Abstract
Aims
Obesity is associated with the development of atrial fibrillation (AF), and both obesity and AF are independently associated with the development of heart failure with preserved ejection fraction. We tested the hypothesis that sleep apnea (SA) would have a body mass index (BMI) independent association with adverse left ventricular (LV) remodeling and clinical outcomes in patients with AF and preserved LV function.
Methods and results
From 720 consecutive patients with AF, 403 patients without myocardial disease (preserved LV function) were identified and followed up for 3.3 ± 1.5 years. The primary outcome was a combination of all-cause mortality/heart failure hospitalization. Left ventricular mass and LV mass-to-volume ratio were higher in patients with SA and obesity (P < .0001 for all). Body mass index (β per log = .47; P < .0001) and SA (β = .05; P = .045) were independently associated with LV mass index. Patients with treated SA had a lower LV mass index (but not LV mass-to-volume ratio) compared with untreated (P = .002). In a best overall multivariable model, SA therapy (β = −.129; P = .001) and BMI (β per log = .373; P = .0007) had opposing associations with LV mass index. Sleep apnea (hazard ratio [HR] = 2.94; P = .0004) and BMI (HR per 1 kg/m2 = 1.08; P = .004) were associated with clinical outcome in unadjusted analysis. Only SA was associated with clinical outcome in a best overall multivariable model (HR = 2.14; P = .02).
Conclusion
Sleep apnea and obesity are independently associated with adverse LV remodeling and clinical outcomes in patients with preserved LV function, whereas continuous positive airway pressure therapy is associated with a beneficial effect on LV remodeling. Research investigating SA therapies in patients at high risk for LV remodeling and heart failure is warranted.
Approximately half of patients with newly diagnosed heart failure (HF) are classified as HF with preserved ejection fraction (HF-pEF). Contemporary treatments for HF-pEF remain limited, and therapy is directed primarily at underlying comorbidities. Multiple associations with HF-pEF exist, including obesity, hypertension, diabetes, and atrial fibrillation (AF). There is a complex interplay between these risk factors; obesity is associated with the development of AF,1 and both obesity and AF are independently associated with the development of HF-pEF.2 Furthermore, animal and small physiologic studies demonstrate a dose-dependent effect of obesity on myocardial remodeling,3 suggesting an independent role for obesity and obesity-related cardiovascular illness in the pathogenesis of incident HF.
Among contributors to obesity-related heart disease, sleep apnea (SA) appears to play a role in integrating factors critical to the development of HF-pEF, including AF,4–6 systemic hypertension,7 vascular stiffness,8 and left ventricular hypertrophy.9 Interventions such as continuous positive airway pressure (CPAP) are associated with improvement in diastolic function and reduction in recurrent AF,4,10–15 both contributors to the progression to HF. Given the influence of AF on HF-pEF, investigating a possible body mass index (BMI) independent association of SA with adverse left ventricular (LV) structure and function and clinical outcome in patients with AF may establish a rationale for more aggressive SA screening and treatment.
To address the independent contributions of SA and obesity on LV structure in AF, we performed a prospective observational cohort study of patients referred for cardiac magnetic resonance (CMR) imaging before AF ablation. Given their potential for additive effect on LV structure, we hypothesized that both BMI and SA would be associated with LV mass and concentric LV remodeling (by LV mass-to-volume ratio). Furthermore, we investigated the association of both obesity and SA on all-cause mortality and HF hospitalization.
