Abstract
Treatments for IBD, IBS, FD or motility disorders are not adequate, and purinergic drugs offer exciting new possibilities. GI symptoms that could be targeted for therapy include visceral pain, inflammatory pain, dysmotility, constipation and diarrhea. The focus of this review is on potential for developing purinergic drugs for clinical trials to treat GI symptoms. Purinergic receptors are divided into adenosine P1 (A1,A2A,A2B,A3), ionotropic ATP-gated P2X ion channel (P2X1–7) or metabotropic P2Y1,2,4,6,11–14 receptors. There is good experimental evidence for targeting A2A, A2B, A3, P2X7, P2X3 receptors or increasing endogenous adenosine levels to treat IBD, inflammatory pain, IBS/visceral pain, inflammatory-diarrhea and motility disorders. Purine genes are also potential biomarkers of disease. Advances in medicinal-chemistry have an accelerated pace toward clinical trials: Methotrexate and sulfasalazine, used to treat IBD, act by stimulating CD73-dependent adenosine production. ATP protects against NSAID-induced enteropathy and has pain-relieving properties in humans. A P2X7R antagonist AZD9056 is in clinical trials for CD. A3 AR drugs target inflammatory diseases (e.g. CF101; CF102). Dipyridamole, a nucleoside uptake-inhibitor, is in trials for endotoxemia. Drugs for pain in clinical-trials include P2X3/P2X2/3(AF-219) and P2X7(GSK1482160) antagonists and A1(GW493838) or A2A(BVT.115959) agonists. IberogastR is a phytopharmacon targeting purine-mechanisms with efficacy in IBS and FD. Purinergic drugs have excellent safety/efficacy profile for prospective clinical trials in IBD, IBS, FD and inflammatory-diarrhea. Genetic polymorphisms and caffeine consumption may affect susceptibility to treatment. Further studies in animals can clarify mechanisms and test new-generation drugs. Finally, there is still a huge gap in our knowledge of human pathophysiology of purinergic signaling.
Keywords: Purinergic drugs, clinical trials, inflammatory diarrhea, visceral pain, endogenous adenosine, A3 receptors, ATP-gated P2X ion channels, Inflammatory Bowel Disease, Irritable Bowel Syndrome
1.0 Introduction
The purinergic field has come a long way since the original experiments done to identify the non-adrenergic, non-cholinergic (NANC) inhibitory neurotransmitter in the gut. 1 Recent studies challenge the view that adenosine triphosphate (ATP) is the main purinergic transmitter involved in gut neuromuscular transmission in mice, primates and humans. 2–5 Cloning experiments roughly 2 decades ago identified four G protein-coupled adenosine (P1) receptors (A1, A2A, A2B, A3), seven subtypes of P2X ion channel receptors for nucleotides (P2X1–7) and 8 G protein-coupled receptors for nucleotides (P2Y1,2,4,6, 11–14 receptors). 6 The P2X receptor channels consist of subunit trimers, which often are heterogeneous combinations of the various P2X proteins. Therefore, the medicinal chemistry of the P2X system is more challenging than the adenosine and P2Y receptors. Nevertheless, advances in medicinal chemistry are providing highly selective compounds for all 3 families of purinoceptors (P1, P2X and P2Y) to study the pharmacology, pathophysiology and therapeutic potential of targeting such receptors. A recent comprehensive review by Burnstock 7 covers purinergic signaling in the GI tract and related organs in health and diseases, in all cell types that express purinergic receptors. The focus of this review is on potential for developing purinergic drugs for clinical trials for GI diseases or disorders, and in particular inflammatory bowel diseases (IBD), irritable bowel syndrome (IBS), functional dyspepsia (FD), motility disorders and diarrheal disorders. Current treatments strategies are inadequate, and purinergic drugs offer exciting new possibilities. GI symptoms that can be targeted for therapy include visceral pain, dysmotility, constipation, gastroparesis and diarrhea.
Purinergic signaling plays an important role in gut neural reflexes, and most if not all purinergic receptors have been identified in the gut, differentially distributed on different cellular components of the gut, including several types of neurons in the enteric nervous system (ENS, sensory, interneurons and motor neurons), epithelial cells, immune/inflammatory cells, enterochromaffin cells (EC), glial cells, interstitial cells of Cajal and smooth muscle. 8,9 Purines in the gut are involved in secretion, immunomodulation, synaptic transmission, neuromuscular transmission, gliotransmission, and visceral sensation. Key studies utilizing gene knockout models to investigate the role of purines in IBD or functional GI disorders (e.g. IBS and FD) are referenced in Table 1. As discussed later, purines act as ‘danger signals’ and are very sensitive to inflammation or the health-disease state of tissues and organs. Therefore, abnormalities in purinergic signaling are a hallmark of IBS, IBD, chronic inflammation, diabetic neuropathy or other disease-states. Purines are important regulators of the neurophysiology of the gut, and therefore such abnormalities are linked to significant pathophysiology. Abnormalities in purinergic signaling are summarized in Table 2.
Table 1.
Key studies in IBD or functional GI disorders in mouse knockout models targeting purine genes
| Model | Cellular Target | Functional Consequence | Disease/Functional disorder |
|---|---|---|---|
| P2X7 −/− KO (DNBS, DSS, Oxazolone, IL-10−/−) | nNOS + Myenteric Neurons | Prevents neuronal apoptosis and protects against IBD inflammation-induced colonic motor dysfunction | IBD 73 |
| Mouse Jejunum, Serum & Peritoneum (T spirallis) & P2X7−/− KO | Macrophages | Increased IL-1β-mediated mechanosensitivity in mesenteric afferents, not present in KO | IBS 212 |
| P2X2/3−/− KO (T spirallis) | Small Intestinal Afferent Nerves | Increased afferent hypersensitivity. | IBS 164 |
| Heterozygote and P2X3 −/− KO (zymosan) | DRG Neurons | Reduced VMR to CRD Absent hypersensitivity in zymosan-treated P2X3−/− KO |
IBS 165 |
| A2AAR−/− KO (H. felis), IL-10 −/− KO (H. pylori) | Blood & Mucosal Th cells | Exacerbated gastritis with no difference in colonization (Diminished with ATL313 in IL-10 deficient) | GERD 133 |
| A2AAR −/−KO and A2BAR −/−KO (DSS) | Epithelial cells | Increased severity of DSS colitis and loss of mucosal IL-10 expression in A2BAR −/−KO | IBD 68 |
| A2BAR −/− KO (DSS, TNBS, S typhimurium) | Epithelial Cells | Reduced inflammatory response Possible abnormal circular muscle |
IBD 98 |
| CD39-null (TNBS, Oxazolone) | Lamina Propria | Decreased severity of disease (TNBS but no Oxazolone) | CD & UC 52 |
| CD39 null & heterozygote (DSS), Colon | Leukocytes | Increase severity of DSS colitis Increase leukocyte infiltration |
IBD 26 |
| A3AR−/−KO(DSS) | Mucosa | Decreased severity of disease (prevent diarrhea, weight loss, inflammation) vs WT | IBD 29 |
| A3AR −/−KO (DSS) | Epithelial Cells, ENS/Glial cells | Increased intestinal transit and colonic evacuation | IBD 29 |
| A3AR−/−KO (DSS) | Mucosa | Moderate protection against colitis; impaired innate immune response | IBD 30 |
| CD73 −/− KO (DSS), Colon | Epithelial Cells | High susceptibility to DSS-induced colitis. Increase TLR9 (mRNA), IL-IB, TNFα (ELISA), constitutive activation of NF-κB | IBD 213 |
| CD73 −/− KO (TNBS) | Mucosa | Increase severity of colitis (weight loss, colon shortening) Downregulation of INFα | IBD 64 |
| P2ry1 −/−KO | Circular Muscle Cells | fIJP completely absent and delayed colonic transit | Analysis of phenotype 4 |
| P2ry1−/− KO | Circular Muscle Cells | Absent purinergic IJP Absence of spontaneous IJP |
Analysis of phenotype 214 |
DSS; Dextran Sodium Sulfate, DNBS; 2,4-Dinitrobenzene Sulfonic Acid, fIJP; Fast Inhibitory Junction Potential, IBD; Inflammatory Bowel Disease, IBS; Irritable Bowel Syndrome, TNBS; 2,4,6-Trinitrobenzenesulfonic acid, VMR to CRD; Visceromotor Reflex to Colorectal distension, S typhimurium; Salmonella typhimurium, T spiralis; Trichinella spiralis
Table 2.
Abnormalities of purinergic signaling in disease
| Model | Target | Δ Gene expression | Functional Consequence | Disease |
|---|---|---|---|---|
| Mouse Colon (Zymosan) | Colonic DRG | ↑P2X3R (Increased Function) | Increased excitability and enhanced purinergic signaling | IBS 215 |
| Rat (Acetic acid) | DRG from LS but not TL | ↑P2X3R | In association with visceral hypersensitivity | IBS 166 |
| Rat colon and Spinal Cord (CRD) | Whole Colon, Neurons | ↑P2X4R (Immunoreactivity) | In association with visceral hypersensitivity | IBS 216 |
| Mouse Small Intestine (T spiralis) | Visceral Afferents | ↑Purinergic Component (PPADS Sensitive) | Increased afferent sensitivity small intestine. | IBS 164 |
| Rat (STZ) | DRG L4-6 | ↑P2X2R, ↑P2X3R (mRNA) | In association with Neuropathic Pain | Diabetic Neuropathy 217 |
| Rat (STZ) | DRG L4-6 (hind-paw labeled) | ↑P2X3R (Protein Trafficking) | In association with Neuropathic Pain | Diabetic Neuropathy 218 |
| Rat (STZ) | Microglia from Spinal Cord | ↑P2X4R | In association with Neuropathic Pain | Diabetic Neuropathy reviewed in 219 |
| Human biopsies (ganglionic & aganglionic regions) | Myenteric and Submucose and Nerve Fiber in Muscle Layers | ↓P2Y1R ↓P2Y2R |
Decrease in immunoreactivity for P2Y1/P2Y2 occurs in the aganglionic segment | Hirschprung’s Disease 220 |
| Mouse Ileum (S mansoni) | Longitudinal Muscle | ↓A1AR | Impaired inhibitory adenosinergic modulation of cholinergic transmission | Chronic Inflammation 221 |
| Rat colon (DNBS) | LMMP | ↑A3AR ↑ADA (mRNA) |
Decreased cholinergic contraction in nflamed tissue | IBD 145 |
| Mouse Colon (DSS) | Epithelium | ↑P2Y2R ↑P2Y6 R (mRNA) |
Proinflammatory effect | IBD 53 |
| Rat (DNBS) | LMMP | ↑A2AAR ↑CD73 (mRNA) A1AR (Not Affected) |
Inhibitory control of motor function converted from a predominant A1 to A2A dependent regulation | IBD 149 |
| Mouse (DSS and IEC-6) | Epithelial Cells | ↑P2Y2R (mRNA) | Exacerbate inflammation | IBD 144,222 |
| Guinea pig (LMMP) (TNBS) | Smooth Muscle | Augmented Release of ATP, ADP, AMP, ADO, β-NAD (by HPLC) | Impaired purinergic fIJP via P2Y1R receptors | IBD 223 |
| Mouse(DSS), Guinea pig (TNBS) | Smooth Muscle | ↓ATP & ADP Release Stimulus-Induced | Reduced IJP and propulsive motility | IBD 223 |
| OVX Female rats (TNBS-EtOH) | DRG neurons | ↓P2X3R in association with ↓VMR | P2X3 and hyposensitivity in OVX rats reverted after Estrogens treatment | IBD 161 |
| Mouse Colon (DSS) | Macrophages (F4/80 +) Submucosal Arterioles | ↑CD39 (ENPD1)(Protein & mRNA) | Linked to impaired arterioles-constriction | IBD 75 |
| Rat Ileum/jejunum (TNBS) | Whole Tissue | ↓A1AR ↓A2AAR ↓A3AR (mRNA) |
Altered nerve mediated cholinergic contractions | IBD 191 |
| Rat (TNBS) | Longitudinal Muscle | ↑A2AAR | Facilitate inhibition of cholinergic transmission | IBD 224 |
| Mouse Mast cell Deficient, Colon (TNBS, DSS) | Mononuclear Cells | ↓P2X7R ↓ATP |
Amelioration of colitis (↑P2X7R & ATP in WT) | IBD 54 |
| Guinea pig (TNBS) | Submucosal Neurons | ↑P2X function (fEPSP) | Submucosal synapses | IBD 225 |
| Human mucosa & Rat (DSS) | Mucosa & EC | ↑A2BAR ↑HIF-1a (mRNA, protein) |
Exacerbation of inflammation by increased 5HT release via A2BAR | CD 129 |
| Human PBMC | Mononuclear cells | ↑A3AR (Protein) | Biomarker of disease and therapeutic target | CD 45 |
| Human biopsies | Epithelial Cells | ↑P2Y2R ↑P2Y6R (mRNA) |
Increased Neutrophils infiltration | CD & UC 53 |
| Human whole colonic biopsies | Colon | ↑CD39 (Inactive CD vs UC) Variable in animal models |
Exacerbation of inflammation | CD 52 |
| Human whole colonic biopsies | Colon | ≈CD39 (Inactive UC) | Not difference | UC 52 |
| Human Serum/Neutrohil | Neutrophils | ↑tADA, ↑ADA2 (Serum), ↑ADA1(Netrophils) | Increased inflammatory response (Possible biomarker of inflammation - Active Disease-) | CD 50 |
| Human colonic biopsies | Myenteric Neurons | ↑P2X3R (Protein) | Dysmotility and Pain | CD & UC 162 |
| Human UC (colon) vs control | EC (5-HT+ cells) | ↓P2X3+/5-HT+ cells from 15% to <1% | Expected to alter fast purinergic regulation of 5-HT release | UC 130 |
| Human mucosal biopsies | Mucosa | ↓ADORA3, ↑ADORA2A, ↓AMPD3, ↑ADAR, ↓P2RY13, ↓P2RY14, ↑NT5E, etc | Unique purine dysregulation profile for CD/distinguishes between CD and UC (biomarker of disease) | CD (biomarker of disease) 47 |
| Human mucosal biopsies | Mucosa | ↑ADORA3, ↑AMPD3, ↑P2RY13, ↑P2RY14, ↑DPP4, ↓P2RY6, ↑NT5E, etc | Unique purine dysregulation profile for UC | UC (biomarker of disease) 47 |
| Human PBMC | Mononuclear Cells | ↑ADORA2B, ↑ADORA2A, ↑AMPD3, ↓ADAR, ↓DPP4, ↓P2RX5, ↑P3RY5, ↑AMPD2, etc | Unique purine dysregulation profile for CD/distinguishes between CD and UC | CD (biomarker of disease) 47 |
| Human PBMC | Mononuclear Cells | ↓ADORA2B(w), ↓ADORA2A(w), ↑AMPD2↑ADAR, ↑DPP4, ↑P2RX5, ↓P2RX1, ↓P2RX2, ↓P2RX3, etc | Unique purine dysregulation profile for UC | UC (biomarker of disease) 47 |
| Human samples | Peripheral Blood | P2X7R (loss of function Arg307Gln, P=0.06) | Polymorphism is not a susceptibility factor for CD | CD 19 |
| Human colon and ileum | Epithelial Cells | ↓P2X7R (Protein) ↑P2X7R (mRNA) |
Increased PMNL transepithelial migration. Amplified inflammatory loop. |
CD 51 |
| Human colonic biopsies and TNBS & DSS mice | Mast Cells | ↑P2X7R | Associated with aggravation of intestinal inflammation | CD (biomarker of disease) 54 |
| Human Colonic biopsies and TNBS & DSS mice | Mast Cells | No change in P2X7R vs control | Not difference | UC 54 |
ADA1 and 2; ADA Izoenzimes 1 and 2, CFA; Complete Freud’s Adjuvant, CRD; Colorectal Distension, DNBS; 2,4-Dinitrobenzene Sulfonic Acid, DRG; Dorsal Root Ganglia, DSS; Dextran Sodium Sulfate, GERD; Gastroesophageal Reflux Disease, H felis; Helicobacter felis, H pylori: Helicobacter pylori, HIF-1a; Hypoxia-inducible factor 1-alpha, induces transcription and increases the activity of 59ecto-nucleotidase (CD73), the enzyme that converts AMP to adenosine. CD73 also regulates transcription of the ADORA2B receptor while suppressing transcription of the adenosine re-uptake transporters (ENT1 and 2), IBD; Inflammatory Bowel Disease, IBS; Irritable Bowel Syndrome, LS; Lumbosacral, NCI; Nerve Chronic Constriction Injury, PBMC; Peripheral blood mononuclear cells, S mansoni; Schistosoma mansoni, STZ, Streptozotocin (Model of diabetes and neuropathic pain), S typhimurium; Salmonella typhimurium, tADA; (CD26) total Adenosine deaminase, TNBS; 2,4,6-Trinitrobenzenesulfonic Acid, TL; Toracolumbar, T spiralis; Trichinella spiralis, W; Woman
There is good experimental evidence for targeting A2A, A2B, A3, P2X7, P2X3 receptors or increasing adenosine levels to treat IBD, inflammatory pain, visceral pain in IBS or FD and inflammatory-diarrhea, and key studies will be discussed. More comprehensive reviews have been written on the basic physiology, pathophysiology and signaling mechanisms of purinergic signaling in the GI tract. 7,10–14 There is also an expanding list of ‘purinergic drugs’ in clinical trials for relevant diseases that will be reviewed, and several drugs have clinical efficacy in IBD, IBS or FD. Some of the challenges of developing purinergic drugs are given special consideration.
