Abstract
Chemoresistance is mediated, in part, by the inhibition of apoptosis in tumor cells. Survivin is an antiapoptotic protein that blocks chemotherapy-induced apoptosis. To investigate whether blocking survivin expression enhances docetaxel-induced apoptosis in patients with non–small-cell lung cancer (NSCLC), we compared the antitumor activity of the survivin inhibitor LY2181308 plus docetaxel with docetaxel alone. We used change in tumor size (CTS) as a primary endpoint to assess its use in early decision-making for this and future studies of novel agents in NSCLC. Patients (N = 162) eligible for second-line NSCLC treatment (stage IIIB/IV) with an Eastern Cooperative Oncology Group performance status of 0 to 1 were randomized 2:1 to receive LY2181308 (750 mg intravenously, weekly) and docetaxel (75 mg/m2 intravenously, day 1) or docetaxel alone every 21 days. CTS from baseline to the end of cycle 2 was compared between the two treatment arms. The mean (SD) tumor size ratio for LY2181308/docetaxel and docetaxel was 1.05 (0.21) and 1.00 (0.15) (p = 0.200), respectively, suggesting no significant improvement in antitumor activity between the arms. Because there was also no significant difference between the two arms for progression-free survival (PFS) (2.83 months with LY2181308/docetaxel and 3.35 months with docetaxel [p = 0.191]), both arms were combined. Using the combined arms, CTS correlated with PFS (PFS = 4.63 months in patients with decreased CTS compared with 2.66 months in patients with increased CTS), supporting its use in early decision-making in phase II studies.
Keywords: Change in tumor size, Non–small-cell lung cancer, Docetaxel, Survivin, LY2181308
Lung cancer is one of the most common malignancies worldwide, and is the leading cause of cancer death in most countries.1 Currently, patients with advanced or metastatic non–small-cell lung cancer (NSCLC) who fail first-line treatment have several treatment options, including docetaxel, which has a median overall survival (OS) of 7.9 months and a median progression-free survival (PFS) of 2.9 months.2 This limited response to second-line treatment results from chemoresistance associated with the inhibition of apoptosis.3 One protein implicated in chemoresistance and failure to undergo apoptosis is the antiapoptotic protein, survivin.4
In preclinical models, the survivin inhibitor, LY2181308, a second-generation antisense oligonucleotide, showed enhanced antitumor activity when combined with docetaxel.5 These observations led to the clinical evaluation of a combination of LY2181308 and docetaxel in cancer patients.6 It was hypothesized that blocking the expression of survivin with LY2181308 would restore default cell-death checkpoints and selectively eliminate cancer cells, leading to improved outcomes for NSCLC patients.5
To investigate the hypothesis that the combination of LY2181308 plus docetaxel is safe and potentially efficacious, we designed an open-label, randomized phase II study of second-line treatment in patients with NSCLC. Based on a previous publication,7 the primary endpoint in this trial was change in tumor size (CTS) from baseline assessed at the end of cycle 2.
PATIENTS AND METHODS
Patients
Patients were at least 18 years of age and had a histological diagnosis of NSCLC (stage IIIB or IV at entry) that had progressed after one line of systemic chemotherapy. This study was conducted in accordance with the ethical principles originating in the Declaration of Helsinki, good clinical practice, and all applicable laws and regulations. The ethical review board at each site approved the study, and all patients provided written informed consent before undergoing any study procedure.