Methods
Study population
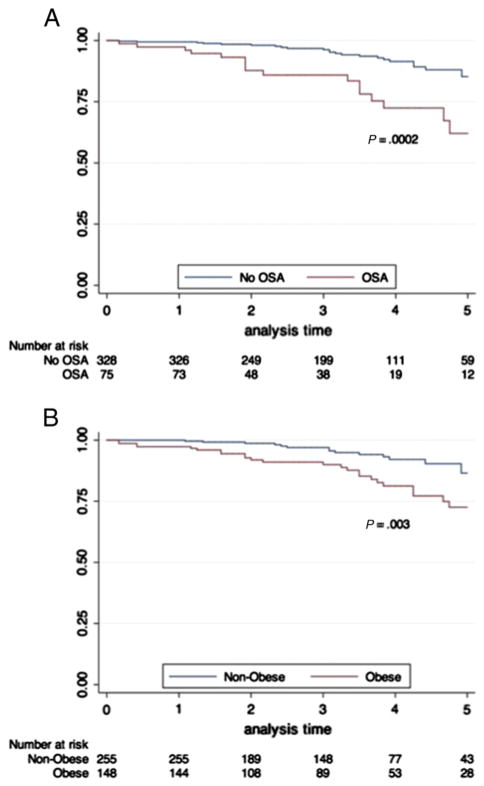
We studied 403 patients undergoing CMR before pulmonary vein isolation at the Brigham and Women’s Hospital between September 2005 and June 2011. Patients with evidence of prior myocardial infarction (MI) (defined by clinical evidence of MI per history, electrocardiographic criteria, or late gadolinium enhancement by CMR) were excluded. Given our focus on HF-pEF, patients with reduced left ventricular ejection fraction (LVEF) by CMR (LVEF <50%) were excluded. All patients had either paroxysmal AF (AF terminating spontaneously <7 days after onset) or persistent AF (AF >7 days) as an indication for AF ablation. Heart failure was defined by clinical history in the medical record by a cardiologist (TGN) blinded to all imaging variables. Obesity was defined as a BMI ≥30 kg/m2. The presence or absence of SA was prospectively determined (and blinded to the results of the CMR) as part of the institutional screening process before anesthesia. All patients diagnosed with SA had undergone polysomnography. The diagnosis of SA reflected the sleep study criteria recommended by the American Academy of Sleep Medicine.16 Data regarding the extent of use of CPAP were obtained from each patient from follow-up phone interviews. Therapy with CPAP was defined as >4 hours of continuous CPAP per night on average. The human subjects’ research review committee of our institution approved the study protocol (See Figure).
Figure.
Kaplan-Meier estimates of survival free of death or HF hospitalization to 5 years, stratified by SA (A) and obesity (B).
CMR protocol
Cardiac magnetic resonance imaging was used to measure LV end-diastolic mass, end-systolic and diastolic volumes, and LVEF using standard techniques. Cardiac magnetic resonance was performed on either a 1.5- or 3.0-T system (SignaHDxt; General Electric Healthcare, Waukesha, WI and Tim Trio; Siemens, Erlangen, Germany, respectively), using electrocardiographic or pulse gating during breath hold. The CMR protocol consisted of cine steady-state free precession imaging for cardiac function (typical repetition time; 3.4 ms and echo time; 1.2 ms and in-plane spatial resolution; 1.6 × 2 mm) and mass, pulmonary vein anatomy imaging, and late gadolinium enhancement imaging, as has been described by our laboratory.17 Cine images were obtained in 8 to 14 matching short axis (8-mm thick; 0-mm spacing) and 3 radial long-axis planes with full ventricular coverage. Left ventricular function and mass were quantified by Simpson’s technique. We indexed LV mass and volumes to height2.7, as previously reported. 18 Images were analyzed using Mass Research (Leiden University Medical Center, Leiden, Belgium) or CMR42 (Circle Cardiovascular Imaging, Calgary, Canada).
Adjudication of clinical events
Patients were followed up postprocedure at 3- to 6-month intervals via clinic visits. Our end point was a composite of all-cause mortality and HF hospitalization. We assessed mortality via the Social Security Death Index and electronic medical records. When medical records provided insufficient follow-up information, the patient and the primary provider was contacted. Heart failure hospitalization was confirmed by a review of all medical records from hospitalizations occurring after the index CMR scan, including clinical notes and radiography.