2.0 Medicinal chemistry
Adenosine (1, or derivatives) is used as a drug since the 1990’s. Adenocard and adenoscan are the generic forms of adenosine used for the treatment of supraventricular tachycardia and for cardiac stress testing, respectively. The medicinal chemistry of adenosine A1, A2A and A3 receptors (P1 family) is well developed and many selective ligands are available for ligand binding, pharmacological analysis, in vivo studies in animal models of disease, and a significant number are being pursued in human clinical trials. Advances in medicinal chemistry are providing a pipeline of new potential drugs for testing in animals and humans. Regadenoson (68, Lexiscan, Astellas Pharma) is the first selective A2A agonist approved by the FDA, which acts as a potent vasodilator. Drugs targeting adenosine receptors or which elevate endogenous adenosine levels are currently in advanced clinical trials as treatment for chronic heart failure, inflammatory and autoimmune disorders, dry eye syndrome, neurological disorders (e.g. Parkinson’s disease), hepatocellular carcinoma, uveitis, cardioplegia, neuropathic pain, FD, IBS, perioperative pain as well as stress/diagnostic agents. The medicinal chemistry of P2X and P2Y receptors is not as well-developed 15–17, although various P2X and P2Y drugs have progressed into clinical trials, and P2Y12 antagonists including clopidogrel (Plavix; Sanofi-Aventis/Bristol-Myers Squibb) is FDA approved and used widely to block platelet aggregation in the management of clot related cardiovascular events. 18
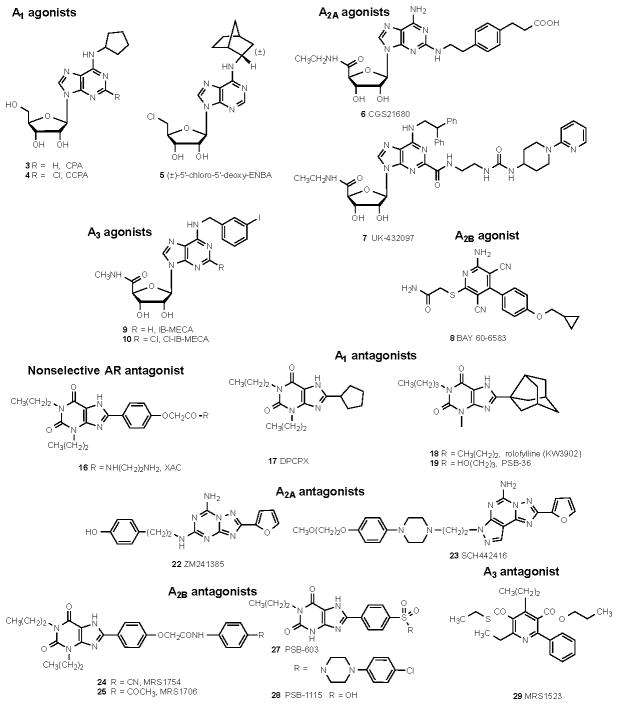
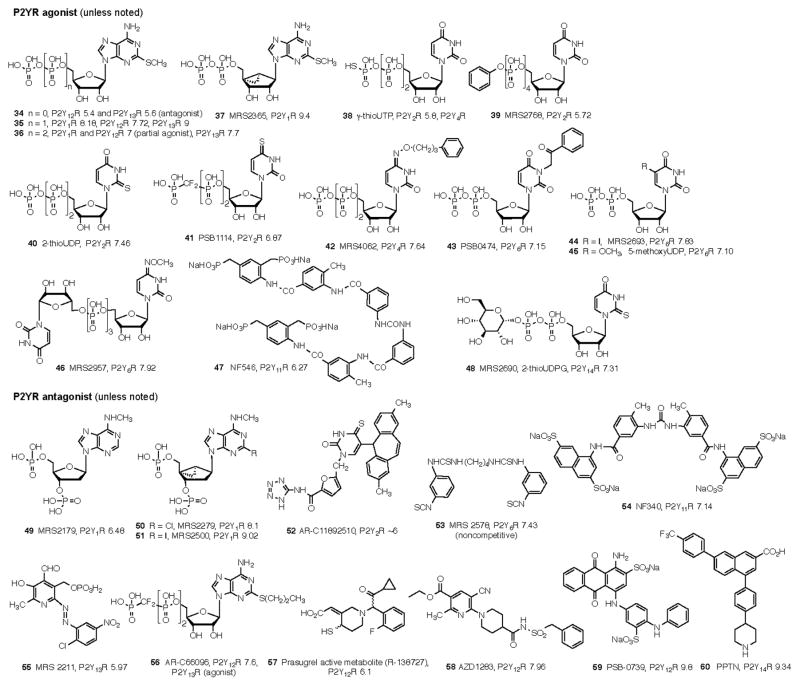
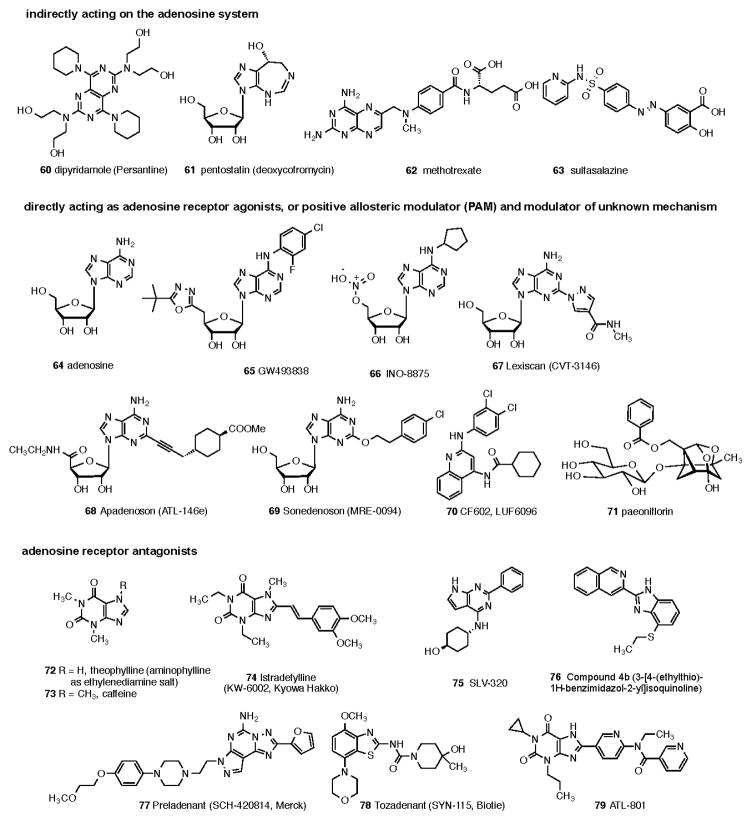
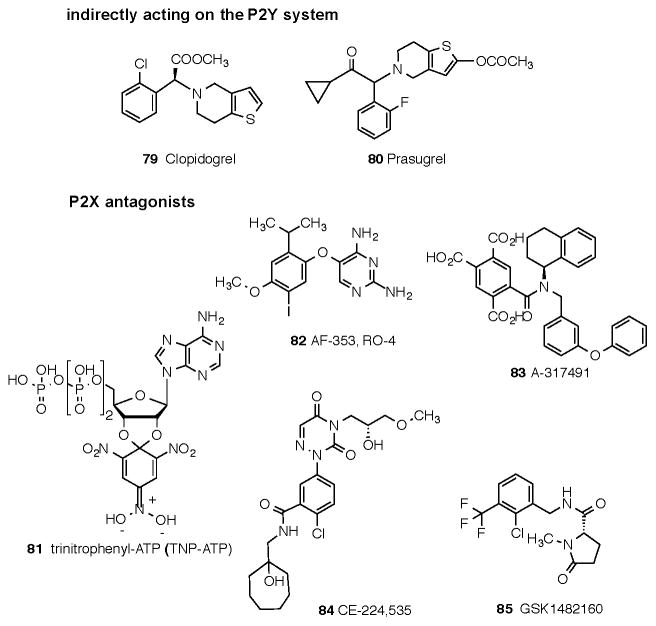
The affinities of commonly used adenosine receptor ligands for studying adenosine receptors are summarized in Table 3. Structures of commonly used ligands of adenosine (A1, A2A, A2B, A3) and P2Y1,2,4,6,11–14 receptors are shown in Figures 1A and 1B, respectively. Some of those compounds are currently in clinical trials, but the structures of most of the compounds mentioned in this review in a clinical context are shown in Figure 2A (adenosine system) and Figure 2B (P2X and P2Y systems). Table 4 summarizes selected purinergic drugs in clinical trials found on ClinicalTrials.gov that are described throughout the review. Table 5 lists selected ligands that represent new generation drugs that have been shown to have efficacy in animal models of IBD or IBS but have not yet made it to clinical trials.
Table 3.
Affinities of orthosteric adenosine receptor ligands that are commonly used as pharmacological probes. Affinity is shown at the human adenosine receptors, unless noted. Structures are shown in Figures 1A and 2A.
| No. | Compound | Affinity, pKia | |||
|---|---|---|---|---|---|
| A1 | A2A | A2Bb | A3 | ||
| Adenosine receptor agonists | |||||
| 1 | adenosineb | ~7 7.14 (r) |
6.49 6.82 (r) |
4.82 5.29 (r) |
6.54 5.19 (r) |
| A1-selective | |||||
| 2 | R-PIA | 8.69 8.92 (r) |
6.66 (r) | 3.82 | 7.48 6.80 (r) |
| 3 | CPA | 8.64 | 6.10 | 4.73 | 7.14 |
| 4 | CCPA | 9.08 8.89 (r) |
5.64 6.02 (r) |
4.73 | 7.42 6.63 (r) |
| 5 | 5′-Cl-5′-deoxy-ENBA | 9.29 | 5.89 | 5.87 | 5,56 |
| A2A-selective | |||||
| 6 | CGS21680 | 6.54 5.74 (r) |
7.57 7.72 (r) |
<5 <5 (r) |
7.17 6.23 (r) |
| 7 | UK432,097 | ND | 8.4 | ND | ND |
| A2B-selective | |||||
| 8 | BAY 60-6583 | <5b | <5b | 8–8.5 | <5b |
| A3-selective | |||||
| 9 | IB-MECA (CF101) | 7.29 | 5.54 | 4.96 | 8.74 |
| 10 | Cl-IB-MECA (CF102) | 6.66 6.55 (r) |
5.27 6.33 (r) |
<5 | 8.85 9.48 (r) |
| 11 | thio-Cl-IB-MECA | 6.71 | 6.63 | ND | 9.42 |
| 12 | MRS5698 | <5 | <5 | <5 | 8.5 |
| Adenosine receptor antagonists | |||||
| Non-selective | |||||
| 13 | Caffeine | 4.97 4.39 (r) |
5.02 4.32 (r) |
4.98 4.52 (r) |
4.88 <4 (r) |
| 14 | Theophylline | 5.17 5.06 (r) |
5.17 25,300 (r) |
5.04 4.82 (r) |
4.65 4.07 (r) |
| 15 | CGS15943 | 8.46 8.19 (r) |
8.92 | 7.49 | 7.46 |
| 16 | XAC | 7.54 8.92 (r) |
9.0 7.20 (r) |
7.91 | 7.04 |
| A1-selective | |||||
| 17 | DPCPX (CPX) | 8.52 9.0 (r) |
7.22 6.30 (r) |
7.29 6.73 (r) |
6.61 4.37 (r) |
| 18 | PSB-36 | 9.2 9.91 (r) |
6.01 6.26 (r) |
6.73 | 5.64 5.19 (r) |
| A2A-selective | |||||
| 19 | KW6002 | 6.08b 6.64(r)b |
7.92 8.66 (r) |
<5b | 5.35b |
| 20 | CSCd | 4.55 (r) | 7.27 (r) | 5.09 | <5 (r) |
| 21 | ZM241,385 | 6.11 | 8.80 | 7.12 | 6.13 |
| 22 | SCH442,416 | 5.95 | 8.39 | <5 | <5 |
| A2B-selective | |||||
| 23 | MRS1754 | 6.39 7.77 (r) |
6.30 6.21 (r) |
8.70 7.89 (r) |
6.24 |
| 24 | MRS1706 | 6.80 | 6.91 | 8.86 | 6.64 |
| 25 | MRE2029-F20 | 6.70 | <6 | 8.26 | <6 |
| 26 | PSB-603 | <5 <5 (r) |
<5 <5 (r) |
9.26 | <5 |
| 27 | PSB-1115 | <5 5.66 (r) |
4.62 (r) | 7.27 | <5 |
| A3-selective | |||||
| 28 | MRS1523 | <5 4.81 (r) |
5.44 5.69 (r) |
<5 | 7.72 6.95 (r) |
| 29 | PSB-10 | 5.77 6.09 (r) |
5.57 5.22 (r) |
ND | 9.36 |
| 30 | VUF5574 | ≤5 (r) | ≤5 (r) | ND | 8.39 |
| 31 | MRS1191 | <5 4.40 (r) |
<5 <5 (r) |
<5 | 7.50 5.73 (r) |
| 32 | MRS1334 | <5 (r) | <5 (r) | ND | 8.57 |
Figure 1.
Structures of commonly used ligands for adenosine and P2Y receptor families are shown. 1A. Selective agonist - and antagonist - probes of the adenosine receptors that are readily available as pharmacological probes. The compounds are numbered consecutively to correspond to the list in Table 3. For additional details refer to a review by Müller et al. 211 1B. Selective agonist - and antagonist - probes of the P2Y receptors that are readily available as pharmacological probes. The in vitro pEC50 or pIC50 is indicated at each relevant subtype. Compound 57 is the active metabolite of 80 (see Figure 2B). The compounds are numbered consecutively from the end of Figure 1A. More detail is available in reference 15 and on the website. 17
Figure 2.
Structures of ligands of clinical interest acting directly or indirectly through the adenosine receptor system (Fig. 2A), or through the P2X and P2Y receptor families (Fig. 2B). The compounds are numbered consecutively from the end of Figure 1B. Many of these compounds are proprietary
Table 4.