Treatment and Study Design
Patients were randomized in a 2:1 ratio to the experimental combination (LY2181308 and docetaxel arm) or standard-of-care treatment (docetaxel arm). Before cycle 1, patients in the LY2181308/docetaxel arm received two consecutive loading doses of 750 mg LY2181308 intravenously for 3 hours each one and two days prior to day 1 of cycle 1.8 A third loading dose was administered on day 1 of cycle 1, followed by weekly maintenance doses (on days 6 and 14) of 750 mg LY2181308. On day 1 of cycle 1, patients received 75 mg/m2 docetaxel (administered over approximately 1 hour) 1 hour after LY2181308 infusion. Beginning with cycle 2 (and beyond), LY2181308 was administered on days 1, 8, and 15, with docetaxel 75 mg/ m2 on day 1 of each cycle until disease progression. Each cycle started with the administration of docetaxel and lasted 21 days (see Supplementary Fig. 1, Supplemental Digital Content 1, http://links.lww.com/JTO/A645 for study diagram).
The primary objective was to evaluate the antitumor activity of LY2181308 in combination with docetaxel using CTS from baseline to the end of cycle 2 as an endpoint, as proposed by Karrison et al.9 The approach of using tumor size measurements as a continuous rather than categorical variable for assessing antitumor activity preserves information at the patient level and increases statistical efficiency as suggested by Lavin et al.10 Secondary objectives included a comparison of the following between treatment arms: PFS, OS, objective response rate, safety, pharmacokinetic profiles, and tumor markers associated with tumor progression, such as plasma CK19 fragment (CYFRA21-1) and carcinoembryonic antigen.
Endpoint Assessments
For the analysis of CTS, tumors were measured per Response Evaluation Criteria in Solid Tumors 1.111 using serial high-resolution computed tomography imaging. Scans of the chest/abdomen/pelvis were performed at baseline and at every other cycle. All images were collected at 3-mm slices or with higher resolution, read by local investigators and by a central assessment (VirtualScopics, Rochester, NY). Safety was assessed in every cycle by analyzing adverse events (AEs, graded per Common Terminology Criteria for Adverse Events v4.0) and serious AEs. CYFRA21-1 and carcinoembryonic antigen were measured in plasma at baseline and before each cycle at a central laboratory (Quintiles, Durham, NC).
Statistical Analyses
Patients who received at least one dose of study drug were included in all statistical analyses. All tests of treatment effects were conducted at a two-sided alpha level of 0.05.
The analysis of the primary endpoint (i.e., CTS) was planned to occur after 120 patients received at least two cycles of therapy. Using simulations, chances of detecting a difference in CTS at the 5% significance level with this sample size were 100%, 99%, or 86%, if the assumed differences in CTS were 60%, 50%, and 40%, respectively. CTS, analyzed as the log of the ratios of tumor size (defined as the sum of the longest diameters) at visit 2 to tumor size at baseline, follows a normal distribution and was compared between the treatment arms using a t test. The primary analyses were based on the measurements obtained from the central imaging assessment. Kaplan–Meier curves were produced for each time-to-event variable,12 and the differences between arms were assessed with the log-rank test. The effect of prognostic factors on PFS was assessed using a Cox proportional hazards model.13
RESULTS
Patient Disposition
The study was conducted from May 2010 to June 2012. A total of 207 patients entered the study, of which 120 were randomized to LY2181308/docetaxel and 60 to docetaxel (docetaxel monotherapy) (Supplementary Fig. 2, Supplement al Digital Content 2, http://links.lww.com/JTO/A646). Of the enrolled patients, 90% (162 of 180) were eligible for the study evaluation (especially for CTS assessment). Patient demographics were similar between the two arms with respect to age, race, sex, and Eastern Cooperative Oncology Group performance status (Supplementary Table 1, Supplemental Digital Content 3, http://links.lww.com/JTO/A647).
Change in Tumor Size
Based on central imaging data, the mean (SD) tumor size ratio at cycle 2 to that at baseline was 1.05 (0.21) with LY2181308/docetaxel and 1.00 (0.15) with docetaxel (p = 0.200). These data coincided with the investigator-assessed CTS evaluation (Table 1) (1.07 [0.28] with LY2181308/docetaxel versus 1.04 [0.28] with docetaxel; p = 0.666). A waterfall plot for CTS was produced for the treatment groups based on the central imaging data (Supplementary Fig. 3, Supplemental Digital Content 4, http://links.lww.com/JTO/A648). Tumor size diameter by visit and treatment is depicted in Supplementary Figure 4 (Supplemental Digital Content 5, http://links.lww.com/JTO/A649).
