Introduction
Drug-drug interactions (DDIs) are an important clinical and public health concern. Although DDI screening now occurs during drug development, it is difficult to predict clinical importance based on in vitro experiments. Further, older drugs that were not screened may have as-yet unidentified interactions. In this commentary, we review the importance of DDIs, and argue that a translational research approach is needed to produce clinically actionable information as well as generalizable biologic knowledge.
The clinical and public health importance of drug-drug interactions
Fifty-six percent of all adult Americans and 90% of Americans age 65+ take at least one prescription drug in any given month.(1) Known drug-drug interactions (DDIs) are responsible for 13% of adverse drug events in community-dwelling older adults (2) and 3% of all hospital admissions.(3) As these figures include only well-documented DDIs, they are undoubtedly underestimates. Given the continued development of new drugs, the rising frequency of polypharmacy and the aging of the population, the clinical and public health importance of DDIs will continue to grow. In a 2002 public opinion poll, 70% of respondents indicated that, if hospitalized, they would be “concerned about receiving two or more medicines that interact in a negative way.”(4) Over 100,000 two-drug DDIs have been hypothesized,(5) and many more potential DDIs may exist. However, the mechanisms and clinical importance very few of these have been studied thoroughly, and largely uninformative papers predominate in the literature. For example, approximately 70% of the primary literature on warfarin DDIs consists of case reports.(6) As a consequence of this lack of informative data, the extent of disagreement among major DDI compendia is truly startling. For example, one study found that only 2.2% of the interactions listed as “major” in any of four major compendia were listed as such among all four; 72% were listed as major in only one compendium.(7) Further, current clinical decision support systems are plagued by a high number of DDI alerts that clinicians perceive to be unimportant. Thus, both physicians and pharmacists typically override about 90% of DDI alerts. Clearly, clinicians need to be provided with much better tools to identify, evaluate, and manage potential DDIs.
Translational research
With the establishment of the Clinical and Translational Science Award program by the National Institutes of Health, translational research has become a major focus of federally-funded biomedical research in the US. Although a major goal of translational research is timely passage of therapeutic agents from basic science into clinical practice, translational research is not just about product development. It is also about passing hypotheses and knowledge across different research settings, and back and forth between research and clinical care (8). Unfortunately, because of structural issues in biomedical research including a lack of integration among disciplines (i.e., a siloed approach to science), such passage is often impaired, sometimes with famously catastrophic effect. For example, impaired translation of hypotheses and findings across animal studies, experimental human pharmacologic studies and pharmacoepidemiologic studies is thought to be largely responsible for the considerable delay in identifying the risk of myocardial infarction associated with rofecoxib and other cyclooxygenase-2 inhibitors. One important goal of translational research is to reduce such delays in the identification of clinically important pharmacologic effects. One area where such an approach is acutely needed is DDIs. This need is discussed below.
The need for translational research on drug-drug interactions
The goals of DDI research include screening for previously unanticipated DDIs; elucidating their potential pharmacokinetic and/or pharmacodynamic mechanisms; predicting and examining their effects on pharmacokinetic and clinical outcomes; and developing and evaluating approaches to manage their risks in clinical settings. Figure 1 depicts many of the research approaches used to study DDIs. These approaches include systems pharmacology approaches such as mining of published pharmacologic data; mining of spontaneous adverse drug event reports; mining of organized health care data such as electronic health records; in vitro studies of enzymes, microsomes or hepatocytes; in silico simulation of pharmacokinetic parameters; in vivo studies of pharmacokinetic parameters; population pharmacokinetic studies (i.e., analyzing pharmacokinetic data obtained in the course of clinical care); pharmacoepidemiologic studies of clinical outcomes; and development and evaluation of approaches to avoid DDIs or manage their risks in clinical settings. Importantly, each of these approaches provides a different kind of information about potential DDIs, and all are complementary. Thus, the biomedical research disciplines needed to study DDIs and their management are quite diverse, and include basic and clinical pharmacology, biomedical informatics, pharmacoepidemiology, design and evaluation of clinical decision support tools, and others. Given the heterogeneity of these disciplines, translation of knowledge and hypotheses across them is challenging but crucial. Researchers in these disciplines have different scientific orientations and use different vocabularies. There is therefore a great need to translate (both figuratively and literally) among them. Further, research on DDIs contributes to broader pharmacologic knowledge about the drugs involved and the biological pathways involved in their kinetics and dynamics, thus yielding generalizable biologic knowledge.