Drugs in clinical trials targeting purinergic receptors
| ID # | Drug | Target | Disease/Symptom | Sponsor | Phase | Status | Efficacy |
|---|---|---|---|---|---|---|---|
| NCT00376454 | GW493838 (66) | A1AR Agonist | Neuropathic Pain | GlaxoSmithKline | II | Completed | Results not available |
| NCT01123772 | INO-8875 (67) | A1AR Agonist | Dose escalation (HV) | Inotek Pharmaceuticals Corporation | I | Completed | Results not available |
| NCT00354458 | KW-3902IV (18) | A1AR Antag | AHF with RI | NovaCardia, Inc. | III | Completed 88 | No (SE, ↑ Stroke) |
| NCT00160134 | SLV320 (74) | A1AR Antag | AHF with RI | Solvay Pharmaceuticals | II | Completed 227 | Yes |
| NCT00744341 | SLV320 (74) | A1AR Antag | AHF with RI | Solvay Pharmaceuticals | II | Completed 228 | Not conclusive |
| NCT00452777 | BVT.115959 | A2AAR Agonist | Diabetic Neuropathic Pain | Swedish Orphan Biovitrum | II | Complete | Results not available |
| NCT00208312 | Regadenoson (68) | A2AAR Agonist | Stress Agent* | Astellas Pharma US, Inc. | III | Completed 229 | Yes |
| NCT00862641 | Regadenoson (68) | A2AAR Agonist | Stress Agent * | Astellas Pharma Inc | IV | Completed 230 | Yes |
| NCT00312364 | MRE0094 (70) | A2AAR Agonist | Diabetic Complications | Pfizer | II | Completed | Results not available |
| NCT01940848 | STW5, Iberogast, BAY98-7411 | A2AAR Antag | IBS | BAYER | III | Recruiting | Results not available |
| N/A | STW5/II | A2AAR Antag | IBS | Steigerwald Arzneimittelwerke GmbH | II | Completed 189 | Yes |
| N/A | STW5 | A2AAR Antag | Functional Dyspepsia | Steigerwald Arzneimittelwerke GmbH | II | Completed 188 | Yes |
| NCT01190735 | Caffeine (13) | A2AAR Antag | Parkinson’s Disease | McGill University Health Center | II | Completed | Results not available |
| NCT01190735 | Caffeine (13) | A2AAR Antag | Parkinson’s Disease | McGill University Health Center | II | Completed | Results not available |
| NCT01155466 | Preladenant (76) | A2AAR Antag | Parkinson’s Disease | Merck | III | Completed | Results not available |
| NCT00006337 | KW-6002 (73) | A2AAR Antag | Parkinson’s Disease | NINDS | II | Completed | Results not available |
| NCT00783276 | SYN115 (77) | A2AAR Antag | Cocaine addiction | National Institute on Drug Abuse (NIDA) | 0 | Completed 231 | Yes |
| NCT01435486 | Caffeine Citrate | A2AAR Antag | Bronchiolitis | Maastricht University Medical Center | N/A | Recruiting | Results not available |
| NCT01034306 | CF101 (9) | A3AR Agonist | RA | Can-Fite BioPharma | II | Recruiting | Results not available |
| NCT00837291 | CF101 (9) | A3AR Agonist | Osteoarthritis of the Knee | Can-Fite BioPharma | II | Not yet Recruiting | Results not available |
| NCT00428974 | CF101 (9) | A3AR Agonist | Plaque-type Psoriasis | Can-Fite BioPharma | II | Completed 35 | Yes |
| NCT00790673 | CF102 (10) | A3AR Agonist | Chronic Hepatitis C | Can-Fite BioPharma | I, II | Completed | Results not available |
| NCT00790218 | CF102 (10) | A3AR Agonist | Hepatocellular Carcinoma | Can-Fite BioPharma | I, II | Unknown | Results not available |
| NCT00349466 | CF101 (9) | A3AR Agonist | Dry Eye Syndrome | Can-Fite BioPharma | II | Completed 37 | Yes (SE, ↓ Intra ocular pressure) |
| NCT01235234 | CF101 (9) | A3AR Agonist | Dry Eye Syndrome | Can-Fite BioPharma | III | Completed 40 | Questionable efficacy |
| NCT01033422 | CF101 (9) | A3AR Agonist | Ocular Hypertension | Can-Fite BioPharma | II | Recruiting | Results not available |
| NCT01905124 | CF101 (9) | A3AR Agonist | Uveitis | Can-Fite BioPharma | II | Not yet Recruiting | Results not available |
| NCT00298636 | Adenosine (1) | AR Agonist | Perioperative Pain | Xsira Pharmaceuticals | II | Completed | Results not available |
| NCT00881686 | Adenosine (1) | AR Agonist | MRI | Xijing Hospital | I, II | Completed | Results not available |
| NCT01123525 | Adenosine (1) | AR Agonist | Cardioplegia | University Hospital of North Norway | I, II | Completed 232 | Yes |
| NCT01022151 | Aminophylline (13) | AR Antag | Recovery from Anesthesia | King Faisal University | II | Completed 233 | Yes |
| NCT01369745 | Dipyridamole (61) | ENT1/2 Inhib | RA | Zalicus | II | Completed | Results not available |
| NCT01091571 | Dipyridamole (61) | ENT1/2 Inhib | Endotoxemia | Radboud University | IV | Completed 82 | Yes |
| NCT01554579 | AF-219 | P2X3R Antag | Osteoarthritis of the Knee | Afferent Pharmaceuticals, Inc. | II | Recruiting | Results not available |
| NCT01569438 | AF-219 | P2X3R Antag | Bladder Pain Syndrome | Afferent Pharmaceuticals, Inc. | II | Recruiting | Results not available |
| NCT01432730 | AF-219 | P2X3R Antag | Chronic Cough | Afferent Pharmaceuticals, Inc. | II | Completed | Results not available |
| D8830C00002& | AZD9056 | P2X7R Antag | CD | Astra Zeneca | II | Completed 234 | Yes |
| NCT00520572 | AZD9056 | P2X7R Antag | RA | Astra Zeneca | II | Completed235 | No |
| NCT00628095 | CE-224,535 (84) | P2X7R Antag | RA | Pfizer | II, III | Completed 187 | No |
| NCT00849134 | GSK1482160 (85) | P2X7R Antag | Inflammatory Pain (HV) | GlaxoSmithKline | I | Completed 236 | Yes (↓ IL1B after LPS) |
| N/A | ATP | P2X? | Postoperative Orofacial Surgery Pain | Multicentre Study (Academic Institutions) | II | Completed 172 | Yes |
| NCT01107912 | Prasugrel (80) vs Clopidogrel (79) | P2Y12R Antag | CAD | Eli Lilly and Company | I | Completed 237 | Yes |
| NCT00557921 | CGT-2168 | P2Y12R Antag | CAD | Cogentus Pharmaceticals | III | Completed 238 | Yes |
| NCT01099566 | Prasugrel (80) | P2Y12R Antag | Sepsis (HV) | Medical University of Vienna | IV | Completed 239 | Yes |
AHF with RI; Acute Congestive/Decompensate Heart Failure with Renal Impairment, Antag; Antagonist, ENT1/2; Extracellular Nucleoside Transporters 1/2, CAD; Coronary Artery Disease, CD; Crohn’s Disease, HV; Healthy Volunteers, IBS; Irritable Bowel Syndrome, Inhib; Inhibitor, MRI; Myocardial Reperfusion Injury, N/A; Information not available, NINDS; National Institute of Neurological Disorders and Stroke, RA; Rheumatoid Arthritis, SE; Side-effects,
Stress Agent for Myocardial Perfusion Imaging in Coronary Artery Disease/Asthma and Pulmonary Disease, ID#; ClinicalTrials.gov Identifier,
astrazenecaclinicaltrials.com Identifier
Table 5. Medicinal candidates with efficacy in preclinical models.
| Drug/Treatment | Receptor Target | Cellular Target | Disease | Model | Mechanism | Efficacy |
|---|---|---|---|---|---|---|
| Paeoniflorin (72) | A1AR Agonist | Neurons | IBS | Rat (Maternal Separation-CRD) | Blocks visceral pain (Inhibition of CRD-Glutamate release and action in central structures of pain perception) | Yes 193 |
| FK352, DPCPX (17) | A1AR Antagonist | Possibly Myenteric Neurons | Post-Operative Ileus | Anesthetized Rats (pentobarbital) or Surgical Trauma | Improve propulsive motility (Reversed the slowed colonic propulsion) | Yes 152 |
| STW5 (Iberogast) | A2AAR Antagonist | Possibly Myenteric Neurons | IBD | RAT Ileum/Jejunum (TNBS) | ↑A2AAR (Inhibition of cholinergic transmission) | Yes 191 |
| ATL-313 | A2AAR Agonist | Mucosa | GERD | Mouse IL-10 Deficient (H. Pylori) | ATL-313 Reduce inflammation, bacterial load was increased | Yes 133 |
| Inosine | A2AAR Agonist | Mucosal T-cells | IBD | Rat (TNBS) | Improved leukocyte infiltration and epithelium destruction. Partially Reverted by SCH-442416 (A2AR Antagonist) | Yes 62 |
| ATL-801 (78) | A2BAR Antagonist | Epithelial Cells | IBD | Mouse (DSS), IL-10−/− KO (Piroxicam) | Ameliorate experimental colitis, ↓ Adenosine-mediated cAMP level, Inhibit secretion | Yes 96 |
| A-317491 (83) | P2X3R Antagonist | DRG Neurons | Neuropathic Pain | Rat (CFA & NCI) | Blocks specifically P2X3 & P2X2/3R | Yes 240,241 |
| AF-353 (82) | P2X3R, P2X3/2R Antagonist | Neurons | Pharmacokinetic Profile (rats) | Recombinant Expression of Human and Rat P2X3 in CHOK-K1 | Blocks specifically P2X3 & P2X2/3R | Yes 175 |
| Diaminopyrimidines | P2X3R, P2X3/2R Antagonist | DRG Neurons | Inflammatory Pain | Recombinant Expression of P2X3 | Blocks specifically P2X3 & P2X2/3R | Yes 176 |
| AZ004 | P2X3R, P2X3/2R Antagonist | Neurons | Inflammatory & Neuropathic Pain | Inflammatory Pain Model | Blocks specifically P2X3 & P2X2/3R | Yes 163 |
| Electroacupunture at He- Mu points | P2X4R | Neurons from Colon and Spinal Cord | IBS | RAT (CRD) | ↓P2X4R: Decrease Visceral Hypersensitivity | Yes 216 |
CFA; Complete Freud’s Adjuvant, CRD; Colorectal Distension, DRG; Dorsal root ganglia, GERD; Gastroesophageal Reflux Disease, IBD; Inflammatory Bowel Disease, IBS; Irritable Bowel Syndrome, NCI; Nerve Chronic Constriction Injury, TNBS; 2,4,6 Trinitrobenzenesulfonic acid
Drug License: STW5; BAYER, ATL-313; Santen Pharmaceutical Co, ATL-801; Adenosine Therapeutics, AF353; Afferent Pharmaceuticals Inc, AZ004; AstraZeneca
Polymorphisms of genes involved in purinergic signaling are important considerations in designing clinical trials to test safety and efficacy of new potential drugs. Important genetic variants, such as for ADOA2AR, P2X7R, CD39 and PON1, can alter susceptibility to disease or efficacy to treatment, which will be further discussed latter. 18–26
3.0 Adenosinergic drugs
3.1 A3 AR medicinal candidates in Inflammatory Bowel Diseases
3.1a) Experimental therapeutics of A3 AR
Adenosine receptors (A1, A2a, A2B and A3) are being investigated as therapeutic targets for chronic inflammatory disorders including IBD, autoimmune disorders and cancer. Adenosine is a potent anti-inflammatory agent and its actions are mediated in part through A3AR activation. A3AR agonists have been shown to be beneficial in experimental models of colitis. 27,28 In a model of colitis induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS), the prototypical A3AR agonist IB-MECA (9, CF101) was very effective in ameliorating colitis in rats treated with 3mg/kg IB-MECA i.b.d. for 7 days, and the drug protected animals against weight loss, developing GI symptoms (diarrhea, occult blood, mucosal inflammation) and prevented changes in gene-expression profiles associated with chronic mucosal inflammation. 28 The beneficial effect of IB-MECA in murine models of colitis (including IL-10 KO mice and dextran sodium sulfate [DSS]-induced colitis) was less impressive, and species or model differences may explain the outcomes – this deserves further consideration. Differences in different experimental models of IBD may be a result of differential sensitivity to IB-MECA in models of Crohn’s disease (CD) (e.g. TNBS colitis with transmural inflammation) and ulcerative colitis (UC) (e.g. DSS colitis with mucosal inflammation ). This may be important because there are seversal clincial phenotypes of UC and CD, and it is possible that IB-MECA would be more effective in CD than UC, and in a particular phenotype.
Recent studies in A3−/− deficient mice suggested instead a pro-inflammatory role for A3 AR activation during development of colitis. 29 Mice lacking a functional A3AR (A3−/− AR phenotype) was less susceptible to DSS-induced colitis 29, and mice were protected against development of severe colitis. The implication is that A3AR activation contributes to the development of colitis under these experimental conditions. In another study by Butler et al 30, A3-deficient mice exhibited reduced colon pathology and decreased levels of myeloperoxidase, but the degree of protection was not as pronounced as that seen by Ren et al 29. Another difference in the latter study is that by day 21 wild-type animals recovered, whereas A3 deficient mice displayed significantly greater inflammation and a significantly higher burden of tissue-associated bacteria. Together with other findings, their data suggested that disruption of the A3 AR interferes with neutrophil migration, and impairment of innate immunity prevents the clearance of invading microorganisms in the intestinal mucosa. This suggests that clinical use of A3 drugs (agonists or antagonists) in IBD or gut infection induced inflammation, could potentially raise the risk of opportunistic infections, 30 making them more risky to use in the clinical setting. Differences in outcomes in the two separate A3−/− knockout studies may be due to environmental variations, and housing of animals in different facilities could result in variations in microbiota that are known to affect the severity of various colitis models. 31,32 Differences in protocols or mouse strains could also contribute to outcomes. It should also be pointed out that adaptations in other adenosine receptors can occur with global KO mice of adenosine receptors – For instance, A2a −/− AR KO mice exhibit A2B up-regulation. 33 If such adaptation in other receptors occurs in A3- deficient mice, it could serve to explain outcomes in colitis. More studies with agonists and antagonists of A3 receptors and conditional KO mice are needed to clarify the mechanisms of protection.
Our preliminary findings (AGA abstract form) indicate that Cl-IB-MECA (10, CF-102) is more effective than IB-MECA (9) in attenuating mouse DSS colitis, but both are less effective than A3 −/−AR KO mice. Clearly, more studies in experimental models of IBD and conditional KO mice are warranted to clarify the use of A3AR agonists or antagonists to treat IBD; as are more studies on therapeutic effect of A3 drugs in experimental colitis models. What is most encouraging is that use of an A3AR agonist is beneficial in both animal and human studies of patients with inflammatory diseases (namely RA). 34
3.1b) Clinical trials with A3 AR agonists
Studies in animals and humans (clinical trials) indicate that A3AR is a therapeutic target in inflammatory diseases including rheumatoid arthritis, psoriasis and possibly dry eye syndrome, and A3 drugs have an excellent safety profile. 34 There is good evidence that the A3AR expression level is a useful indicator or predictor of a patient’s eligibility for treatment with the A3AR agonist in these diseases. 34
Can-Fite BioPharma has several pipeline drugs targeting A3 AR with a good safety profile and oral availability, currently at various stages of development for inflammatory diseases. The following compounds are A3 agonists and allosteric modulators: CF101 (9, IB-MECA) is a prototypical directly-acting A3 agonist. Results from a phase IIa study in RA patients indicate that the drug has anti-inflammatory activity and is efficacious in RA patients failing methotrexate therapy. Thus far, CF101 has shown a 20% improvement in disease symptoms. An exploratory randomized phase II clinical trial was conducted in 75 patients to evaluate the safety and efficacy of the drug in treating patients with plaque-type psoriasis. CF101 was safe and well tolerated. A 2mg dose given orally twice daily for 12 weeks resulted in progressive improvement in the severity of plaque psoriasis. 35 Another Phase II randomized, double-blind and placebo controlled trial showed that CF101 is beneficial in dry eye syndrome. Notably, doses that are shown to improve dry eye syndrome, do not cause cardiovascular or other side effects. 36,37 CF602 is a new generation drug, a positive allosteric modulator of A3AR that is being developed as a second-generation anti-inflammatory drug, its efficacy to enhance the protective action of agonist Cl-IB-MECA was shown in an animal model of cardiac ischemia 38. Other possible indications of A3 drugs are in the treatment of hepatocellular carcinoma and hepatitis (Phase II trial), as well as in analgesia to control murine and rat chronic neuropathic pain. 34,39
Recent updates 40 by OphthaliX (subsidiary to Can-Fite) indicate that the CF101 drug failed to meet primary efficacy endpoint in a phase III study for dry eye syndrome; it was however well tolerated. This was a 24-week, placebo-controlled phase III study of 237 patients with moderate to severe dry eye syndrome. Patients received two oral doses of CF101 (0.1mg or 1.0mg) or placebo. As a follow up, OphthaliX is planning a phase III retrospective analysis on the basis of A3 AR adenosine biomarker status. Can-Fite also announced positive data for a phase II trial in RA. A positive interim analysis was disclosed for a separate phase II/III clinical trial in patients with Psoriasis. Ophthalix is developing the CF101 for uveitis as well but no results are yet available.
There are currently no reported clinical trials with A3AR drugs in CD, UC, or bacterial induced colitis. Such studies are worth pursuit given the safety and tolerability of these drugs in clinical trials in health subjects or in treating disease. 36,41 Studies are also needed to clarify the cellular mechanisms of A3AR in healthy and inflamed gut tissues, including human surgical specimens or mucosal biopsies.
Other adenosine receptor agonists are of interest for pain treatment, including selective A1 (GW493838, 66) or A2A (BVT.115959, structure not disclosed) agonists.
3.2 Biomarkers of disease
3.2a) A3AR as a biomarker of disease
A3AR overexpression occurs in inflammatory cells of both experimental animal models of inflammation and humans. A3 AR expression was up-regulated in the colons of rats with TNBS-colitis 28 and in the lungs of LPS-induced pulmonary inflammation. 42 Over-expression of A3 AR was also detected in synovial cells from patients with RA, and in animals with adjuvant-induced arthritis (in synovial cells, paw tissue and drainage lymph nodes). 43 In comparison to healthy control patients, there was over-expression of the receptor in tissues derived from eyes of patients with pseudoexfoliation syndrome. 44 Higher expression of the A3AR was also found in patients with autoimmune inflammatory diseases including CD, RA and psoriasis 45,46 and animal models of RA. 43 In a phase II clinical trial with CF101 in RA, A3AR expression levels at baseline was a good predictor of patients responses to the drug in predicting clinical response/efficacy. 31 Finally, a retrospective analysis of gene expression data in mucosal biopsy from CD patients also indicated that the chronicity of the disease (ranging from 2 to 20 years after diagnosis) was inversely related to the A3AR expression. 47 However, a much bigger cohort study is needed to confirm this finding, if so it needs to be given consideration in any future potential clinical trial with A3AR drugs.
3.2b) ADA activity as a biomarker of disease
Adenosine deaminase (ADA) is the enzyme involved in the metabolism of adenosine and its conversion to the inactive (or less active) metabolite inosine. It is a marker of inflammation and activated leukocytes, 48 and inhibition of ADA in animal models has been suggested to be a potential therapeutic strategy in IBD. 49 A recent study showed that ADA activity in patients with CD could distinguish between active and non-active disease. In this study the activity of total ADA (tADA) and its isoenzymes, ADA1 and ADA2, were measured in serum and neutrophils (mucosal infiltration in response to inflammation) obtained from 20 patients with active CD, 20 patients in remission, and 15 healthy controls. It is claimed that tADA and ADA2 are serum biomarkers of inflammation, and may provide a useful indicator of CD activity, since their levels decrease approaching normal values in patients who go into remission. 50 These findings are potentially very important, and deserve further consideration and confirmation.