Statistical analysis
Continuous data are presented as median and interquartile range (IQR) stratified by obesity, with intergroup comparison using the Wilcoxon rank sum test. Categorical covariates were compared with χ2 testing. To measure the association of BMI and LV remodeling, we calculated Spearman rank-order correlation coefficients between BMI and parameters of LV structure and compared LV structural parameters by SA status using Wilcoxon rank sum testing. Given potential confounding by factors related both to obesity and SA, we constructed multivariable linear regression models for height-indexed LV mass and LV mass-to-volume ratio (dependent variables), adjusted for age, gender, hypertension, diabetes, and prior coronary revascularization, with inclusion of SA (as a binary covariate) and BMI. In the subset of patients with SA (n = 75), we performed additional stepwise multivariable linear regression modeling (model entry P < .05; model retention P < .05) using identical covariates as above, including the presence of SA therapy to identify an association of SA therapy with LV remodeling indices. Height-indexed LV parameters and BMI were log-transformed to establish normality. Finally, to measure the association of BMI and SA with all-cause mortality/HF hospitalization, we used univariable and multivariable Cox proportional hazards regression modeling out to maximum follow-up. We conducted a best overall, backward multivariable Cox model (model entry P < .05; model retention P < .05), including all covariates used in univariable models. Proportional hazards assumptions were assessed for each covariate. Kaplan-Meier methods were used to estimate censored event-free survival for the primary and secondary outcomes stratified by obesity and SA status separately, with comparison of survival by the log-rank test. A 2-tailed P value of < .05 was considered significant, and statistical analysis was performed in SAS version 9.3 (SAS Institute Inc, Cary, NC).
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Results
Clinical and demographic characteristics
Baseline characteristics are shown in Table I. Overall, 290 (72%) patients were male, with a median age of 57 years. All patients had a history of AF, with 138 (34%) having paroxysmal AF and 267 (66%) with persistent AF. Cardiometabolic risk was prevalent in this population, including hypertension (47%), diabetes (14%), and SA (19%). Obesity was present in 148 patients (37%), with a median BMI 33.8 kg/m2 in the obese (vs 26.5 kg/m2 in nonobese; P < .0001). Obese patients had a greater median systolic blood pressure (131 vs 125 mm Hg in nonobese; P = .008), more prevalent SA (30% vs 12% in nonobese; P < .0001), and a trend toward more prevalent diabetes (19% vs 12% in nonobese; P = .06). History of prior HF was similar across obesity strata (20% vs 14% in nonobese; P = .16), as was AF grade (persistent vs paroxysmal), New York Heart Association functional status, or prior revascularization.
Table I.
Clinical characteristics of our study population stratified by obesity
| Covariate | All patients (N = 403) | Nonobese (n = 255) | Obese (n = 148) | P (obese vs nonobese) |
|---|---|---|---|---|
| Clinical and demographic indices, median (IQR) | ||||
| Age, years | 57 (49–64) | 57 (49–65) | 57 (48–62) | .40 |
| Male, n (%) | 290 (72) | 183 (72) | 107 (72) | 1 |
| Weight, kg | 88.3 (79.3–104.1) | 81.5 (76.6–89.2) | 108.7 (95.1–120.0) | <.0001 |
| Height, m | 1.77 (1.70–1.83) | 1.77 (1.72–1.83) | 1.77 (1.70–1.85) | .46 |
| Body mass index, kg/m2 | 28.7 (25.7–31.8) | 26.5 (24.4–28.3) | 33.8 (31.3–37.4) | <.0001 |