Figure 1.
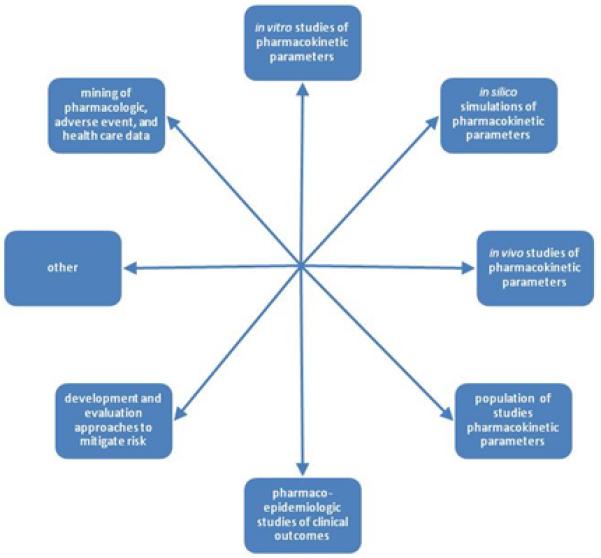
Research designs for studying drug-drug interactions and their management.
Given this background, the paper by Floyd and colleagues (9) in this issue of Clinical Pharmacology and Therapeutics is notable in that it applies three distinct approaches to identify and evaluate the potential DDIs between cerivastatin and clopidogrel. These approaches are methodologically quite varied, consisting of 1) a screening study of epidemiologic and medico-legal data; 2) an analysis of spontaneously reported adverse drug events; and 3) in vitro experiments of enzyme inhibition. As the investigators recognize, their screening case-control study relied on several untestable assumptions, including that cerivistatin-exposed rhabdomyolysis cases who sued the manufacturer consumed the same concomitant medications as cerivastatin-exposed rhabdomyolysis cases who did not sue; that plaintiffs who participated in the study consumed the same concomitant medications as non-participants; that users of atorvastatin consumed the same concomitant medications as users of cerivastatin; and that pharmacy and medical record records of medication use in enrolled cerivastain-exposed rhabdomyolysis cases were equally valid and complete as the medication-bottle-assisted interviews of atorvastatin-taking controls without rhabdomyolysis. Despite reliance on such assumptions, this exercise generated useful hypotheses that were then examined in subsequent studies. As the investigators also recognized, the analysis of spontaneous reports relied on the assumption that use of concomitant medications did not influence reporting of rhabdomyolysis cases. We note that the use of spontaneous reports to follow up of signals from epidemiologic studies is the reverse of the usual sequence used in pharmacovigilance, and that analyses of spontaneous reports can rarely be considered definitive. In addition, there are methodologic pitfalls that must be avoided in the laboratory, and care in the extrapolation of in vitro data is essential. It is often the case that an I/Ki threshold is used as to predict the clinical importance of a potential DDI, where I is the concentration of inhibitor in the circulation, and Ki is the equilibrium constant of that inhibitor for a recombinant enzyme. However, concentrations of inhibitor in the blood are often poor predictors of concentrations at the biologic “effect site” at the relevant enzyme or transporter, and there is often an imperfect relationship between drug concentration and effect. Therefore, in vitro data should be interpreted with great caution. Nevertheless, this and another recent paper (10) represent early and promising efforts to employ a translational approach to identify and study potential DDIs, and there is clearly much to be learned by examining laboratory-derived hypotheses through pharmacoepidemiologic studies, and vice versa.