3.2c) Purine gene dysregulation profile as a biomarker of disease
A retrospective analysis of existing gene-array data sets in IBD versus controls, showed that UC and CD could be distinguished based on their unique purine gene dysregulation profiles in mucosal biopsy or polymorphonuclear leukocytes. 47 Therefore, unique changes in the expression profiles occur in UC or CD compared to healthy controls for purine genes, including receptors for P1, P2X and P2Y families, and enzymes involved in purinergic signaling. For example, in UC, there was up-regulation in mRNA levels of ADORA3, AMPD3, P2RY13, P2RY14, DPP4, and NT5E and no change in ADORA2A or ADAR expression. 47 In contrast in CD, there were down-regulation of ADORA3, AMPD3, P2RY14 and P2RY13, and upregulation of ADORA2A and ADAR. Gene expression and dysregulation was strongly associated with mucosal inflammation. 47 Overall, factors that influenced the expression of purine genes were inflammation, severity of inflammation/disease, chronicity of disease, and in some cases sex-dependent differences. Studies in part, supported by the National Institutes of Health and our Neuroscience Signature Program at The Ohio State University Wexner Medical Center, are underway to carry out a prospective analysis in cohorts of IBD and IBS patients to test the suitability of ‘the purine gene dysregulation profile’ as a biomarker of disease that could potentially distinguish UC, CD and IBS.
Expression of other genes for P2Y2, P2Y6, CD39 enzyme, P2X7 or A2B receptor proteins were also shown in different studies to be sensitive to inflammation. 51–54 (Refer to Table 2). In addition to their potential value as biomarkers of disease or inflammation, alterations in the expression of various purine genes are likely to contribute significantly in the pathophysiology of GI diseases, especially IBD. 7,14 The functional significance of these changes deserves further study. Definitive information on purine gene dysregulation and their functional consequences in human gut of UC or CD patients in comparison to control, is necessary to fully appreciate the potential for targeting these receptors to treat IBD patients. Given the variability in disease models and responses observed, such translational studies become increasingly more important.
3.3 Clinical Relevance of other ‘adenosinergic drugs’ to block inflammation and diarrhea
The potential of adenosine receptors as therapeutic targets has been the subject of numerous reviews. 10,12,55–57 These articles cover the biology of adenosine signaling in health and disease, biomedical implications in a broad range of diseases including inflammatory diseases. A recent Nature review describes the challenges in developing drugs for adenosine receptors. 58 Ongoing efforts in medicinal chemistry are helping tremendously in drug discovery for adenosinergic or purinergic drugs by generating selective ligands for the human variants of the receptors, ligands with positron-emitting radioisotopes can be used to monitor in vivo occupancy of adenosine receptors in vivo, improved bio-distribution and tissue selectivity.
3.3a) Clinical trials with methotrexate and sulfasalazine, modulation of extracellular levels of adenosine by ectoenzymes
Purinergic signaling is involved in intestinal inflammation associated with IBD and with severe hypoxia of the inflamed mucosa. 59 The role of hypoxia in the regulation of extracellular adenosine production and signaling in intestinal inflammation was the subject of a recent review by Eltzschig et al. 55 In patients with intestinal inflammation such as occurs in IBD, profound hypoxia of the mucosa induces Sp1-dependent activation of CD39 60 and a hypoxia-inducible factor (HIF) dependent induction of CD73 signaling 61 that favors extracellular adenosine production and signaling. In experimental colitis, enhancement of extracellular adenosine levels attenuates intestinal inflammation. Adenosine deaminase (ADA) catalyzes the breakdown of adenosine to inosine, generally considered to be an inactive metabolite. However, inosine also has an anti-inflammatory and protective effect against TNBS-induced colitis, mediated by adenosine A2A AR and uric acid, a metabolite of inosine. 62 The A2A receptor is unique perhaps in that several distinct ligands can activate it in situ, e.g. adenosine, inosine, or 5′AMP (neural activation). 62,63
The ectoenzymes ectonucleoside triphosphate diphosphohydrolase (CD39) and ecto-5′-nucleotidase (CD73) regulate nucleotide phosphohydrolysis of ATP and ADP to AMP, and conversion of AMP to its active metabolite adenosine, respectively. Studies in knockout mice of CD39−/− and CD73−/− highlight the importance of extracellular adenosine signaling in protecting against development of inflammation in pathologic situations. CD39 or CD73 deletion exacerbates experimental murine colitis (see Table 1). 26,64 In CD39−/− mice, reduction in extracellular adenosine level together with increase in ATP and ADP is suggested to result in increase in susceptibility to developing pathologic inflammation in disease states. Regulatory T-cells (CD4+ T lymphocytes) inhibit antigen-specific T-cell responses and prevents colitis. Therefore they are critical players in suppressing intestinal inflammation. Adenosine generation produced by activation of CD39 and CD73 expressed on regulatory T-cells leads to immune suppression. 65 CD73 is a critical enzyme in PMN-mediated human intestinal epithelial Cl− secretion. 66 A2A and A2B receptors are implicated in the protective effects of endogenous adenosine – the A2AAR has a critical role in T-cell mediated regulation of colitis. 67–69
Methotrexate (64) or sulfasalazine (65) are 2 commonly used drugs to treat IBD, autoimmune disease and rheumatoid arthritis. Their anti-inflammatory actions involve in part enhanced release of extracellular adenosine via a CD73-dependent signaling pathway. 70,71 Sulfasalazine acts through multiple mechanisms, by inhibiting 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) transformylase, via A2 receptors on inflammatory cells to attenuate inflammation, and enhances release of adenosine at inflamed sites. An adenosine A2A agonist regadenoson (68) is the first approved drug for clinical use in stress echocardiography.
In contrast to adenosine, ATP (the precursor of adenosine), is involved in purinergic chemotaxis, the process of cell migration during host defense responses by neutrophils to engulf and destroy foreign microorganisms. 72 Furthermore, during colitis inflammatory cells (platelets or epithelial cells) release nucleotides to activate P2 receptors, and in particular a neuronal P2X7-pannexin-1 signaling pathway leading to death of enteric neurons and exacerbation of tissue injury and inflammation. 73
Studies in animals or humans provide compelling evidence that enhancing the production of extracellular levels of adenosine (rather than ATP) in inflamed states provides protection against intestinal inflammation and injury. Furthermore, it is worth further investigation into drug strategies favoring a shift in the balance from pro-inflammatory nucleotide signaling (P2/P2X7 activation) to anti-inflammatory adenosinergic signaling (via A2A, A2B or perhaps A3 receptors).
Human polymorphisms in the noncoding region of CD39 cause a decrease in ectonucleotidase expression that leads to an increased susceptibility to IBD and multiple sclerosis. 25,26 In Chagas disease (caused by infection with the protozoan parasite Trypanosoma cruzi) E-NTPDase 1 (CD39) and ectoadenosine deaminase activity are reduced in lymphocytes of patients with the disease. 74 Upregulation in CD39 is also implicated in the loss of purinergic vascular regulation in the colon during colitis. The resulting impaired regulation of GI blood flow may have contributed to a more compromised permeability barrier provided by epithelial cells lining the gut mucosa. 75
3.3b) Clinical trials for dipyridamole
Adenosine plays an important role in gut immune and inflammatory responses. 12 As discussed, adenosine is involved in the therapeutic actions of anti-inflammatory drugs such as methotrexate or sulfasalazine. 76 Adenosine inhibits cytokine production, reduces neutrophil activity and increases the production of IL-10, an anti-inflammatory cytokine. 49,67,77 Adenosinergic drugs are potential anti-diarrheal drugs by virtue of their anti-inflammatory properties. Drugs shown to have efficacy in experimental models of colitis include ATL-146e (69, agonist of A2A AR), ATL-801 (78, A2B antagonist), IB-MECA (9, A3AR agonist), pentostatin (62, adenosine deaminase inhibitor) or dipyridamole 61 (a nucleoside transport inhibitor). Some inconsistencies in the literature on the efficacy of these drugs in pre-clinical models suggest further work is needed to clarify mechanisms and sites of action of A2A, A2B and A3 receptors. However, overall, the potential of adenosinergic agents as anti-diarrheal drugs should be further explored in clinical trials, not done to date.
Dipyridamole acts as a nucleoside uptake inhibitor by blocking the equilibrative nucleoside transporters (ENT1 and ENT2). Dipyridamole is particularly effective when there is an increased extracellular level of endogenous adenosine, such as in inflammation or hypoxia. Blockers of ENT are shown to reduce the severity of tissue injury in models of inflammation. Effects are mediated via A2A AR since antagonists could abolish the therapeutic effects of dipyridamole. 78–81
Seven-day oral treatment with dipyridamole increased circulating adenosine concentration, and augmented the anti-inflammatory response in experimental human endotoxemia. 82 Dipyridamole treatment enhanced the anti-inflammatory IL-10 response during endotoxemia that is produced by cells of the innate immune system, and it was able to inhibit production of proinflammatory cytokines like TNFα. These effects were also seen in human cultured mononuclear cells, and in patients undergoing coronary bypass surgery, dipyridamole inhibited post-op PBMC cell adhesion to endothelial cells. 83–85 Further studies in systemic inflammatory diseases, including IBD and inflammatory diarrhea are suggested, given that it has limited side effects (e.g. oral drug is associated with bleeding tendency; intravenous drug can cause chest pain and angina in patients with coronary artery disease).
Dipyridamole is a coronary vasodilator used in patients for pharmacological stress echocardiography or to prevent platelet aggregation (to protect after cardiac bypass surgery, NCT01295567). Dipyridamole is also in clinical trial as a supplement with prednisolone in RA (NCT01369745). The drug dipyridamole could easily be used to enhance the anti-inflammatory actions of endogenous adenosine in IBD, in both pre-clinical and clinical trials. 83 Considering the widespread distribution of adenosine receptors, targeting receptors with agonist or antagonist drugs could have a wide range of effects in cells and tissues including potential untoward side effects. Therefore, it is possible that using drugs that elevate endogenous adenosine levels locally at sites of release (e.g. with dipyridamole) may offer potential advantages over drugs targeting a specific receptor. As such, despite encouraging pre-clinical pharmacology, several phase III clinical trials of A2A receptor antagonists in Parkinson’s Disease were with little or insufficient clinical efficacy. 86,87 The largest clinical Phase III trial (PROTECT) of the A1 receptor antagonist rolofylline (18) in acute heart failure has failed because of its toxicity, 88 but we still don’t know if its toxicity this is specific to the drug in the disease or a result of general targeting of the A1 receptor. 57
3.3c) Caffeine (methylxanthines) use in humans, and clinical trials
Caffeine (13, a methylxanthine) has biological effects as an antagonist at adenosine receptors, e.g. used to treat premature apnoea, chronic obstructive pulmonary disease (POCD), cardiac ischemia/reperfusion injury. Caffeine’s actions can be explained in part by its effects to reduce adenosine receptors to ~50% the normal expression levels. 89 It has been estimated that the doses consumed in coffee are sufficient to exert biological effects at adenosine receptors. Long-term use of caffeine in coffee in sufficient doses to influence behavior 90 is not associated with any severe side effect(s) or increased morbidity. This knowledge is encouraging, and may suggest that long-term use of drugs like caffeine that act as antagonists at adenosine receptors, especially the A2A AR can be fairly safe. 58 In a recent nature review 58 it was stressed that clinical trials of adenosine drugs (and in particular A2A drugs) need to take caffeine consumption into considerations in clinical trials. As a cofounding variable, caffeine is indicated for headache and fatigue, but it can cause CNS excitation.
Single nucleotide polymorphisms (SNPs) in ADORA2A are associated with age of onset of Huntington’s disease, and reduced risk of Parkinson’s disease. Caffeine and others A2A antagonists, having a common target, have similar pharmacological effects in brain. The identification of SNPs in association with caffeine consumption (in relationship to Parkinson’s disease), suggests that clinical trials should consider subdividing patients according to their genotypes for A2A, CYP1A1, CYP1A2 genes, etc. 58 Genetic studies with caffeine may offer unique prospects for individualized medicine by identifying useful pharmacogenetic markers to predict individual responses to caffeine and adenosine drugs in clinical trials. An earlier example, arguing for individualized medicine, is the use of A3AR upregulation as a predictor of susceptibility to treatment with an A3AR agonist (CF101).
3.3d) Experimental therapeutics with purinergic drugs in diarrheal diseases targeting ENaC
UC and CD and infection-induced inflammation causes diarrhea. There is potential to exploit purinergic signaling-mechanisms in the treatment of inflammatory diarrhea. In normal colon and rectum, the electrogenic Na+ absorption via the epithelial Na+ channel (ENaC) accounts for the lumen-negative transmucosal electrical potential difference (PD). In active UC, TNFα/IFNγ cause down-regulation of the ENaC leading to impairment of electrogenic Na+ absorption and consequent reduction/loss of PD. 91,92 In UC reduction in Na absorption could also be involved from impairment in electroneutral NaCl transport in colon. ENaC absorption of Na+ is also impaired in CD in non-inflamed sigmoid colon of patients with active CD of the terminal ileum. Therefore, impaired absorption of Na+ is likely to contribute to the pathogenesis of diarrhea in CD and UC. 93 The potential application of purinergic compounds as anti-diarrhea drugs was the subject of a recent review by Sandle and co-workers. 94 Drugs shown in Table 6 have efficacy in experimental models of gut inflammation–induced diarrhea, which may act in part to restore normal Na+ absorption. Studies with purinergic drugs targeting the ENaC are worth pursuit.
Table 6.
Purinergic drugs for treating inflammation-induced diarrhea in experimental models
| Drug | Purinergic target | Model | References |
|---|---|---|---|
| ATL-801 (78) | A2B antagonist | DSS colitis, IL-10 KO spontaneous colitis | Kolachala V et al, 2008 96 (ameliorates colitis) |
| PSB-1115 (27) | A2B antagonist | DSS colitis | Frick JS et al, 2009 68 (exacerbates colitis) |
| IB-MECA (9) | A3 agonist | IL-10 KO, DSS colitis | Gessi S et al, 2008 242 |
| ATL-146e (69) | A2A agonist | Rabbit colitis, spont. Ileitis in SAMP1/YitFc mice | Odashima M et al, 2005132 |
| 4-amino-2-(2-hydroxyl-1-decyl) pyrazole[3,4-d]pyrimidine (APP) (63) | AdoDase inhibitor | DNBS-induced colitis | Antonioli L et al, 2007 49 |
| Pentostatin (62) | AdoDase inhibitor | Severe IL-10−/− colitis with piroxicam-induced colitis | Brown JB et al, 2008 243 |
| Dipyridamole (61) | ENT1, ENT2 inhibitor | LPS/phytohaemagglutinin-induced gut mononuclear cells from CD patients | Poturoglu S et al, 2009 83 |
| MRS2500 (51) | P2Y1 antagonist | Neurogenic secretion model (in vitro) | Fang X et al, 2006 103 |
3.3e) Epithelial A2B in diarrhea and inflammation
The role of A2BAR in immunity and inflammation has been comprehensively reviewed. 95 We will restrict our focus to studies indicating a role of A2BAR as a therapeutic target in IBD and inflammatory diarrhea. Briefly, during active intestinal inflammation, polymorphonuclear leukocytes transmigrating into the lumen, release 5′AMP that is converted to adenosine, which then activates electrogenic Clsecretion via apical A2BAR that likely contributes to secretory diarrhea. 67,96 A2B AR blockade by pharmacological antagonism 97 or A2B −/− gene deletion 98 suppresses gut inflammation and ameliorates murine colitis. A2BAR regulates Cl− secretion from intestinal epithelial cells, a process that is critical in the development of diarrhea. Stimulation of A2B AR increases cAMP and triggers the release of IL-6. Neutrophil-epithelial crosstalk at the intestinal luminal surface involves reciprocal secretion of adenosine and IL-6 99 thus providing an amplification mechanism for intestinal inflammation. Furthermore, TNF-α upregulates the A2B receptor gene in gut tissue in human IBD and murine colitis, propagating a vicious cycle of inflammation in the intestinal tract. 100 Protection afforded by A2BAR inactivation is associated with a decrease in the production of IL-6, a reduction in neutrophil infiltration in mucosal tissues, and keratinocyte – derived chemokine.
It is notable that not all studies have yielded consistent results. In contrast to the above mentioned studies, a separate study by Frick et al 68 found that A2B −/− deletion or an A2B antagonist PSB-1115 (27) increased the severity of DSS colitis. It remains puzzling as to why one study reveal an anti-inflammatory and tissue-protective role of A2B AR, whereas others indicate a pro-inflammatory effect of A2B AR in colitis. Differences in murine strains of genetic deleted mice or bacterial flora of the mice were offered as potential explanations. More studies are warranted to identify the mechanism, and to test whether the A2B AR is a viable therapeutic target in a mucosal inflammatory disease like IBD.
