- Van Houdt R., Moons P., Buj M. H., Michiels C. W. (2006). N-acyl-L-homoserine lactone quorum sensing controls butanediol fermentation in Serratia plymuthica RVH1 and Serratia marcescens MG1. J. Bacteriol. 188 4570–4572 10.1128/JB.00144-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houdt R., Moons P., Jansen A., Vanoirbeek K., Michiels C. W. (2005). Genotypic and phenotypic characterization of a biofilm-forming Serratia plymuthica isolate from a raw vegetable processing line. FEMS Microbiol. Lett. 246 265–272 10.1016/j.femsle.2005.04.016 [DOI] [PubMed] [Google Scholar]
- Vivijs B., Moons P., Aertsen A., Michiels C. W. (2014a). Acetoin synthesis acquisition favors Escherichia coli growth at low pH. Appl. Environ. Microbiol. 80 6054–6061 10.1128/AEM.01711-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivijs B., Moons P., Geeraerd A. H., Aertsen A., Michiels C. W. (2014b). 2,3-butanediol fermentation promotes growth of Serratia plymuthica at low pH but not survival of extreme acid challenge. Int. J. Food Microbiol. 175 36–44 10.1016/j.ijfoodmicro.2014.01.017 [DOI] [PubMed] [Google Scholar]
- White D. (2000). The Physiology and Biochemistry of Prokaryotes. New York: Oxford University Press. [Google Scholar]
- Xiao Z., Xu P. (2007). Acetoin metabolism in bacteria. Crit. Rev. Microbiol. 33 127–140 10.1080/10408410701364604 [DOI] [PubMed] [Google Scholar]
- Yoon S. S., Mekalanos J. J. (2006). 2,3-butanediol synthesis and the emergence of the Vibrio cholerae El Tor biotype. Infect. Immun. 74 6547–6556 10.1128/IAI.00695-06 [DOI] [PMC free article] [PubMed] [Google Scholar]