| Systolic blood pressure, mm Hg | 127 (117–138) | 125 (116–135) | 131 (118–141) | .0075 |
| Diastolic blood pressure, mm Hg | 75 (68–82) | 75 (67–81) | 75 (69–84.5) | .19 |
| Heart rate, beat/min | 67 (57–76) | 65 (56–74) | 69.5 (60–80) | .0005 |
| QRS duration, ms | 92 (86–100) | 92 (86–100) | 94 (88–102) | .07 |
| NYHA class, n (%) | .01 | |||
| I | 211 (52) | 146 (57) | 65 (44) | |
| II | 188 (47) | 108 (42) | 80 (54) | |
| III | 4 (1) | 1 (0.4) | 3 (2) | |
| Medical history, n (%) | ||||
| Revascularization | 13 (3.2) | 7 (2.8) | 6 (4.1) | .56 |
| Paroxysmal AF | 138 (34) | 89 (35) | 49 (33) | .74 |
| Persistent AF | 267 (66) | 167 (66) | 100 (68) | .74 |
| Prior AF ablation | 99 (25) | 62 (24) | 37 (25) | .90 |
| Hypertension | 189 (47) | 103 (40) | 86 (58) | .0006 |
| Diabetes | 58 (14) | 30 (12) | 28 (19) | .06 |
| SA | 75 (19) | 31 (12) | 44 (30) | <.0001 |
| Prior HF | 65 (16) | 36 (14) | 29 (20) | .16 |
Abbreviations: kg, Kilogram; m, meter, kg/m2, kilogram per square meter; mm Hg, millimeter of mercury; beat/min, beats per minute; ms, millisecond; NYHA, New York Heart Association. All values are expressed as median (IQR), and P values are calculated by a Wilcoxon rank sum test or χ2 test where appropriate.
Cardiac structure and function
Cardiac magnetic resonance results are shown in Table II. As expected, overall LVEF was preserved (median 60.4; IQR: 56.2–64.3). Obese individuals had a trend toward higher LV end-diastolic volume index (P = .05), a higher LV mass (P < .0001) and LV mass-to-volume ratio (P < .0001), greater maximal left atrial volume (P = .009), and trend toward higher right ventricular end-diastolic volume index (P = .05). Of note, both LVEF and right ventricular ejection fraction were similar between obese and nonobese subjects (P = .62 and .85, respectively).
Table II.
Indices of ventricular structure and function by CMR
| Covariate | All patients (N = 403) | Nonobese (n = 255) | Obese (n = 148) | P |
|---|---|---|---|---|
| LVEF | 60 (56–64) | 60 (57–64) | 61 (56–64) | .62 |
| LV end-diastolic volume indexed (mL/m2.7) | 34.6 (29.7–40.3) | 34.3 (29.4–39.2) | 35.9 (30.3–41.9) | .05 |
| LV mass indexed (g/m2.7) | 30.8 (26.9–35.5) | 28.6 (25.8–33.3) | 33.9 (29.8–38.7) | <.0001 |
| LV mass-to volume index | 0.9 (0.8–1) | 0.8 (0.7–1) | 0.9 (0.8–1.1) | <.0001 |
| Maximal left atrial volume (mL) | 107.6 (87.2–132.6) | 102.7 (84.7–130.4) | 113.1 (95.8–134.7) | .009 |
| RV ejection fraction, % | 53 (49–57) | 53 (49–57) | 53 (49–58) | .85 |
| RV end-diastolic volume indexed (mL/m2.7) | 34.4 (29.1–40.1) | 34.2 (28.6–39.3) | 35.6 (29.9–41.4) | .05 |
Abbreviations: mL/m, Milliliter per meter; g/m, gram per meter; RV, right ventricular. All values are expressed as median (IQR), and P values are calculated by a Wilcoxon rank sum test.
Independent association of BMI and SA with cardiac structure and LV remodeling
In addition to the differences in cardiac structure and function observed with obesity stratified around 30 kg/ m2 (Table II), there were associations between BMI and indexed LV mass (Spearman ρ = 0.50; P < .0001) and LV mass-to-volume ratio (Spearman ρ = 0.23; P < .0001). Similarly, patients with SA had a higher indexed LV mass (median 29.6 g/m2.7 without SA vs 34.3 kg/m2.7 with SA; P < .0001) and higher LV mass-to-volume ratio (median 0.85 without SA vs 0.96 with SA; P = .009). However, both SA and obesity were strongly associated with cardiometabolic diseases implicated in LV remodeling (eg, diabetes, hypertension), suggesting the possibility of residual confounding.