3.3f) Potential for purinergic drugs targeting neurogenic diarrhea
The ENS is important for secretion, mixing, and propulsion of intestinal contents. 101,102 Fluid secretion involves a predominant neurogenic component. Estimates suggest that neurogenic secretion 101–104 is responsible for >60% of that to luminal secretagogues 104 and excessive secretion is often associated with clinical symptoms of diarrhea, whereas low rates of secretion may be a contributing factor in constipation. 104 Diarrhea is a prominent feature of IBD, ranging in frequency from >50% to 99% of acute flare-ups of CD or UC, respectively and is often a leading symptom of distress in these patients. 104 Diarrhea-predominant IBS (D-IBS) occurs in a subset of IBS patients. 105 Colonic inflammation or agents like immune/inflammatory mediators that cause ENS excitation increase fluid volume and liquidity of luminal contents, ion secretion and the potential for neurogenic diarrhea. 106–108 A better understanding of purinergic mechanisms regulating human gut reflexes is necessary to fully-understand the basis of disturbances in secretomotor function in UC, CD or D-IBS. Both EC 109–114 and ENS 101,103 are implicated in the pathophysiology of intestinal secretory states suggesting potential new sites of action for drugs to treat diarrhea or constipation. 106 Use of P2X antagonists as drugs 13,115 to target motility and slow intestinal transit is another approach, since activation of P2X receptors in the ENS is expected to have pro-kinetic effects. 12,116 Of the many purine receptors known to exist in the human gut, P2Y1 (stimulation) and A3 AR (inhibition) are primary regulators of neurogenic secretion and early human data supports it. 12,101,108,117–120 P2X2 and P2X3 ion channel receptors are expressed on human submucousal neurons 121, and they are involved in stimulatory purinergic transmission in human ENS (Linan-Rico, Wunderlich and Christofi, unpublished observations). Release of ATP or a related nucleotide evokes a slow EPSP response in secretomotor neurons via P2Y1 receptors resulting in increase in fluid and electrolyte secretion. 103 Mechanically evoked reflex electrogenic chloride secretion in rat distal colon is triggered by endogenous nucleotides acting at P2Y1, P2Y2, and P2Y4 receptors. 117 Mechanical stimulation also releases nucleotides that activate P2Y1 receptors to trigger neural reflex chloride secretion in guinea pig distal colon. 119 Antagonist drugs at P2Y1 or P2X (or agonists at A3 AR) could suppress intestinal secretion by acting at both EC and ENS, and lead to harder, drier stools and could be beneficial for neurogenic diarrhea –studies in animals on mucosal diarrhea and fluid secretion are needed to prove their efficacy in vivo.
3.3g EC cell signaling in IBD
Enterochromaffin cells (EC) lining the intestinal mucosa release serotonin (5-HT) to regulate gut secretion, motility, pain signaling to the brain, nausea and immune modulation in IBD. 122 Alterations in 5-HT signaling are associated with IBD and IBS in both animals and humans. 109 5-HT signaling is tightly regulated by adenosine and ATP. 10,12,123
Hypoxia is a key feature of IBD that can activate HIF-1α signaling and 5-HT release from EC cells isolated from the human GI tract. 124 Responses are augmented by inflammation. Hypoxia stimulates release of adenosine 125,126 and it also acts to stabilize HIF-1α. 127 Hypoxia induces 5-HT synthesis and secretion from EC cells. Adenosine acts to decrease serotonin transporter (SERT) activity 128 that would serve to increase 5-HT signaling in the gut. Activation of A2B AR via MAPK/CREB and TPH-1 signaling amplifies the effect of hypoxia in human EC cells. 129 Overall, effects of adenosine in IBD are very complex, and much more work needs to be done, but targeting this pathway in EC cells is of potential interest as a therapeutic target in IBD.
Adenosine A1, A2A, A2B and A3 ARs provide fine tune modulation and autocrine regulation of 5-HT release from EC cells in response to mechanical stimulation. 123 Comprehensive reviews have been written on the role of purinergic signaling in health and disease of the GI tract, mechanosensory reflexes and secretomotor function. 10,12 Recent findings indicate that ATP-gated P2X3 channels and metabotropic P2Y1 receptors provide fast and slow – regulation of mechanically evoked 5-HT release, respectively. A putative P2Y12 receptor provides inhibitory modulation of 5-HT secretion. Therefore, these receptors are likely to play a critical role in the physiological regulation of peristaltic and secretory reflexes. 130 Any change in the expression of these receptors or signaling pathways in disease states such as IBD (or IBS) would be expected to have significant consequences. So for instance, P2X3 – immunoreactivity is normally expressed in 15% of human EC cells lining the colonic mucosa. However, in patients with ulcerative colitis (UC), P2X3 could no longer be detected by a selective P2X3 antiserum indicating that the fast-purinergic autocrine regulation of 5-HT release is impaired. 130 This needs confirmation in functional studies, but if so, it may be an important mechanism in the pathophysiology of UC. Alternatively, impairment in the P2X3 mechanism may actually be a compensatory mechanism in a futile attempt to try and restore normal 5-HT signaling that is known to be altered in IBD (and IBS).
3.3h) Immunomodulation via A2A AR and experimental therapeutics
Adenosine accumulation in inflamed (or hypoxic) tissues occurs via a two-enzyme dephosphorylation process involving CD39 (nucleoside triphosphate dephosphorylase) that converts ATP to ADP then to 5′AMP. Next, CD73 (a 5′ectonucleotidase) converts 5′AMP to adenosine. 26,131 It is well known that activation of A2A AR attenuates gut inflammation in animal models of IBD. 132 A2A AR is expressed on several types of immune cells involved in the mucosal inflammatory response in IBD, including myeloid cells, endothelial cells, T-lymphocytes. Adenosine analogs can ameliorate colitis and Clostridium difficile toxin-induced diarrhea, as well as gastric mucosal inflammation. 133 A2AAR−/− mice exhibit a more inflamed phenotype, for example after infection with Helicobacter pylori that causes gastritis. Activation of A2A AR on CD4+T (Th) cells causes an anti-inflammatory response. A2A receptors play a critical role in mucosal immune regulation by suppressing T-cell cytokine production including TNFα, IFNγ and IL-2 and it regulates Helicobacter-induced gastritis and bacterial persistence. In IL-10 KO mice, the inflammatory response is sufficient to clear (resolve) H. pylori infection. Infection of mice lacking the A2A −/−AR exacerbates the inflammation/gastritis in comparison to wild type mice. Administration of an A2A agonist ATL313 during infection suppresses inflammatory responses of Th cells, and reduces gastritis, but it also impairs immunity to H. pylori infection that could favor persistence observed as an increase in bacterial load. 133 Notwithstanding this potential ‘risk’ in its activity as an immunomodulator, adenosine’s A2A AR anti-inflammatory properties are worth pursuit in IBD. It remains unknown whether A2A agonists are effective in clinical trials of IBD.
It is not yet clear whether adenosine receptor heterodimerization with other purine or different types of receptors represents a significant challenge to the use of adenosine drugs and their clinical pharmacology (e.g. A2A-A2B; A2A-D2; A2A-A1). 134–139
3.3i) Immune modulation in epithelial cells
Epithelial cells respond to invading pathogens by producing inflammatory mediators. Perception of microbial molecular recognition receptors with various pattern recognition receptors (PRRs) stimulates the production of inflammatory mediators that can recruit and activate innate and adaptive immune responses. The immune response to non-pathogenic bacteria (e.g. commensal flora) is normally regulated to avoid a state of chronic inflammation. ATP has been proposed to serve as an endogenous ‘danger signal’ of adaptive immunity. 140 ATP was shown to alter human epithelial responses to commensal bacterial products in vivo, provoking an inappropriate immune response that could potentially favor development of IBD. 141 Activation of P2X7 receptors by ATP induces apoptosis and autophagy (possibly via production of free radicals) in human epithelial cells, an effect that could have implications for gut inflammation. 142 The epithelial P2X7 is suggested to play a critical role in initiating a positive amplification loop of polymorphonuclear leukocyte recruitment into the intestinal mucosa during the acute phase of inflammation. It was inferred from that study that dysregulation of the P2X7 apoptotic mechanism could result in the development of chronic IBD. 51 Other purinoceptors are implicated in IBD as well. Therefore, inflammatory stress associated with IBD elevates extracellular nucleotide concentrations at tissue sites of inflammation, in association with increased P2Y2 mRNA expression in colonic epithelia from mice with experimental colitis or from patients with Crohn’s Disease (CD) and UC. 143 P2Y2 expression is regulated by an NF-kB dependent mechanism and it is suggested it may contribute to IBD or other inflammatory diseases by stimulating prostaglandin release. C/EBPβ is a regulator of P2Y2 expression. 144 Further studies are needed to explore the pathophysiology and therapeutic potential of targeting epithelial P2X7, P2Y2 or other nucleotide receptors. 10,12
4.0 A3, A1, A2A, P2X and P2Y1 receptors and implications for motility disorders, constipation and diarrhea
Neural A3AR are involved in the regulation of both neuromuscular functions 145 and coordination of motility and secretion in the colon. 146 A3AR is distributed throughout myenteric ganglia in the colon, with highest expression in distal colon of the rat. RT-PCR indicated that A3 and ADA mRNA increased in inflamed tissues from experimental colitis. 145 The A3AR – mediated tonic inhibitory control of colonic cholinergic contractions was shown to be impaired in the inflamed bowel despite an increase in functional A3AR.
In a model of neurogenic diarrhea, a mast-cell mediator, histamine (or dimaprit, H2 agonist) was used to activate a stereotype cyclical pattern of chloride secretion that could be sustained for hours in the presence of drug. 146 Endogenous adenosine provided ongoing inhibitory modulation, and A3 antagonists could cause profound augmentation of the secretory response. Neural activation of histamine receptors in submucosal neurons activates a neural program leading to a coordinated motor and secretory response. A3AR tightly regulates the coordinated response to the mast cell mediator histamine. Histamine excites neurons in human submucosal plexus through activation of H1, H2, H3 and H4 receptors. 147 Furthermore, ENS excitation by supernatants collected from biopsy in IBS patients was sensitive to blockade with an H1–H4 antagonist cocktail, indicating that release of histamine from mucosal mast cells can cause activation of the ENS.
Functional disruption of A3 receptors in A3 −/− mice alters intestinal motility. A3AR-immunoreactivity in the distal colon ≫ proximal colon, by a ratio of 2:1. 146 The receptors in the mouse ENS are restricted to varicose fibers (sites of transmitter release) and glia. Notable species differences exist in the distribution of A3AR. Therefore, in rat unlike the mouse, cell bodies of enteric neurons (postsynaptic sites in transmitter release) highly express the A3AR. 146 Interestingly, as in the mouse the highest expression of A3 is in the distal colon. Intestinal transit and colonic evacuation reflex were accelerated in A3−/− mice, and stool retention was lower in these mice. 29 It was suggested that activation of A3AR by eADO attenuates the evacuation reflex and slows down intestinal transit, colonic emptying and mass movement in the colon. Therefore, the A3AR is a potential target for motility disorders. The A3−/− phenotype also protected against colitis, diarrhea, occult fecal blood, weight loss, neutrophil or CD4+ infiltration, and tissue injury. 29
Overall, taken together, these studies support the concept that A3AR selective agonists are potential therapeutic agents for the management of diarrhea and abnormal bowel motor activity or secretion associated with IBD, IBS or neurogenic diarrhea. A3AR selective agonists are already in clinical trials for inflammatory diseases, and their safety profile is excellent. It remains to be shown whether adenosine can suppress ENS activation evoked by supernatants from mucosal biopsies collected from patients with IBS (or IBD).
In contrast to A3AR, A2BAR plays a key role in regulating distal colon relaxation, and the mechanisms is linked to NO signaling 148 It has been suggested that targeting colonic A2B AR could represent a therapeutic strategy to treat constipation. A2B−/− AR mice have a constipated phenotype whereas A3−/− AR mice have accelerated motility and colorectal evacuation reflex. 29 Therefore, an A2BAR agonist or A3AR antagonist are potential drugs for constipation by promoting motility. Further animal studies with A3AR antagonists are needed to confirm the physiological relevance of A3 in motility and its efficacy in ameliorating colitis. For both A2BAR and A3AR, more studies are needed to further clarify the mechanism of action on motility and secretion.
In chemically induced colitis in the rat, there is also molecular and functional rearrangement of neural A1 and A2A receptors, favoring A2A receptor regulation of inhibitory control of colonic neuromuscular activity. Both A1 and A2A receptors contribute to inhibitory neuromuscular control in normal bowel. In inflamed bowel, neuronal A1 receptor function is lost, and A2A function becomes more prominent, in part due to A2A and CD73-dependent upregulation. 149 A1 and A2 receptors mediate inhibitory effects of adenosine on motor activity of human colon. 150
To date, a single study has been published on the direct effects of activating adenosine receptors in the human ENS. A study by Wunderlichet al 108 provided proof for inhibitory A3AR in human submucous plexus involved in suppressing synaptic neurotransmission. A3AR inhibited nucleotide or cholinergic synaptic transmission in the human ENS. Neural A1 receptors could not be revealed in contrast to animal studies. 151 In the study of Antonioli et al 149, the inhibitory effect of A1 activation on motor activity in human colon also differs from that in rodents – they found that A1 inhibition was restricted to the muscle, while A2A receptors operated through inhibitory nitrergic nerve pathways. 150 Species differences in other purinergic receptors are also known to occur between the mouse, rat and guinea-pig (e.g. for P2X2 and P2X3 receptors). For the development of purinergic drugs for motility disorders or neurogenic diarrhea for instance (associated with specific phases of IBD), it is imperative that translational studies in human surgical specimens are done to fully characterize receptors, enzymatic pathways and inactivation mechanisms for adenosine receptors, as well as P2X1–7 and P2Y1,2,4,6,11–14 receptors in both normal and inflamed gut. It is also necessary to identify receptors that are conserved in mouse and human ENS-muscular tissues for pre-clinical testing of drug candidates for IBD or IBS. Our unpublished observations indicate that endogenous adenosine and nucleotides play a critical role in purinergic regulation of neurotransmission in the human ENS (Linan-Rico, Wunderlich and Christofi, unpublished observations).
An earlier study provided encouraging results on the use of an A1 AR antagonist to treat post-operative ileus (POI) in a rat model. 152 The selective A1AR antagonist DPCPX (17, or CPX) reversed the slowed colonic propulsion in the rat. The sites or mechanisms of action are not understood, and more studies are warranted. Alterations in the purinergic pathway also occur in POI 153 (e.g. increase in ATP production in myenteric neurons and P2Y expression on smooth muscle) and drugs targeting these pathways may be relevant in alleviating POI. Little is known about purinergic signaling in postoperative ileus. In ulcerated regions of inflamed guinea-pig distal colon, neuromuscular transmission and propulsive motility are attenuated, an effect that is associated with a decrease in the purinergic component of the descending inhibitory limb of the peristaltic reflex. 154
Derivatives of benzimidazol-2-ylquinoline and benzimidazol-2-ylisoquinoline are selective A1AR antagonists with stimulant activity on human colon. Nanomolar concentrations (~10 nM) of these compounds enhanced EFS – contractions of the human colon, and this property makes them highly attractive agents for stimulating motility in humans. 155 As pointed out, this is a first step to developing new drugs for the therapeutic management of digestive disorders that are characterized by alterations GI propulsion (e.g. idiopathic chronic constipation, post-operative paralytic ileus and IBS). Their suitability in these disorders could be rather limited if inflammation (as occurs in post-operative paralytic ileus) or the disease impairs A1 regulation of neuromuscular transmission as occurs in experimental colitis. 149 Furthermore, A1 receptors were identified on smooth muscle but not in human ENS. Perhaps targeting other neural adenosine receptors might be a better option, and they should be given further consideration.
Studies in P2ry1−/− knockout mice demonstrated the physiological relevance of P2Y1 purinoceptors in inhibitory motor control of murine colonic excitability and transit. 4 Overall, inhibitory neuromuscular transmission is mediated via P2Y1 purinergic receptors in mouse, guinea pig, primate and humans. 4,5,156,157 It is not clear what effects P2Y1 receptor antagonists would have on in vivo transit or constipation, but P2ry1−/− KO mice should prove helpful in such studies in animal models of constipation. In Hirschsprung’s Disease, P2Y1 and P2Y2 receptors were absent in the aganglionic segment in both myenteric and submucous plexuses. P2Y1 receptors are involved in inhibitory transmission to smooth muscle of the human colon 5,156 and their absence could at least in part, explain the contracted state of the aganglionic gut. Functional studies are needed to test this hypothesis.
The purinergic hypothesis is based on ATP (or a related nucleotide, e.g. ADP or AMP) release as the neurotransmitter at synapses or in neuromuscular transmission. However, a significant body of evidence is emerging to suggest that β-NAD+ and ADP ribose are involved in neurotransmission and inhibitory neuromuscular transmission in rodents, primates and humans. 2–4 For example, in P2ry1−/− KO mice, purinergic fast inhibitory junction potentials (fIJPs) and responses to β-NAD+ or ADP ribose were abolished, whereas those to ATP or ADP were retained. The findings of the group at Reno support the intriguing hypothesis that β-NAD+ or ADPR meet the criteria for a neurotransmitter in neuromuscular transmission. 3 Gallego et al 5 concluded from their study that β-NAD+ only partially fulfills the criteria for the transmitter involved in inhibitory neuromuscular transmission of the human colon. Nevertheless, the direct actions of these mediators (β-NAD+ or ADP ribose), their involvement in ‘purinergic transmission’, P2Y-inhibitory transmission or P2Y1 stimulatory transmission in the human ENS have yet to be determined.