Table 1.
Ratio of Tumor Sizea at Cycle 2 to That at Baseline
| LY2181308 and Docetaxel (N = 114) |
Docetaxel (N = 48) |
Total (N = 162) |
pb | |
|---|---|---|---|---|
| Investigator-determined (based on case report forms) | ||||
| Number of patients | 85 | 36 | 121 | 0.666 |
| Mean (SD) | 1.07 (0.28) | 1.04 (0.28) | 1.06 (0.28) | |
| Median | 1.03 | 1.00 | 1.02 | |
| Missing | 0 | 1 | 1 | |
| Central assessment of imaging data | ||||
| Number of patients | 82 | 42 | 124 | 0.200 |
| Mean (SD) | 1.05 (0.21) | 1.00 (0.15) | 1.03 (0.19) | |
| Median | 1.02 | 0.97 | 1.02 | |
| Missing | 0 | 0 | 0 | |
The sum of lesion diameters is calculated per Response Evaluation Criteria in Solid Tumors 1.1 guidelines.
p value from comparison using t test. The t test is based on the logarithm of the ratio of tumor size at cycle 2 to that at baseline, as this measure follows a normal distribution.
Progression-Free Survival
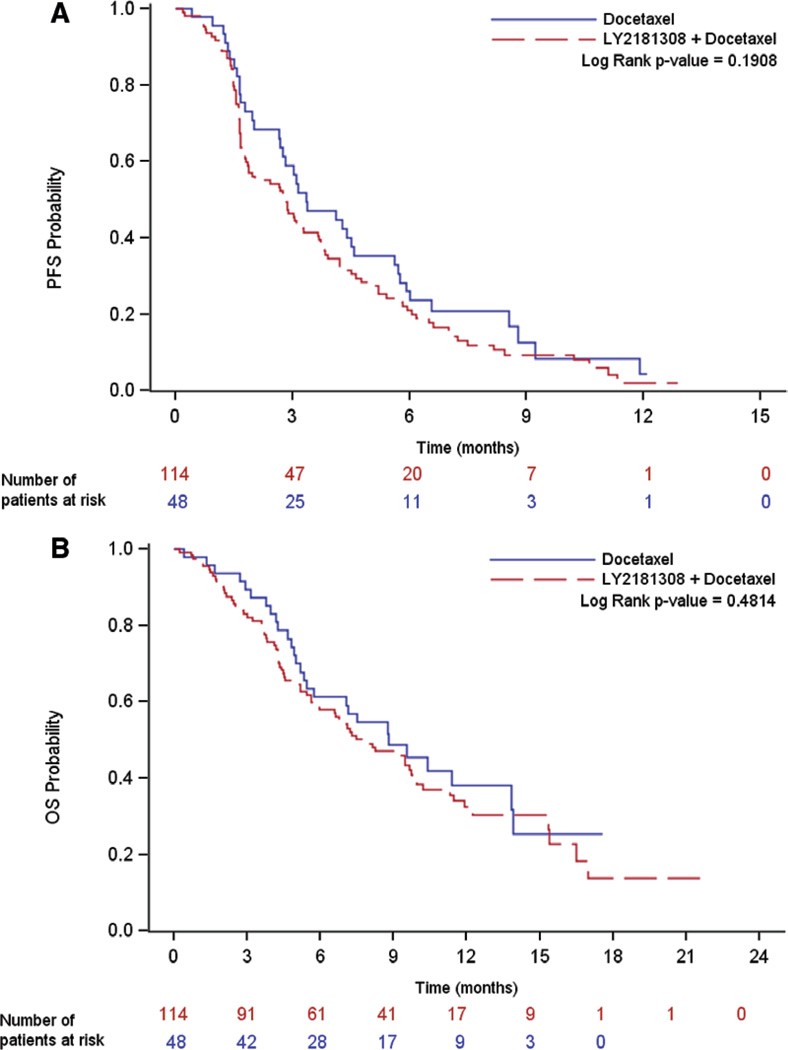
The median PFS was 2.83 (95% confidence interval [CI], 1.84–3.65) months with LY2181308/docetaxel and 3.35 (95% CI, 2.69–4.57) months with docetaxel (p = 0.191) (Supplementary Table 2, Supplemental Digital Content 3, http://links.lww.com/JTO/A647, and Fig. 1A).