Multivariable linear regression models for height-indexed LV mass and LV mass-to-volume ratio (dependent variables), adjusted for well-established risk factors for LV remodeling,3 with inclusion of SA (as a binary covariate) and BMI are shown in Table III. In addition to older age (P < .0001) and the presence of diabetes (P = .007), both a higher BMI (P < .0001) and a diagnosis of SA (P = .04) were independently associated with LV mass index. In a separate model for LV mass-to-volume ratio, hypertension (P = .02), diabetes (P = .02), and BMI (P = .01) but not SA, were independently associated with a greater LV mass-to-volume ratio.
Table III.
Multivariable linear regression models for association with parameters of LV remodeling, including LV mass indexed to height (g/m2.7), LV end-diastolic volume (g/m2.7), and LV mass to volume ratio
| Covariate | LV mass index
|
LV mass to volume
|
||
|---|---|---|---|---|
| β | P | β | P | |
| Age, per year | −.0005 | <.0001 | .002 | .06 |
| Male gender | .03 | .1 | .05 | .06 |
| Prior HF | .02 | .39 | −.01 | .68 |
| Prior revascularization | −.04 | .36 | −.05 | .46 |
| Hypertension | .03 | .09 | .06 | .024 |
| Diabetes | .06 | .007 | .08 | .017 |
| SA | .05 | .04 | .04 | .25 |
| BMI (per 1 log kg/m2) | .47 | <.0001 | .19 | .01 |
Both dependent variables were log-transformed to establish normality.
Association of SA therapy with LV mass and concentric LV remodeling
Of the 75 patients who had SA, 37 (50%) were receiving CPAP therapy. Relative to those without therapy, patients receiving CPAP were similar in age, BMI, systolic blood pressure, and had no difference in medication, New York Heart Association functional status, history of HF, diabetes, hypertension, or gender. Structurally, patients receiving SA treatment had no difference in LV/right ventricular volumes or ejection fraction. Although LV mass-to-volume ratio was similar by treatment, LV mass index was lower in treated SA relative to untreated SA (37.8 g/m2.7 vs 31.8 g/m2.7; P = .002). In a best overall multivariable linear regression among patients with SA (n = 75), only SA therapy (β = −.129; P = .001) and BMI (β per log = .373; P = .0007) remained in the model with independent, opposing effects on LV mass index.
Association of BMI and SA with adverse outcomes
Complete follow-up was available for all patients. The mean follow-up in our cohort was 3.3 ± 1.5 years. Kaplan-Meier estimates of event-free survival to 5 years stratified by SA and obesity are shown in the Figure. The overall annual rate of all-cause mortality/HF hospitalization was 3.4% per patient-year (46 events over 1,344 patient-years). The annual event rate was higher in obese versus nonobese individuals (5.1% vs 2.5%/patient-year; P < .0001 by χ2) and in individuals with SA versus those without SA (7.3% vs 2.6%/patient-year; P < .0001).
Sleep apnea (HR = 2.94; 95% CI 1.61–5.35) (P = .0004) and body mass index (HR per 1 kg/m2 = 1.08; 95% CI 1.03–1.14) (P = .004) were significantly associated with our primary composite end point (Table IV) in addition to clinical risk factors well established to predict cardiovascular risk (eg, age, diabetes, hypertension, history of HF). However, in a backward selection multivariable model, in addition to age, history of HF, the presence of AF, and diabetes, the presence of SA (but not BMI) was associated with an independent 2-fold higher risk of the composite end point (HR = 2.14, 95% CI 1.16–3.98) (P = .02).
Table IV.