P2X agonists on the ENS may enhance GI transit and secretion and they could be useful in treating constipation or constipation-predominant IBS. Alternatively, P2X antagonists could be useful in treating diarrhea-predominant IBS or the neurogenic component of inflammation–induced diarrhea, since majorities of the secretory diarrhea observed in IBD or infection-induced gut inflammatory states is estimated to be neurogenic in nature. 104 Blockade of excitatory P2Y1 receptors on secretomotor neurons in animals 103 or humans 108 is another target to regulate neurogenic diarrhea, and studies in P2ry−/− mice can provide molecular proof.
5.0 ATP and P2X ion channel receptors antagonists as potential analgesic drugs for visceral pain in IBD and IBS
P2X ion channel receptors are distributed on subsets of myenteric and submucous neurons of the ENS, glia, ICC, smooth muscle, epithelia and EC cells, and immunochemical studies have revealed their discrete localization in subsets of neurons with distinct chemical coding and function. Electrophysiological and calcium imaging studies confirmed the role of P2X ion channel receptors in excitatory neurotransmission and information transfer between neurons and glia. 158 ATP is a potent stimulus for electrolyte secretion in the GI tract (colon, gall bladder, pancreatic duct, and from bile duct) and its release is likely mediated from both local epithelial cells and nerves to modulate peristalsis, secretion and nociception. ATP also exerts fine tune modulation of EC-cell function and 5-HT secretion that also triggers intrinsic gut and nociceptive reflexes. 130 Enteric glia express P2X7 and (P2Y4 receptors). 14 The ectoenzyme NTPDase2 is exclusively localized to glia in the ENS (where as NTPDase1 is localized to neurons) to regulate the availability of ATP 159 and gliotransmission. P2X7 receptors (and P2X2 receptors) are expressed on NOS-positive inhibitory neurons, cholinergic secretomotor neurons, and intrinsic sensory neurons. 115
5.1 Pain and visceral hyperalgesia
Abnormalities in P2X signaling are implicated in diverse diseases such as IBS, IBD, Chaga’s Disease, Hirschsprung’s Disease and non-erosive esophagitis. Recent reviews have addressed the role of purines in gastrointestinal diseases and inflammation, and pain control. 7,14,158,160 IBS is more prevalent in females than males and severity of pain seems to fluctuate with the menstrual cycle. This implies that sex hormones could affect perception of painful stimuli, although a causal relationship between sex hormones and IBD is not yet clearly evident. However, P2X3 mediated nociception in a colitis model is closely related to endogenous estrogen modulation. 161 P2X3 channels on sensory EC cells are down-regulated in UC 130 and up-regulated in enteric neurons in IBD 162 that would impact on both P2X signaling in intrinsic neural reflexes and nociceptive reflexes via the dorsal root ganglia to the brain.
Visceral pain is a debilitating symptom of IBD, IBS, FD and dysmotility disorders (e.g. gastroparesis), pseudo-obstruction, and GERD. The visceral purinergic system is a therapeutic target for pain control, and the experimental evidence support the hypothesis that mechanosensory signaling via P2X receptors can trigger visceral pain in visceral hollow organs (tubes or sacs) that include the bladder, ureter and the GI tract. 160,163 It is proposed that during distension, ATP released from sub-epithelial visceral afferents conveys pain information to the brain via the dorsal root ganglia. P2X3 and P2X2/3 receptors on low threshold fibers are involved in physiological reflexes whereas such receptors on high threshold sensory fibers transmit pain. P2X2/3 receptors contribute to small intestinal afferent hypersensitivity in post-infectious bowel disease. 164 IBS is a functional GI disorder associated with pain and hypersensitivity in the absence of colonic inflammation or obvious structural changes. Several studies indicate that P2X receptors are involved in colonic hypersensitivity. 165,166 Visceral hypersensitivity in non-erosive reflux disease (in the absence of esophageal mucosal injury) may involve ATP sensitization of P2X3 receptors. 160,167 Mice lacking P2X3−/−, P2X2−/− or double knockout mice P2X2/P2X3−/− have provided proof for the involvement of P2X2 and P2X2/3 receptors in inflammatory pain, physiological voiding and bladder inflammation (in a model of interstitial cystitis using cyclophosphamide). 168–170
5.2 Pain – relieving effects of intravenous ATP in chronic intractable orofacial pain
An earlier clinical study provided proof of concept in a small cohort of patients (n=16) that intravenous infusion of ATP at a rate of 100μg·Kg−1·min−1 over 2 h has pain-relieving effects in chronic intractable orofacial pain. 171 They found that ATP caused a reduction of the VAS scores for spontaneous pain and allodynia by 82%±15% and 74%±9% respectively. These beneficial effects of ATP outlasted the infusion period (for medians of 7 and 12 h respectively). A later study by the same group conducted a double blind placebo controlled study to evaluate the effects of intraoperative intravenous ATP on postoperative pain in 30 patients scheduled for sagittal split ramus osteotomy. In this study the ATP infusion rate was 160μg·Kg−1·min−1 throughout surgery. Data suggested that ATP infusion could blunt hemodynamic responses to surgical stimulation, and it produced prolonged analgesia in patients undergoing such orofacial surgery. ATP reduced the cumulative morphine consumption for 72 h postoperatively by 47% compared to placebo, and no adverse effect of ATP was reported. 172 These are compelling studies, albeit in small numbers of patients, but they provide proof of concept that the cognate ligand ATP can provide effective analgesia. Further studies are needed to determine the sites of action of ATP and whether P2X2/3, P2X or multiple receptors are targeted by the non-selective ligand ATP, therefore, larger multi-center studies are warranted. Several medicinal candidate drugs exist for P2X3, P2X2/3 and P2X7 for trials. A larger Phase II, double - blind, placebo-controlled, dose-response trial involved intravenous adenosine for perioperative analgesia in 166 subjects (125 subjects received adenosine and 41 received placebo). Women undergoing major gynecological surgery were randomized to receive 25, 50, 100 or 200μg·Kg−1·min−1 or placebo. Adenosine was not different from placebo with respect to efficacy and safety for perioperative analgesia. 173 It would be important to ascertain whether adenosine’s lack of effect is because ATP or a related nucleotide (ADP or AMP) is a requirement for efficacy.
5.3 P2X2/3, P2X3 and P2X7 antagonists are potential drugs for visceral pain
In the last decade advances in medicinal chemistry offer new selective P2X3 and P2X2/3 compounds with suitable potency, selectivity and bioavailability, to consider testing in clinical trials. These compounds are being developed by several pharmaceutical companies including Evotec, AstraZeneca, Merck and Shionogi. 174 Recent development of orally bioavailable P2X2 and P2X2/3 antagonists targeting these receptors makes these compounds potential therapeutic agents in treating visceral pain. A-317491 (83) is a selective P2X2/3 and P2X3 antagonist synthesized by Abbott Laboratories – it allowed studies to validate the role of these receptors in neuropathic and chronic inflammatory pain. AF-353 (82) is an antagonist at these receptors that is bioavailable and stable in vivo. 175 It is synthesized originally by Roche Palo Alto and is now being developed by Afferent Pharmaceuticals. Trinitrophenyl-ATP (81, TNP-ATP) is a high affinity antagonist at P2X3 and P2X2/3 that requires much higher concentrations to activate P2X2 receptors. Several diaminopyridines were shown to be selective antagonists at P2X3 and P2X2/3 receptors, and had in vivo efficacy in a pain model. 176
Clinical trials for some P2X compounds are in progress, but there is no information yet on the efficacy of these drugs to relieve pain. 175 The P2X3 antagonist AF-219 (an aryloxy-diaminopyrimidine) from Afferent Pharmaceuticals is the first compound in clinical trials. 163 Several Phase I clinical studies in healthy volunteers indicate good safety and tolerability. In addition, several Phase II studies began in 2011 including a study in suppressing chronic cough (airway sensitization), joint pain (knee osteoarthritis) and visceral pain (in bladder pain syndrome). P2X3 immunoreactivity is elevated in lingual mucosa in patients with burning mouth syndrome, suggesting that P2X3 may be a therapeutic target for treating this type (trigeminal) of neuropathic pain. 177 Another P2X antagonist for treating pain is an orally available negative allosteric modulator of the P2X7 (GSK1482160, 85).
5.4 Potential for P2X7 receptor antagonist drugs
P2X7 receptor plays an important role in inflammation and immunity. The P2X7 receptor antagonist is a potential therapeutic target for inflammatory diseases including rheumatoid arthritis, IBD and glomerulonephritis, and for treating inflammatory pain, and amelioration of the pro-inflammatory phase of sepsis. This is supported by animal studies with P2X7 −/− deletion or pharmacological studies with selective P2X7 receptor antagonists. More than a dozen phase I and phase II clinical trials are ongoing or completed on the use of selective P2X7 antagonists in the treatment of pain or inflammation in patients with RA. These trials are truly in the early stages of development, but the safety and tolerability of the drugs, and early results on efficacy are encouraging, to justify further study. For a comprehensive review of P2X7 receptor antagonists in treating inflammatory diseases and clinical trials refer to Arulkumaran et al 2011. 178
A number of patents have been filed for P2X7 receptor antagonists for neuropathic pain and inflammatory disorders. 179 In addition, nociceptive signaling is dually modulated by Gi- and Gq-coupled P2Y receptors (ADP activated); Gq-coupled P2Y1 activation is required for full expression of inflammatory hyperalgesia, while agonists for Gi-coupled P2Y receptors (P2Y12–14) cause reduction in hyperalgesia. 180 Overall, a number of candidate drugs for P2X receptors (antagonists) are available for future clinical trials to treat painful conditions.
5.5 Inflammatory and neurological diseases
There is good evidence that the P2X7 receptor has a pathogenic role in inflammatory glomerulonephritis, and pre-clinical studies in animal models suggest a possible therapeutic role of P2X7 antagonists in the treatment of inflammatory renal diseases. 178 P2X7 is important in the defense mechanism against Mycobacterium tuberculosis. 181 It was shown that the crucial bactericidal step of mycobacteria following phagocytosis by macrophages is P2X7-mediated apoptosis of the macrophage. And, a polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis by 3.5 fold. 181,182 Some data also suggest that targeting P2X7 receptors might be a therapeutic option for treating COPD. Furthermore, extracellular ATP activation of P2X7 receptors contributes to cigarette-smoke induced lung inflammation and emphysema. P2X7 KO mice have reduced pulmonary inflammation after acute cigarette smoke exposure. The anti-nociceptive properties of P2X7 antagonists have been researched extensively, and reports indicate reduction or amelioration of chronic inflammatory and neuropathic pain. 183,184 The levels of purines and pyrimidines in synovial fluid of patients with RA are high, and these nucleotides can produce joint inflammation through production of cytokines (IL1β, TNF-α, IL-2 and IL-6). 185,186
P2X7 receptors are implicated in the course/progression of Alzheimer’s disease and of other neurodegenerative diseases via ATP-mediated cortical cell death and free radical release. In the gut, P2X7−/− mice are protected against colitis. The mechanism involves P2X7-pannexin 1 signaling, pore formation and caspase-3 leading to neuronal death in gut in animal models of IBD or CD 73 and may be relevant to human IBD. The P2X7 receptor is expressed on enteric glia, NOS-positive inhibitory neurons, cholinergic secretomotor neurons and intrinsic sensory neurons. 7
Therefore, P2X7 activation in the inflamed state is likely to contribute to the symptoms of IBD, including motor abnormalities, diarrheal state, and visceral pain. The actions of P2X7 receptor activation are not restricted to neurons and glia. Activation of the P2X7 receptor leads to activation of inflammasome and release of interleukin-1β. 186 Recent findings also indicate that the extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors, and suggests that antagonists of P2X7 receptors are potential therapeutic targets in both IBD and IBS (or other functional GI disorders) where mast cells are implicated in the immune/inflammatory response. In mast cells, P2X7 receptors induce inflammatory cytokines, chemokines and leukotrienes. Activated MC’s exacerbate inflammation by also recruiting neutrophils to produce TNFα. 54
5.6 Clinical trials with P2X7 antagonists
Phase I and II clinical trials on the safety and efficacy of P2X7 antagonist drugs are ongoing. AZD9056, CE-224, 535 (84) and GSK1482160 (85) are compounds in phase II studies targeting IBD, RA and COPD (Table 4). These drugs are fairly well-tolerated and no serious concerns have been raised regarding their safety thus far. The main adverse events of these drugs are gastrointestinal (nausea, diarrhea and vomiting), dizziness and headaches. However, these side - effects were reported most frequently at the higher doses. Newer antagonists are entering clinical trials, and it is too early to draw conclusions. These are patients receiving background treatment with methotrexate or/and sulfasalazine without symptomatic relief (see Table 4).
CE-224, 535 (84) represents another antagonist drug of P2X7 receptors, but its effect was not shown to be better than placebo for the treatment of RA in patients with an inadequate response to methotrexate. CE-224,535 exhibited an acceptable safety and tolerability profile. 187
5.7 First clinical trial in CD patients with P2X7 antagonist
AZD9056 is an adamantane amide and selective P2X7 antagonist (structure not disclosed), being evaluated for safety and efficacy in causing clinical remission in CD patients – This is the first clinical trial in IBD patients with a P2X antagonist. It represents a phase II double blind, placebo-controlled, parallel group, and multicenter international study. The drug AZD9056 is from Astra Zeneca (study code D8830C00002 2008 available from www.astrazenecaclinicaltrials.com). A 200 mg of AZD9056 once daily is given for 4 weeks to adult patients with active CD compared to placebo – 10 centers in 5 countries are participating in this first study in IBD patients. The aim of the study is to evaluate the safety and benefit of the drug in reducing the CD Activity Index (CDAI) score from a moderate to severe index (CDAI ≥ 220) to clinical remission (CDAI≤150) after 4 weeks of treatment in ileum and/or colon. Forty patients were enrolled, and 30 of the patients were randomized (20 to AZD9056 and 10 to placebo). Initial results were promising, and there was improvement in CDAI compared to placebo. Vital signs and laboratory values remained unchanged. The proportion of CD patients with a clinical response and those in remission was greater in the AZD9056; improvements in the IBD questionnaire score were seen in the ADZ group. Three of 4 patients who discontinued the study (of 30 patients) due to adverse effects were in the AZD9056 group. Abdominal pain was common and reported in both treatment groups. Also, GI disorders that included diarrhea were more frequent after treatment with the drug (54%) versus 30% with placebo. Overall, the drug was well – tolerated to continue development of the drug. Despite encouraging results in CD patients, AZD9056 did not show significant efficacy in the treatment of RA, and targeting the P2X7 with this antagonist does not appear to be a therapeutically useful target in RA.
5.8 P2X7 polymorphisms
Functional P2X7 receptor polymorphisms have been identified in patients with CD. 19 These include a gain-of-function single nucleotide polymorphism (SNP) His155Tyr and a loss-of-function SNP Arg307Gln and Glu496Ala. There is evidence for P2X7 polymorphisms that renders an increased susceptibility to Alzheimer’s disease, bipolar affective disorders or major depressive illness, multiple sclerosis and diabetes or resistance to infection with Chlamydia trachomatis. 20–24 However, association analysis indicated that these SNP’s of the P2X7 receptor are not a susceptibility factor for CD.
6.0 Herbal medicines are natural purinergic drugs with efficacy in FD and IBS
6.1 STW 5 (IberogastR)
STW 5 is a liquid formulation of nine herbs (phytopharmacon) shown to be effective in randomized, double bind placebo controlled multi-center clinical trials in functional dyspepsia 188,189 and IBS 190. Adenosine A2A receptors contribute to the anti-inflammatory effect of Iberogast in rat TNBS colitis. 191 A double blind, randomized, placebo-controlled phase III study is ongoing on the efficacy of Iberogast (BAY98-7411) to reduce pain intensity in patients with IBS. No data is yet available.
6.2 Paeoniflorin
Natural products are also a potential source to obtain P2X antagonists for use in clinical applications as analgesics. 192 Several natural products are shown to cause analgesia on inflammatory pain or neuropathic pain by inhibiting P2X3 or P2X7 mechanisms, although the selectivity of these compounds for specific P2X receptors remains unclear. Paeoniflorin (72) is a chief ingredient in the root of Paeonia lactiflora Pall, and it has been shown to be effective in relieving colorectal distension induced visceral pain and hyperalgesia in a rat model of IBS (neonatal maternal separation). The effect is mediated through the adenosine A1AR to inhibit glutamate/NMDA receptor – dependent ERK signaling. 193 Much more work is needed to validate these products as viable alternative ‘medicinal candidates’ for clinical trials.