Figure 1.
Kaplan–Meier graphs of PFS and OS. A, Kaplan–Meier graph for PFS, all treated patients; (B) Kaplan–Meier graph for OS, all treated patients. PFS, progression-free survival; OS, overall survival.
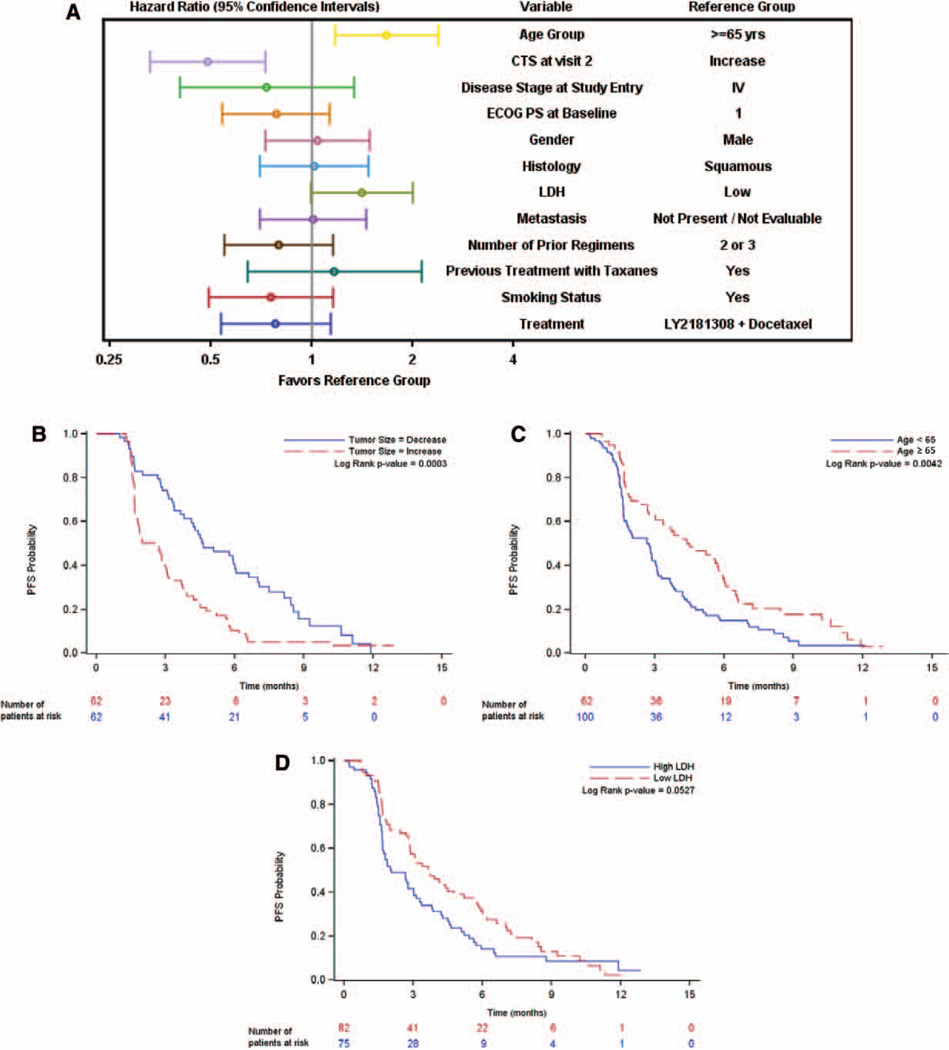
As depicted in a forest plot (Fig. 2A), CTS (decrease versus increase), age (<65 versus ≥65), and lactate dehydrogenase (high versus low) were identified as factors affecting PFS (Figs. 2B–D). For both arms combined, CTS reduction translated to longer PFS (PFS of 4.63 months in patients with decreased CTS compared with 2.66 months in patients with increased CTS).