Univariable and best overall multivariable Cox models for a composite outcome of all-cause mortality or HF hospitalization
| Covariate | Univariable
|
Multivariable
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age, per year | 1.07 | 1.04–1.11 | <.0001 | 1.07 | 1.03–1.11 | .0002 |
| Male gender | 1.28 | 0.64–2.59 | .49 | – | – | – |
| BMI, per kg/m2 | 1.08 | 1.03–1.14 | .004 | – | – | – |
| History of HF | 2.77 | 1.47–5.19 | .002 | 2.55 | 1.35–4.83 | .004 |
| Hyperlipidemia | 1.31 | 0.71–2.43 | .73 | – | – | – |
| Hypertension | 2.03 | 1.12–3.68 | .02 | – | – | – |
| Diabetes | 3.45 | 1.88–6.34 | <.0001 | 2.75 | 1.47–5.14 | .002 |
| SA | 2.94 | 1.61–5.35 | .0004 | 2.14 | 1.16–3.98 | .02 |
| LVESVI (per 1 mL/m2.7) | 1.06 | 0.98–1.14 | .14 | – | – | – |
| LVEF, per 1 % | 1.00 | 0.95–1.05 | 1.00 | – | – | – |
| LVMI (per 1 g/m2.7) | 1.04 | 1.00–1.09 | .07 | – | – | – |
| LV mass-to-volume ratio | 2.00 | 0.74–5.40 | .17 | – | – | – |
| RVEF, per 1% | 1.00 | 0.95–1.05 | 1.00 | – | – | – |
Abbreviations: LVESVI, Left ventricular end-systolic volume index; LVMI, left ventricular myocardial infarction; RVEF, right ventricular ejection fraction.
Discussion
In a population free of prior MI or LV dysfunction referred for AF ablation, we found that obesity was associated with greater cardiometabolic risk, higher prevalence of SA, and more adverse LV remodeling. The association between LV mass and both BMI and SA remained independent of diabetes, hypertension, and age, whereas BMI (but not SA) was associated with concentric LV remodeling. Furthermore, patients with treated SA had a lower LV mass index relative to the untreated, even after adjustment for BMI, hypertension, diabetes, and other clinical risk factors. These abnormalities in LV structure translated into a higher annual rate of all-cause mortality or HF hospitalization for individuals with either obesity or SA. At a mean follow-up >3 years, SA (but not BMI) was independently associated with a >2-fold hazard of all-cause mortality or HF hospitalization, after adjustment for previous HF, age, and diabetes. These results highlight a role for SA independent of BMI, suggesting that targeting SA in addition to weight reduction may mitigate adverse LV remodeling and improve clinical outcome.
Although obesity has been independently associated with SA, HF-pEF, and AF in community-based studies,1,2 the independent role for SA in myocardial remodeling is less well studied. Despite the recognition that SA affects myocardial physiology central to HF-pEF,4,7–9,19 SA remains underappreciated and undertreated: in 1 study, <5% of obese, diabetic patients with clinically significant SA at risk for HF-pEF were treated 1 year after diagnosis.20 Although earlier studies suggested that obesity and weight reduction were associated with LV mass improvement,21 more recent studies suggest that SA itself may be independently harmful to the ventricle10. In addition, some reports have demonstrated a weight and metabolic syndrome independent association of SA with LV structure.22,23 Our results extend the current literature on SA, obesity, and cardiac remodeling in a large group of patients with AF at risk for HF-pEF by establishing the independent association of both SA and BMI with LV mass, a prognostically important index of LV remodeling.24
The cross-sectional association with LV mass suggests the potential for benefit with CPAP therapy to reverse ventricular remodeling. In the largest study of cardiac structure with CMR in SA, Colish et al25 investigated 47 patients with SA undergoing CPAP therapy, finding a reduction in body surface area-indexed LV mass (159 ± 12 g/m2–141 ± 8 g/m2) at 6 months after CPAP initiation with sustained benefits to 1 year. Left ventricular mass regression occurred coordinately with improvements in pulmonary, biatrial, and biventricular function, suggesting a global benefit of CPAP on myocardial structure and function. Our cross-sectional results in a group of 75 patients with SA add to these results by demonstrating (1) a higher LV mass in those individuals with SA not receiving CPAP and (2) independent and opposing effects of SA therapy and BMI on LV mass after multivariable adjustment. Collectively, these findings highlight the distinct effects of BMI and SA on LV remodeling and suggest the possibility of significant reverse remodeling by SA therapy regardless of BMI.