7.0 GI side effects of the P2Y12 antagonist clopidogrel (Plavix)
Clopidogrel (79) is a thienopyridine class antiplatelet drug used to inhibit vascular clot formation. After preactivation in the liver, its active metabolite binds irreversibly to P2Y12 receptors on platelet membranes and prevents platelet aggregation. Typically patients with significant coronary artery disease who undergo percutaneous intervention and coronary artery stent placement begin dual anti-platelet therapy immediately following the procedure. 194 Adverse effects of clopidogrel include bleeding (3–10%), hypersensitivity reactions, thrombotic thrombocytopenic purpura, neutropenia. Among others, significant gastrointestinal symptoms are discomfort (27.1%), diarrhea (4.5%), dyspepsia (5.2%), nausea (3.4%), and abdominal pain (5.6%). CNS side effects also occur: headache 7.6%, dizziness 6.2% and occasional vertigo, numbness, neuralgia and paresthesias. It is likely that GI side-effects of Plavix are linked to receptors localized in the GI tract, and pharmacological studies suggest inhibitory P2Y12 receptors on EC cells modulate mechanosensitive 5-HT release. 130 P2Y12 immunoreactivity is distributed throughout the ENS on enteric neurons (Christofi, unpublished observations). Irreversible antagonist binding to P2Y12 receptors would lead to dis-inhibition and over-activation of mucosal reflexes and perhaps activate nociceptive reflexes as well. Therefore, receptors on EC or other sites in the ENS deserve further consideration, in better understanding the actions of Plavix and to test their hypothesis.
8.0 ATP protects against NSAID-induced enteropathy in humans
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely prescribed medications for their analgesic and anti-inflammatory properties. NSAIDs such as naproxen, ibuprofen and indomethacin have an elevated risk of mucosal damage in the GI tract. A significant increase in mucosal permeability (leakiness) could be the underlying cause of such enteropathy. 195,196 A clinical study in healthy humans showed that topical ATP administration into the duodenum during a short challenge of NSAID attenuates the NSAID-induced increase in intestinal permeability in vivo. This finding may have potential implications for the treatment of other intestinal diseases or disorders associated with an increase in mucosal permeability. 197 For instance, infection with Helicobacter pylori increases intestinal permeability of the stomach and intestine. 198 In CD, gut mucosal permeability changes are associated with increase in the severity of the disease and is an early predictor of relapse. 199–202 An increase in mucosal permeability is also implicated in Celiac Disease 203 and IBS 204. Further studies are needed to prove the validity of this approach in animal models of disease, healthy humans and GI diseases, but certainly it would be a worthy pursuit.
The therapeutic potential of ATP as an immune modulator in the treatment of HIV/AIDS, in combination with highly active antiretroviral therapies has recently been described. 205 This intriguing possibility awaits a pilot proof of concept clinical study.
9.0 Neuroprotection of the Enteric Nervous System
There are 2 potential receptor targets for neuroprotection of the ENS in lipid induced enteric neuropathy 206 and IBD. 73 The neuroprotective effects observed in P2X7 null mice have already been discussed elsewhere in this review, and provide experimental proof to suggest that P2X7 antagonists may be beneficial in protecting the ENS against apoptosis of the neurons, 73 and in immunomodulation in mast cells. Another receptor with pro-apoptotic properties is the P2Y13 receptor. 207 A new study indicates that a P2Y13 receptor antagonist such as MRS2211 (55) could prevent neuronal loss caused by fat-diet and palmitic acid induced neuronal loss in mice. 206 The ADP sensitive P2Y13 receptor is a potential therapeutic target in lipid-induced enteric neuropathy. Briefly, animals fed a high fat diet for 6 months developed enteric neuropathy and cell damage, whereas P2Y13−/− litermates were protected against neuropathy and the loss of myenteric neurons. Therefore, activation of the P2Y13 receptor is important in the development of enteric neuropathy and apoptosis of the neurons.
10.0 Extracellular ATP as ‘alarmin’ or danger signal in IBD
In animals fed a diet supplemented with nucleosides and nucleotides, chemical induced colitis and colonic injury was exacerbated, in association with increased leukocyte, macrophage and lymphocyte infiltration of the colonic mucosa. 208,209 UDP activation of P2Y6 receptors is involved in the innate mucosal response of the gut and it regulates T cell activity in chronic colitis 210 Intestinal inflammation has been shown to increase the expression of P2Y2 and P2Y6 receptors on epithelial cells and the release of CXC chemokine ligand 8 by UDP, the cognate ligand of the P2Y6 receptor. 143 Extracellular nucleotide signaling is therefore involved in the progression of intestinal inflammation. Extracellular nucleotides can act via P2X1–7 ligand-gated cation channels and G protein-coupled P2Y1,2,4,6,11–14 receptors. P2Y2,4,6 receptors regulate Cl−, Na+ and K+ secretion in the intestinal tract and absorption mechanisms. 10 In the inflamed state, P2Y receptors stimulate production and secretion of cytokines, proinflammatory molecules and cell adhesion molecules, and induce cell migration, immune cell recruitment and proliferation and differentiation processes. Overall, it has been suggested that extracellular nucleotides are ‘alarmins’ or danger signals that can be rapidly released to enhance the activity of the innate immune system of the gut. 143 It is too early to know whether nucleotide receptors are promising therapeutic targets in IBD.
Conclusions
Purinergic drugs methotrexate, sulfasalazine, Adenocard (adenosine), dipyridamole, caffeine, and many newer generation drugs developed as a result of progress in medicinal chemistry (targeting A3, A2A, P2Y12, P2X2/3, P2X3, P2X7 receptors) and several phytopharmaca have an excellent safety/efficacy profile for potential future clinical trials in IBD, IBS, FD and inflammatory diarrhea. The future for purinergic drugs on clinical trials seems hopeful, although it may be a bit risky, and somewhat of a ‘balancing act’ to obtain the benefit of treatment without compromise the physiology of the patient that is dually targeted by receptor drugs. Genetic polymorphisms, caffeine consumption, and other individual traits or differences in behavior between patients may potentially affect susceptibility to treatment. Therefore, a personalized medicine approach may ultimately be a suitable option to tailor treatment in every patient. Future studies in animals are needed to further clarify cellular and molecular mechanisms and to test new-generation drugs. There is still a huge gap in our knowledge of human pathophysiology of purinergic signaling, and such translational studies are of critical importance given that significant species differences likely exist in purinergic signaling between animals and humans and the receptors are differentially regulated in disease states.
Overall, given the safety, tolerability, efficacy of several purinergic drugs in clinical trials, a rather compelling case can be made for going ahead with FDA approved, designed clinical trials to treat GI symptoms in patients with IBD and IBS.
Acknowledgments
Current support from the National Institutes of Health on R01 DK093499; strategic initiative research funds from the Department of Anesthesiology & Wexner Medical Center at The Ohio State University to F.L.C; R01 DK044179 11–15; DK04417915S and NCRR S10RR11434 to F.L.C. Support for from “the NIDDK, NIH Intramural Research Program” to K.A.J. for research in the Laboratory of Bioorganic Chemistry & Molecular Recognition Section, Bethesda, MD USA. Thank you to Iveta Grants for technical editing and formatting of the manuscript.
Abbreviations
- ADA
Adenosine deaminase
- AR
Adenosine receptors
- eADO
Endogenous adenosine
- tADA
total adenosine deaminase
- CNS
Central nervous system
- CD
Crohn’s Disease
- COPD
Chronic obstructive pulmonary disease
- CDAI
Crohn’s Disease activity index
- CD39
Ectoucleoside triphosphate diphosphohydrolase (ENTPDase, Nucleotidase CD39)
- CD73
ecto-5′-nucleotidase (ecto-5′-NT)
- CYP1A1
Cytochrome P450, 1A1
- CYP1A2
Cytochrome P450, 1A2
- DSS
Dextran sulfate sodium salt
- EC
Enterochromaffin cell
- EFS
Electrical field stimulation
- ENaC
Epithelial Na+ ion channel
- ENS
Enteric nervous system
- ENT1/2
Extracellular Nucleoside Transporters 1/2
- FD
Functional disorder
- GI
Gastrointestinal
- GERD
Gastroesophageal reflux disease
- HIF
Hypoxia-inducible factor
- 5-HT
5-Hydroxytryptamine (Serotonin)
- IBD
Inflammatory Bowel Disease
- IBS
Inflammatory Bowel Syndrome
- ICC
Interstitial cells of Cajal
- IL
Interleukine
- INF-γ
Interferon-γ
- KO
Knock-out
- LPS
Lipopolysaccharides
- NSAID
Non-steroidal anti-inflammatory drugs
- NANC
non-adrenergic, non-cholinergic
- PMN
Polymorphonuclear lukocytes
- PBMC
Peripheral blood mononuclear cells
- POI
Post-operative ileus
- PON1
Paraoxonase-1
- RA
Rheumatoid Arthritis
- RT-PCR
Reverse transcription polymerase chain reaction
- SNP
Single nucleotide polymorphism
- TNFα
Tumor-necrosis Factor α
- TNBS
2,4,6-Trinitrobenzenesulfoni acid
- TPH-1
Tryptophan hydroxylase 1
- UC
Ulcerative Colitis
- UDP
Uridine-5′-diphosphate
- VAS
Visual analog scale pain score
References
- 1.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 2.Durnin L, Hwang SJ, Ward SM, et al. Adenosine 5′-diphosphate-ribose is a neural regulator in primate and murine large intestine along with [beta]-NAD+ J Physiol (Lond) 2012;590:1921–1941. doi: 10.1113/jphysiol.2011.222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang SJ, Durnin L, Dwyer L, et al. β-Nicotinamide Adenine Dinucleotide Is an Enteric Inhibitory Neurotransmitter in Human and Nonhuman Primate Colons. Gastroenterology. 2011;140:608–617. e6. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang SJ, Blair PJ, Durnin L, et al. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol. 2012;590:1957–1972. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallego D, Gil V, Aleu J, et al. Pharmacological characterization of purinergic inhibitory neuromuscular transmission in the human colon. Neurogastroenterology & Motility. 2011;23:792–e338. doi: 10.1111/j.1365-2982.2011.01725.x. [DOI] [PubMed] [Google Scholar]
- 6.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal. 2013 doi: 10.1007/s11302-013-9397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G. Purinergic receptors as future targets for treatment of functional GI disorders. Gut. 2008;57:1193–1194. doi: 10.1136/gut.2008.151134. [DOI] [PubMed] [Google Scholar]
- 9.Peri LE, Sanders KM, MutafovaYambolieva VN. Differential expression of genes related to purinergic signaling in smooth muscle cells, PDGFR[alpha]-positive cells, and interstitial cells of Cajal in the murine colon. Neurogastroenterology & Motility. 2013;25:e609–e620. doi: 10.1111/nmo.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christofi FL. Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signal. 2008;4:213–236. doi: 10.1007/s11302-008-9104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonioli L, Fornai M, Colucci R, et al. Pharmacological modulation of adenosine system: novel options for treatment of inflammatory bowel diseases. Inflamm Bowel Dis. 2008;14:566–574. doi: 10.1002/ibd.20316. [DOI] [PubMed] [Google Scholar]
- 12.Antonioli L, Fornai M, Colucci R, et al. Regulation of enteric functions by adenosine: Pathophysiological and pharmacological implications. Pharmacol Ther. 2008;120:233–253. doi: 10.1016/j.pharmthera.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Burnstock G. Targeting the visceral purinergic system for pain control. Current Opinion in Pharmacology. 2012;12:80–86. doi: 10.1016/j.coph.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Roberts JA, Lukewich MK, Sharkey KA, et al. The roles of purinergic signaling during gastrointestinal inflammation. Curr Opin Pharmacol. 2012;12:659–666. doi: 10.1016/j.coph.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson KA, Jayasekara MP, Costanzi S. Molecular Structure of P2Y Receptors: Mutagenesis, Modeling, and Chemical Probes. Wiley Interdiscip Rev Membr Transp Signal. 2012;1:WMTS68. doi: 10.1002/wmts.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.North RA, Jarvis MF. P2X receptors as drug targets. Mol Pharmacol. 2013;83:759–769. doi: 10.1124/mol.112.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.IUPHAR/BPS. [Accessed April 8, 2014];Guide to Pharmacology. Available at: http//www.guidetopharmacology.org/
- 18.Bouman HJ, Schömig E, van Werkum JW, et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med. 2011;17:1044–1042. doi: 10.1038/nm.2281. [DOI] [PubMed] [Google Scholar]
- 19.Haas SL, Ruether A, Singer MV, et al. Functional P2X7 receptor polymorphisms (His155Tyr, Arg307Gln, Glu496Ala) in patients with Crohn’s disease. Scand J Immunol. 2007;65:166–170. doi: 10.1111/j.1365-3083.2006.01876.x. [DOI] [PubMed] [Google Scholar]
- 20.Fuller SJ, Stokes L, Skarratt KK, et al. Genetics of the P2X7 receptor and human disease. Purinergic signalling. 2009;5:257–262. doi: 10.1007/s11302-009-9136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parvathenani LK, Tertyshnikova S, Greco CR, et al. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 22.Barden N, Harvey M, Gagné B, et al. Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24. 31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2006;141:374–382. doi: 10.1002/ajmg.b.30303. [DOI] [PubMed] [Google Scholar]
- 23.Elliott JI, Higgins CF. Major histocompatibility complex class I shedding and programmed cell death stimulated through the proinflammatory P2X7 receptor: a candidate susceptibility gene for NOD diabetes. Diabetes. 2004;53:2012–2017. doi: 10.2337/diabetes.53.8.2012. [DOI] [PubMed] [Google Scholar]
- 24.Yiangou Y, Facer P, Durrenberger P, et al. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fletcher JM, Lonergan R, Costelloe L, et al. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 26.Friedman DJ, Kunzli BM, A-Rahim YI, et al. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mabley J, Soriano F, Pacher P, et al. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide, is protective in two murine models of colitis. Eur J Pharmacol. 2003;466:323–329. doi: 10.1016/s0014-2999(03)01570-x. [DOI] [PubMed] [Google Scholar]
- 28.Guzman J, Yu JG, Suntres Z, et al. ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm Bowel Dis. 2006;12:766–789. doi: 10.1097/00054725-200608000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Ren T, Grants IS, Alhaj M, et al. Impact of disrupting adenosine A3 receptors (A3−/−AR) on colonic motility or progression of colitis in the mouse. Inflamm Bowel Dis. 2011;17:1698–1713. doi: 10.1002/ibd.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler M, Sanmugalingam D, Burton VJ, et al. Impairment of adenosine A3 receptor activity disrupts neutrophil migratory capacity and impacts innate immune function in vivo. Eur J Immunol. 2012;42:3358–3368. doi: 10.1002/eji.201242655. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy TM, Jones RH. Epidemiology of cholecystectomy and irritable bowel syndrome in a UK population. Br J Surg. 2000;87:1658–1663. doi: 10.1046/j.1365-2168.2000.01596.x. [DOI] [PubMed] [Google Scholar]
- 32.Pedrosa E, Loren V, Cabre E, et al. Bacteria and spontaneous experimental colitis: immunological changes. Eur J Clin Invest. 2011;41:1047–1053. doi: 10.1111/j.1365-2362.2011.02499.x. [DOI] [PubMed] [Google Scholar]
- 33.Teng B, Ledent C, Mustafa SJ. Up-regulation of A2B adenosine receptor in A2A adenosine receptor knockout mouse coronary artery. J Mol Cell Cardiol. 2008;44:905–914. doi: 10.1016/j.yjmcc.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fishman P, Bar-Yehuda S, Liang BT, et al. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today. 2012;17:359–366. doi: 10.1016/j.drudis.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361–367. doi: 10.1111/j.1468-3083.2011.04078.x. [DOI] [PubMed] [Google Scholar]
- 36.Silverman MH, Strand V, Markovits D, et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. J Rheumatol. 2008;35:41–48. [PubMed] [Google Scholar]
- 37.Avni I, Garzozi HJ, Barequet IS, et al. Treatment of dry eye syndrome with orally administered CF101: data from a phase 2 clinical trial. Ophthalmology. 2010;117:1287–1293. doi: 10.1016/j.ophtha.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du L, Gao ZG, Nithipatikom K, et al. Protection from myocardial ischemia/reperfusion injury by a positive allosteric modulator of the A(3) adenosine receptor. J Pharmacol Exp Ther. 2012;340:210–217. doi: 10.1124/jpet.111.187559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Z, Janes K, Chen C, et al. Controlling murine and rat chronic pain through A3 adenosine receptor activation. FASEB J. 2012;26:1855–1865. doi: 10.1096/fj.11-201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anonymous. [Accessed April 8, 2014]; Available at www.news-medical.net/news/20131230/CF101-drug-fails-to-meet-primary-efficacy-endpoint-in-phase-III-study-for-Dry-Eye-Syndrome.aspx.