Figure 2.
Evaluation of influence factors on PFS. A, Influence of various baseline and prognostic factors on PFS, patients on therapy; (B) Kaplan–Meier graph for PFS by CTS at visit 2 split at median, patients on therapy; (C) Kaplan–Meier graph for PFS by age group, patients on therapy; (D) Kaplan–Meier graph for PFS by LDH split at median, patients on therapy. CTS, change in tumor size; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; PFS, progression-free survival; IV, intravenous.
In a subgroup of patients (N = 50), CYFRA21-1 levels were available.14 Patients with low baseline CYFRA21-1 levels had a median PFS of 5.62 months (95% CI, 2.66–5.91), whereas patients with high CYFRA21-1 baseline levels had a median PFS of 1.61 months (95% CI, 1.35–2.86).
Overall Survival
The median OS (90% CI) was 7.9 (6.6–9.7) months with LY2181308/docetaxel and 8.8 (5.7–13.8) months with docetaxel (p = 0.481) (Supplemental Table 2, Supplemental Digital Content 3, http://links.lww.com/JTO/A647, and Fig. 1B).
Tumor Response
Partial response was observed in seven patients in the LY2181308/docetaxel arm and one patient in the docetaxel arm, yielding objective response rates (95% CI) of 6.1% (1.7%–10.5%) and 2.1% (2.0%–6.1%), respectively (p = 0.438) (Supplemental Table 2, Supplemental Digital Content 3, http://links.lww.com/JTO/A647).
Safety
Ten (8.8%) patients in the LY2181308/docetaxel arm and three (6.3%) patients in the docetaxel arm discontinued due to serious AEs considered possibly related to study drug. The most frequently reported grade 3/4 AEs were similar between the two treatment arms (Supplemental Table 3, Supplemental Digital Content 3, http://links.lww.com/JTO/A647) and consistent with the known docetaxel toxicity profile.
Pharmacokinetics
Pharmacokinetics of LY2181308 alone, docetaxel alone, and docetaxel in combination with LY2181308 were consistent with their respective known profiles (Supplementary Figs. 5–7, Supplemental Digital Content 6–8, http://links.lww.com/JTO/A650, http://links.lww.com/JTO/A651, http://links.lww. com/JTO/A652).
DISCUSSION
Antitumor activity seen in preclinical models5 did not translate to clinical benefit in the present randomized phase II study comparing LY2181308 and docetaxel with standard docetaxel in patients with NSCLC. A similar observation was made in patients with prostate cancer.6 There are several possible reasons for our findings. First, although the dose and schedule of LY2181308 used in this study were previously shown to reduce survivin,8 tumor tissue was not obtained to confirm target inhibition in lung cancer patients in the current study. Second, this trial did not select patients on the basis of histology or biomarkers of tumor survivin expression, raising the possibility that benefit in a subset of patients was missed. Finally, we cannot exclude that survivin is not a dominant mechanism in the development of chemoresistance in NSCLC.