The relevance of SA across BMI—and potential impact of SA therapy in obese patients—must be tied to clinical outcomes. Specifically, whether SA itself poses a risk independent of obesity remains unknown, especially within populations at high risk for HF-pEF. We addressed this question by selecting a referral population at high risk for incident HF (ie, established AF) and used CMR to exclude patients with established myocardial disease. We found that while both BMI and SA were associated with all-cause mortality or HF hospitalization, only presence of SA, diabetes, history of HF, and age were selected in a multivariable model as independent correlates of outcome. This result suggests that obesity-related illness—SA and diabetes—may be more important than obesity itself. Thus, further study on the impact of obesity independent of its associated comorbidities may be warranted.
The results of our study should be viewed in the context of its design. This was a cross-sectional analysis of patients referred for AF ablation, limiting the generaliz-ability of our findings. However, CMR results allowed us to select carefully a population free from myocardial disease, still at high residual risk for progressive LV remodeling and HF. We did not perform a formal sleep study on all patients referred for pulmonary vein isolation. This approach likely yielded a significant underestimation in the prevalence of SA, which is estimated to be close to 50% in this population.5 We did not measure apnea-hypopnea indices in our cohort, limiting an assessment of the association of SA with remodeling or outcome, although we still observed significant relationships with these end points. Finally, our CMR protocol did not capture emerging parameters relevant to subclinical LV remodeling in obesity and SA (eg, diffuse myocardial fibrosis, arterial stiffness).26 Given the links between obesity and myocardial fibrosis,27 identification of patients with prevalent tissue-level remodeling to target intensity, duration, and mode of therapy is an important emerging area of investigation.
In conclusion, in patients with AF, preserved LV function without prior MI, both obesity and SA are associated with indices of LV remodeling, independent of traditional risk factors for LV remodeling. Patients with SA treated with CPAP have less myocardial hypertrophy relative to those who remain untreated, even after adjustment for BMI. Sleep apnea is a strong and independent predictor of all-cause mortality or HF hospitalization in patients with AF. Future prospective investigation of SA across BMI with and without therapy with a comprehensive assessment of cardiac and vascular structure and function is warranted.
Footnotes
Disclosures
External sources of funding: RVS—American Heart Association (11POST000002) and Heart Failure Clinical Research Network (U01-HL084877). SAA—National Institutes of Health (T32HL094301-02). MJH—National Institutes of Health (RO1HL090634). RYK—National Institutes of Health (RO1HL091157). JAD—National Institutes of Health (T32 AG000158-24). TGN—American Heart Association (12FTF12060588). AM—National Institutes of Health (R01HL090897, K24HL093218, P01HL095491, R01HL110350, UM1HL108724, R01AG035117, and R01HL085188).
Conflict of interest: There are no other relevant conflicts of interest to disclose.