Conclusion
The science of DDIs, including their mechanisms, clinical importance, and optimal management will require integrated research by a wide range of investigators. Further, optimal clinical management of potential DDIs will require knowing much more than simply whether the involved drugs can interact. Other important questions include:
Through what mechanism(s) does the DDI occur?
What events are caused by the DDI?
To what degree does the DDI increase the absolute risk?
Does the risk remain constant or change over time?
What patient factors (e.g., age, co-morbidities, laboratory values, concomitant medications) affect risk?
What management factors (e.g., empiric dosage reduction, therapeutic drug monitoring) affect risk?
Are there reasonable therapeutic alternatives that would avoid this risk?
Answering these questions will require varied research designs and expertise, and will benefit greatly by integration of the various scientific disciplines involved. In addition to helping patients and clinicians, DDI research can yield generalizable biologic knowledge. We look forward to continued integration in the conduct of translational research on DDIs.
Acknowledgements
Dr. Hennessy is supported by grants R01AG025152 and RC1AG035751 from the National Institute on Aging and by grant KL2RR024132 from the National Center for Research Resources. Dr. Flockhart is supported by grants T32-GM08425 from the National Institute of General Medical Sciences and T32-HD069047, U54HD071598 and U10HD063094 from the National Institute of Child Health and Human Development.
Footnotes
Conflict of Interest / Disclosure r. Hennessy has consulted for Bayer and for plaintiff’s attorneys suing Bayer, both unrelated to statins. He is currently an investigator of a study funded by a research contract from Bristol-Myers Squibb to the University of Pennsylvania, unrelated to statins or clopidogrel. Dr Flockhart serves on the scientific advisory boards of Medco Inc and the Corielle Institute
References
- 1.Kit BK, Ogden CL, Flegel KM. Prescription medication use among normal weight, overweight, and obese adults, United States, 2005-2008. Ann. Epidemiol. 2012;22:112–119. doi: 10.1016/j.annepidem.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Gurwitz JH, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289(9):1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 3.Jankel CA, Fitterman LK. Epidemiology of drug-drug interactions as a cause of hospital admissions. Drug Saf. 1993;9(1):51–59. doi: 10.2165/00002018-199309010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Equals Three Communications . Top patient concerns 2002: Omnibus survey results. Equals Three Communications; Bethesda, Maryland: [accessed 13 Nov]. 2002. pp. 1–25. http://www.ashp.org/s_ashp/docs/files/PR_ResearchReport.pdf. [Google Scholar]
- 5.Langdorf MI, Fox JC, Marwah RS, Montague BJ, Hart MM. Physician versus computer knowledge of potential drug interactions in the emergency department. Acad. Emerg. Med. 2000;7(11):1321–1329. doi: 10.1111/j.1553-2712.2000.tb00483.x. [DOI] [PubMed] [Google Scholar]
- 6.Holbrook A, et al. Systematic overview of warfarin and its drug and food interactions. Arch. Intern. Med. 2005;165(10):1095–1106. doi: 10.1001/archinte.165.10.1095. [DOI] [PubMed] [Google Scholar]
- 7.Abarca J, et al. Concordance of major drug interaction classifications among drug interaction compendia. J. Am. Pharm. Assoc. 2004 Mar-Apr;44(2):136–41. doi: 10.1331/154434504773062582. [DOI] [PubMed] [Google Scholar]
- 8.Selker HP. Beyond translational research from T1 to T4: beyond “separate but equal” to integration (Ti) Clinc. Transl. Sci. 2010;3(6):270–1. doi: 10.1111/j.1752-8062.2010.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Floyd JS, et al. A screening study of drug-drug interactions in cerivastatin users: an adverse effect of clopidogrel. Clin. Pharmacol. Ther. 2012 doi: 10.1038/clpt.2011.295. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tatonetti NP, et al. Detecting drug interactions from adverse-event reports: interaction between paroxetine and pravastatin increases blood glucose levels. Clin. Pharmacol. Ther. 2011 Jul;90(1):133–42. doi: 10.1038/clpt.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]