CTS was chosen as a primary endpoint to assess antitumor activity because it can serve as an early evaluation of efficacy in phase II studies.9 Although no statistically significant difference was observed between treatment arms, CTS did emerge as a potentially useful early endpoint across the entire study population, with a significant association between CTS and PFS. Thus, for future clinical trial designs, CTS appears to be an early marker of efficacy, with relatively short time intervals, that can aid in monitoring the efficacy of new treatments and hence accelerate decision-making in early-phase clinical trials.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Vera Westermann, Bonn, Germany, for the scientific advice for the imaging requirements. The authors also thank Michelle Mynderse, PhD, and Noelle Gasco, employees of inVentiv Health Clinical, for writing and editorial support.
Research support for this study (ClinicalTrials.gov identifier NCT01107444) was provided by Eli Lilly and Company.
FB, SD, RR, YS, and DT served on an advisory board for Eli Lilly; SD and YS received travel accommodations for ASCO meeting attendance from Eli Lilly; SG, FG, MPS, and YS have spoken for Eli Lilly; JM received an investigator's fee; YS received funding as a clinical research fellow from Eli Lilly; and VA, SC, ML, and MD are employees and stockholders of Eli Lilly.
Footnotes
Presented in part at the American Society of Clinical Oncology Annual Meeting, Chicago, Illinois, May 31–June 4, 2013.
Disclosure: The other authors declare no conflict of interest.
REFERENCES
- 1.Pisani P, Parkin DM, Bray F, et al. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18–29. doi: 10.1002/(sici)1097-0215(19990924)83:1<18::aid-ijc5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 3.Chang A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer. 2011;71:3–10. doi: 10.1016/j.lungcan.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Mita AC, Mita MM, Nawrocki ST, et al. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 5.Carrasco RA, Stamm NB, Marcusson E, et al. Antisense inhibition of survivin expression as a cancer therapeutic. Mol Cancer Ther. 2011;10:221–232. doi: 10.1158/1535-7163.MCT-10-0756. [DOI] [PubMed] [Google Scholar]
- 6.Wiechno P, Somer BG, Mellado B, et al. A randomised phase 2 study combining LY2181308 sodium (survivin antisense oligonucleotide) with first-line docetaxel/prednisone in patients with castration-resistant prostate cancer. Eur Urol. 2014;65:516–520. doi: 10.1016/j.eururo.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Sung C, Dartois C, et al. Elucidation of relationship between tumor size and survival in non-small-cell lung cancer patients can aid early decision making in clinical drug development. Clin Pharmacol Ther. 2009;86:167–174. doi: 10.1038/clpt.2009.64. [DOI] [PubMed] [Google Scholar]
- 8.Talbot DC, Ranson M, Davies J, et al. Tumor survivin is downregulated by the antisense oligonucleotide LY2181308: a proof-of-concept, first-in-human dose study. Clin Cancer Res. 2010;16:6150–6158. doi: 10.1158/1078-0432.CCR-10-1932. [DOI] [PubMed] [Google Scholar]
- 9.Karrison TG, Maitland ML, Stadler WM, Ratain MJ. Design of phase II cancer trials using a continuous endpoint of change in tumor size: application to a study of sorafenib and erlotinib in non small-cell lung cancer. J Natl Cancer Inst. 2007;99:1455–1461. doi: 10.1093/jnci/djm158. [DOI] [PubMed] [Google Scholar]
- 10.Lavin PT. An alternative model for the evaluation of antitumor activity. Cancer Clin Trials. 1981;4:451–457. [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete oberservations. J Amer Statis Assn. 1958;53:457–481. [Google Scholar]
- 13.Cox DR. Regression models and life-tables. J Royal Statis Soc, Series B (Methodological) 1972;34:187–220. [Google Scholar]
- 14.Ardizzoni A, Cafferata MA, Tiseo M, et al. Decline in serum carcinoembryonic antigen and cytokeratin 19 fragment during chemotherapy predicts objective response and survival in patients with advanced nonsmall cell lung cancer. Cancer. 2006;107:2842–2849. doi: 10.1002/cncr.22330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.