References
- 1.Asghar O, Alam U, Hayat SA, et al. Obesity, diabetes and atrial fibrillation; epidemiology, mechanisms and interventions. Curr Cardiol Rev. 2012;8(4):253–64. doi: 10.2174/157340312803760749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6(2):279–86. doi: 10.1161/CIRCHEARTFAILURE.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turkbey EB, McClelland RL, Kronmal RA, et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3(3):266–74. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence following catheter ablation. J Am Coll Cardiol. 2013;62(4):300–5. doi: 10.1016/j.jacc.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 5.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–7. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 6.Ng CY, Liu T, Shehata M, et al. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol. 2011;108(1):47–51. doi: 10.1016/j.amjcard.2011.02.343. [DOI] [PubMed] [Google Scholar]
- 7.Pedrosa RP, Drager LF, GdPLK, et al. Effects of obstructive sleep apnea treatment on blood pressure in patients with resistant hypertension: a randomized trial. Chest. 2013;144(5):1487–94. doi: 10.1378/chest.13-0085. [DOI] [PubMed] [Google Scholar]
- 8.Jones A, Vennelle M, Connell M, et al. Arterial stiffness and endothelial function in obstructive sleep apnoea/hypopnoea syndrome. Sleep Med. 2013;14(5):428–32. doi: 10.1016/j.sleep.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Baguet JP, Barone-Rochette G, Tamisier R, et al. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol. 2012;9(12):679–88. doi: 10.1038/nrcardio.2012.141. [DOI] [PubMed] [Google Scholar]
- 10.Butt M, Dwivedi G, Shantsila A, et al. Left ventricular systolic and diastolic function in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Circ Heart Fail. 2012;5(2):226–33. doi: 10.1161/CIRCHEARTFAILURE.111.964106. [DOI] [PubMed] [Google Scholar]
- 11.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 12.Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radio-frequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10(3):331–7. doi: 10.1016/j.hrthm.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. doi: 10.1161/JAHA.113.000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chilukuri K, Dalal D, Gadrey S, et al. A prospective study evaluating the role of obesity and obstructive sleep apnea for outcomes after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21(5):521–5. doi: 10.1111/j.1540-8167.2009.01653.x. [DOI] [PubMed] [Google Scholar]
- 15.Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circulation Arrhyth-mia Electrophysiol. 2010;3(5):445–51. doi: 10.1161/CIRCEP.109.858381. [DOI] [PubMed] [Google Scholar]
- 16.Morgenthaler TI, Kapen S, Lee-Chiong T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29(8):1031–5. [PubMed] [Google Scholar]
- 17.Coelho-Filho OR, Seabra LF, Mongeon FP, et al. Stress myocardial perfusion imaging by CMR provides strong prognostic value to cardiac events regardless of patient’s sex. JACC Cardiovasc Imaging. 2011;4(8):850–61. doi: 10.1016/j.jcmg.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20(5):1251–60. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 19.Neilan TG, Mongeon FP, Shah RV, et al. Myocardial extracellular volume expansion and the risk of recurrent atrial fibrillation after pulmonary vein isolation. JACC Cardiovasc Imaging. 2014;7(1):1–11. doi: 10.1016/j.jcmg.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169(17):1619–26. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grandi AM, Laurita E, Marchesi C, et al. OSA, metabolic syndrome and CPAP: effect on cardiac remodeling in subjects with abdominal obesity. Respir Med. 2012;106(1):145–52. doi: 10.1016/j.rmed.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Usui Y, Takata Y, Inoue Y, et al. Coexistence of obstructive sleep apnoea and metabolic syndrome is independently associated with left ventricular hypertrophy and diastolic dysfunction. Sleep Breath. 2012;16(3):677–84. doi: 10.1007/s11325-011-0557-2. [DOI] [PubMed] [Google Scholar]
- 23.Cioffi G, Russo TE, Stefenelli C, et al. Severe obstructive sleep apnea elicits concentric left ventricular geometry. J Hypertens. 2010;28(5):1074–82. doi: 10.1097/hjh.0b013e328336c90a. [DOI] [PubMed] [Google Scholar]
- 24.Choi EY, Bahrami H, Wu CO, et al. N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2012;5(6):727–34. doi: 10.1161/CIRCHEARTFAILURE.112.968701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colish J, Walker JR, Elmayergi N, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141(3):674–81. doi: 10.1378/chest.11-0615. [DOI] [PubMed] [Google Scholar]
- 26.Drager LF, Bortolotto LA, Figueiredo AC, et al. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131(5):1379–86. doi: 10.1378/chest.06-2703. [DOI] [PubMed] [Google Scholar]
- 27.Quillot D, Alla F, Bohme P, et al. Myocardial collagen turnover in normotensive obese patients: relation to insulin resistance. Int J Obes. 2005;29:1321–8. doi: 10.1038/sj.ijo.0803022. [DOI] [PubMed] [Google Scholar]