- 41.Van Troostenburg A, Clark E, Carey W, et al. Tolerability, pharmacokinetics and concentration-dependent hemodynamic effects of oral CF101, an A3 adenosine receptor agonist, in healthy young men. Int J Clin Pharmacol Ther. 2004;42:534–542. doi: 10.5414/cpp42534. [DOI] [PubMed] [Google Scholar]
- 42.Wagner R, Ngamsri K, Stark S, et al. Adenosine receptor A3 is a critical mediator in LPS-induced pulmonary inflammation. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2010;299:L502–L512. doi: 10.1152/ajplung.00083.2010. [DOI] [PubMed] [Google Scholar]
- 43.Fishman P, Bar-Yehuda S, Madi L, et al. The PI3K-NF-kappaB signal transduction pathway is involved in mediating the anti-inflammatory effect of IB-MECA in adjuvant-induced arthritis. Arthritis Research and Therapy. 2006;8:R33. doi: 10.1186/ar1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlotzer-Schrehardt U, Zenkel M, Hofmann-Rummelt C, et al. Functional significance of adenosine receptors in the eye and their dysregulation in pseudoexfoliation syndrome. Ophthalmologe. 2005;102:1074–80. 1082. doi: 10.1007/s00347-005-1216-4. [DOI] [PubMed] [Google Scholar]
- 45.Ochaion A, Bar-Yehuda S, Cohen S, et al. The anti-inflammatory target A(3) adenosine receptor is over-expressed in rheumatoid arthritis, psoriasis and Crohn’s disease. Cell Immunol. 2009;258:115–122. doi: 10.1016/j.cellimm.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 46.Madi L, Cohen S, Ochayin A, et al. Overexpression of A3 adenosine receptor in peripheral blood mononuclear cells in rheumatoid arthritis: involvement of nuclear factor-kappaB in mediating receptor level. J Rheumatol. 2007;34:20–26. [PubMed] [Google Scholar]
- 47.Rybaczyk L, Rozmiarek A, Circle K, et al. New bioinformatics approach to analyze gene expressions and signaling pathways reveals unique purine gene dysregulation profiles that distinguish between CD and UC. Inflamm Bowel Dis. 2009;15:971–984. doi: 10.1002/ibd.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer D, Van der Weyden M, Snyderman R, et al. A role for adenosine deaminase in human monocyte maturation. J Clin Invest. 1976;58:399. doi: 10.1172/JCI108484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antonioli L, Fornai M, Colucci R, et al. Inhibition of adenosine deaminase attenuates inflammation in experimental colitis. J Pharmacol Exp Ther. 2007;322:435–442. doi: 10.1124/jpet.107.122762. [DOI] [PubMed] [Google Scholar]
- 50.Maor I, Rainis T, Lanir A, et al. Adenosine deaminase activity in patients with Crohn’s disease: distinction between active and nonactive disease. Eur J Gastroenterol Hepatol. 2011;23:598–602. doi: 10.1097/MEG.0b013e328346e205. [DOI] [PubMed] [Google Scholar]
- 51.Cesaro A, Brest P, Hofman V, et al. Amplification loop of the inflammatory process is induced by P2X7R activation in intestinal epithelial cells in response to neutrophil transepithelial migration. Am J Physiol Gastrointest Liver Physiol. 2010;299:G32–42. doi: 10.1152/ajpgi.00282.2009. [DOI] [PubMed] [Google Scholar]
- 52.Kunzli BM, Berberat PO, Dwyer K, et al. Variable Impact of CD39 in Experimental Murine Colitis. Digestive Diseases & Sciences. 2011;56:1393–1403. doi: 10.1007/s10620-010-1425-9. [DOI] [PubMed] [Google Scholar]
- 53.Grbic DM, Degagne E, Larrivee JF, et al. P2Y6 receptor contributes to neutrophil recruitment to inflamed intestinal mucosa by increasing CXC chemokine ligand 8 expression in an AP-1-dependent manner in epithelial cells. Inflamm Bowel Dis. 2012;18:1456–1469. doi: 10.1002/ibd.21931. [DOI] [PubMed] [Google Scholar]
- 54.Kurashima Y, Amiya T, Nochi T, et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat Commun. 2012;3:1034. doi: 10.1038/ncomms2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fredholm BB. Adenosine receptors as drug targets. Exp Cell Res. 2010;316:1284–1288. doi: 10.1016/j.yexcr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov. 2013;12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karhausen J, Furuta GT, Tomaszewski JE, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hart ML, Gorzolla IC, Schittenhelm J, et al. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 2010;184:4017–4024. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahimian R, Fakhfouri G, Daneshmand A, et al. Adenosine A2A receptors and uric acid mediate protective effects of inosine against TNBS-induced colitis in rats. Eur J Pharmacol. 2010;649:376–381. doi: 10.1016/j.ejphar.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 63.Gao N, Hu HZ, Liu S, et al. Stimulation of adenosine A1 and A2A receptors by AMP in the submucosal plexus of guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G492–500. doi: 10.1152/ajpgi.00257.2006. [DOI] [PubMed] [Google Scholar]
- 64.Louis NA, Robinson AM, MacManus CF, et al. Control of IFN-alphaA by CD73: implications for mucosal inflammation. J Immunol. 2008;180:4246–4255. doi: 10.4049/jimmunol.180.6.4246. [DOI] [PubMed] [Google Scholar]
- 65.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strohmeier GR, Lencer WI, Patapoff TW, et al. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest. 1997;99:2588. doi: 10.1172/JCI119447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naganuma M, Wiznerowicz EB, Lappas CM, et al. Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. The Journal of Immunology. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 68.Frick JS, MacManus CF, Scully M, et al. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aherne CM, Collins CB, Masterson JC, et al. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut. 2012;61:695–705. doi: 10.1136/gutjnl-2011-300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cronstein BN, Montesinos MC, Weissmann G. Salicylates and sulfasalazine, but not glucocorticoids, inhibit leukocyte accumulation by an adenosine-dependent mechanism that is independent of inhibition of prostaglandin synthesis and p105 of NFκB. Proceedings of the National Academy of Sciences. 1999;96:6377–6381. doi: 10.1073/pnas.96.11.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morabito L, Montesinos MC, Schreibman DM, et al. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest. 1998;101:295. doi: 10.1172/JCI1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Linden J. Cell biology. Purinergic chemotaxis. Science. 2006;314:1689–1690. doi: 10.1126/science.1137190. [DOI] [PubMed] [Google Scholar]
- 73.Gulbransen BD, Bashashati M, Hirota SA, et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Souza, Viviane do Carmo Gonçalves, Schlemmer KB, Noal CB, et al. E-NTPDase and E-ADA activities are altered in lymphocytes of patients with indeterminate form of Chagas’ disease. Parasitol Int. 2012 doi: 10.1016/j.parint.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 75.Neshat S, deVries M, Barajas-Espinosa AR, et al. Loss of purinergic vascular regulation in the colon during colitis is associated with upregulation of CD39. Am J Physiol Gastrointest Liver Physiol. 2009;296:G399–405. doi: 10.1152/ajpgi.90450.2008. [DOI] [PubMed] [Google Scholar]
- 76.Montesinos MC, Takedachi M, Thompson LF, et al. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5′-nucleotidase: Findings in a study of ecto-5′-nucleotidase gene–deficient mice. Arthritis & Rheumatism. 2007;56:1440–1445. doi: 10.1002/art.22643. [DOI] [PubMed] [Google Scholar]
- 77.Csóka B, Németh ZH, Virág L, et al. A2A adenosine receptors and C/EBPβ are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110:2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bodin P, Burnstock G. Increased release of ATP from endothelial cells during acute inflammation. Inflammation Res. 1998;47:351–354. doi: 10.1007/s000110050341. [DOI] [PubMed] [Google Scholar]
- 79.Noji T, Takayama M, Mizutani M, et al. KF24345, an adenosine uptake inhibitor, suppresses lipopolysaccharide-induced tumor necrosis factor-α production and leukopenia via endogenous adenosine in mice. J Pharmacol Exp Ther. 2002;300:200–205. doi: 10.1124/jpet.300.1.200. [DOI] [PubMed] [Google Scholar]
- 80.van Eijk LT, Dorresteijn MJ, Smits P, et al. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers*. Crit Care Med. 2007;35:1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- 81.Riksen NP, Oyen WJ, Ramakers BP, et al. Oral therapy with dipyridamole limits ischemia-reperfusion injury in humans. Clinical Pharmacology & Therapeutics. 2005;78:52–59. doi: 10.1016/j.clpt.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 82.Ramakers BP, Riksen NP, Stal TH, et al. Dipyridamole augments the antiinflammatory response during human endotoxemia. Crit Care. 2011;15:R289. doi: 10.1186/cc10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poturoglu S, Kaymakoglu S, Gurel Polat N, et al. A new agent for tumour necrosis factor-alpha inhibition. Scandinavian Journal of Clinical & Laboratory Investigation. 2009;69:696–702. doi: 10.3109/00365510902989075. [DOI] [PubMed] [Google Scholar]
- 84.Le Vraux V, Chen Y, Masson I, et al. Inhibition of human monocyte TNF production by adenosine receptor agonists. Life Sci. 1993;52:1917–1924. doi: 10.1016/0024-3205(93)90632-d. [DOI] [PubMed] [Google Scholar]
- 85.Chello M, Mastroroberto P, Malta E, et al. Inhibition by dipyridamole of neutrophil adhesion to vascular endothelium during coronary bypass surgery. Ann Thorac Surg. 1999;67:1277–1282. doi: 10.1016/s0003-4975(99)00173-3. [DOI] [PubMed] [Google Scholar]
- 86.Jenner P, Mori A, Hauser R, et al. Adenosine, adenosine A2A antagonists, and Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:406–413. doi: 10.1016/j.parkreldis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Hauser RA, Cantillon M, Pourcher E, et al. Preladenant in patients with Parkinson’s disease and motor fluctuations: a phase 2, double-blind, randomised trial. The Lancet Neurology. 2011;10:221–229. doi: 10.1016/S1474-4422(11)70012-6. [DOI] [PubMed] [Google Scholar]
- 88.Teerlink JR, Iragui VJ, Mohr JP, et al. The safety of an adenosine A(1)-receptor antagonist, rolofylline, in patients with acute heart failure and renal impairment: findings from PROTECT. Drug Saf. 2012;35:233–244. doi: 10.2165/11594680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 89.Yang J, Björklund O, Lindström-Törnqvist K, et al. Mice heterozygous for both A1 and A2A adenosine receptor genes show similarities to mice given long-term caffeine. J Appl Physiol. 2009;106:631–639. doi: 10.1152/japplphysiol.90971.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fredholm BB, Bättig K, Holmén J, et al. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 91.Greig E, Boot-Handford R, Mani V, et al. Decreased expression of apical Na channels and basolateral Na, K -ATPase in ulcerative colitis. J Pathol. 2004;204:84–92. doi: 10.1002/path.1613. [DOI] [PubMed] [Google Scholar]
- 92.Amasheh S, Barmeyer C, Koch CS, et al. Cytokine-dependent transcriptional down-regulation of epithelial sodium channel in ulcerative colitis. Gastroenterology. 2004;126:1711–1720. doi: 10.1053/j.gastro.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 93.Zeissig S, Bergann T, Fromm A, et al. Altered ENaC expression leads to impaired sodium absorption in the noninflamed intestine in Crohn’s disease. Gastroenterology. 2008;134:1436–1447. doi: 10.1053/j.gastro.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 94.Sandle GI. Infective and inflammatory diarrhoea: mechanisms and opportunities for novel therapies. Current Opinion in Pharmacology. 2011;11:634–639. doi: 10.1016/j.coph.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 95.Haskó G, Csóka B, Németh ZH, et al. A2B adenosine receptors in immunity and inflammation. Trends Immunol. 2009;30:263–270. doi: 10.1016/j.it.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kolachala V, Ruble B, Vijay-Kumar M, et al. Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol. 2008;155:127–137. doi: 10.1038/bjp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Strohmeier GR, Reppert SM, Lencer WI, et al. The A Adenosine Receptor Mediates cAMP Responses to Adenosine Receptor Agonists in Human Intestinal Epithelia. J Biol Chem. 1995;270:2387–2394. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- 98.Kolachala VL, Vijay-Kumar M, Dalmasso G, et al. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology. 2008;135:861–870. doi: 10.1053/j.gastro.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sitaraman SV, Merlin D, Wang L, et al. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest. 2001;107:861–869. doi: 10.1172/JCI11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kolachala V, Asamoah V, Wang L, et al. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cell Mol Life Sci. 2005;62:2647–2657. doi: 10.1007/s00018-005-5328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Christofi FL. Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signal. 2008;4:213–236. doi: 10.1007/s11302-008-9104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cooke HJCF. Chapter 27: Enteric Neural Regulation of Mucosal Secretion. In: Jhonson LR, editor. Physiology of the Gastrointestinal Tract. 4. New York: Academic Press; 2006. pp. 737–762. [Google Scholar]
- 103.Fang X, Hu H, Gao N, et al. Neurogenic secretion mediated by the purinergic P2Y1 receptor in guinea-pig small intestine. Eur J Pharmacol. 2006;536:113–122. doi: 10.1016/j.ejphar.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 104.Lundgren O. Enteric nerves and diarrhoea. Pharmacol Toxicol. 2002;90:109–120. doi: 10.1034/j.1600-0773.2002.900301.x. [DOI] [PubMed] [Google Scholar]
- 105.Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- 106.Matkowskyj KA, Nathaniel R, Prasad R, et al. Galanin contributes to the excess colonic fluid secretion observed in dextran sulfate sodium murine colitis. Inflamm Bowel Dis. 2004;10:408–416. doi: 10.1097/00054725-200407000-00012. [DOI] [PubMed] [Google Scholar]
- 107.Seidler U, Lenzen H, Cinar A, et al. Molecular mechanisms of disturbed electrolyte transport in intestinal inflammation. Ann N Y Acad Sci. 2006;1072:262–275. doi: 10.1196/annals.1326.024. [DOI] [PubMed] [Google Scholar]
- 108.Wunderlich JE, Needleman BJ, Chen Z, et al. Dual purinergic synaptic transmission in the human enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2008;294:G554–66. doi: 10.1152/ajpgi.00500.2007. [DOI] [PubMed] [Google Scholar]
- 109.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 110.Christofi FL. Unlocking mysteries of gut sensory transmission: is adenosine the key? News Physiol Sci. 2001;16:201–207. doi: 10.1152/physiologyonline.2001.16.5.201. [DOI] [PubMed] [Google Scholar]
- 111.Cressman VL, Lazarowski E, Homolya L, et al. Effect of loss of P2Y(2) receptor gene expression on nucleotide regulation of murine epithelial Cl(−) transport. J Biol Chem. 1999;274:26461–26468. doi: 10.1074/jbc.274.37.26461. [DOI] [PubMed] [Google Scholar]
- 112.Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum. 2007;50:376–388. doi: 10.1007/s10350-006-0763-3. [DOI] [PubMed] [Google Scholar]
- 113.Kraneveld AD, Rijnierse A, Nijkamp FP, et al. Neuro-immune interactions in inflammatory bowel disease and irritable bowel syndrome: future therapeutic targets. Eur J Pharmacol. 2008;585:361–374. doi: 10.1016/j.ejphar.2008.02.095. [DOI] [PubMed] [Google Scholar]
- 114.Linden DR, Foley KF, McQuoid C, et al. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17:565–574. doi: 10.1111/j.1365-2982.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- 115.Burnstock G. P2X receptors in the gut. Wiley Interdisciplinary Reviews: Membrane Transport and Signaling. 2012;1:269–279. doi: 10.1002/wmts.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodrigues RJ, Almeida T, Richardson PJ, et al. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci. 2005;25:6286–6295. doi: 10.1523/JNEUROSCI.0628-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Christofi FL, Wunderlich J, Yu JG, et al. Mechanically evoked reflex electrogenic chloride secretion in rat distal colon is triggered by endogenous nucleotides acting at P2Y1, P2Y2, and P2Y4 receptors. J Comp Neurol. 2004;469:16–36. doi: 10.1002/cne.10961. [DOI] [PubMed] [Google Scholar]
- 118.Cooke HJ, Wunderlich J, Christofi FL. “The force be with you”: ATP in gut mechanosensory transduction. News Physiol Sci. 2003;18:43–49. doi: 10.1152/nips.01411.2002. [DOI] [PubMed] [Google Scholar]
- 119.Cooke HJ, Xue J, Yu JG, et al. Mechanical stimulation releases nucleotides that activate P2Y1 receptors to trigger neural reflex chloride secretion in guinea pig distal colon. J Comp Neurol. 2004;469:1–15. doi: 10.1002/cne.10960. [DOI] [PubMed] [Google Scholar]
- 120.Xia Y, Hu HZ, Liu S, et al. Clostridium difficile toxin A excites enteric neurones and suppresses sympathetic neurotransmission in the guinea pig. Gut. 2000;46:481–486. doi: 10.1136/gut.46.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wunderlich JE, Ren T, Needleman B, et al. Novel Purinergic P1, P2X and P2Y Receptor Signaling Targets in the Human Enteric Nervous System and Glia. Gastroenterology. 2009;136:A–3. [Google Scholar]
- 122.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 123.Christofi FL, Kim M, Wunderlich JE, et al. Endogenous adenosine differentially modulates 5-hydroxytryptamine release from a human enterochromaffin cell model. Gastroenterology. 2004;127:188–202. doi: 10.1053/j.gastro.2004.04.070. [DOI] [PubMed] [Google Scholar]
- 124.Haugen M, Dammen R, Svejda B, et al. Differential signal pathway activation and 5-HT function: the role of gut enterochromaffin cells as oxygen sensors. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1164–73. doi: 10.1152/ajpgi.00027.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111:904. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Broad RM, Cook MA. Inhibition of neurotransmitter release from enteric nerve endings by 2-[p-(carboxyethyl)-phenethylamino]-5′-N-ethylcarboxamido-adenosine (CGS-21680) and related adenosine analogs: lack of simple competition by antagonists. J Pharmacol Exp Ther. 1993;266:634–641. [PubMed] [Google Scholar]
- 127.Semenza GL. HIF-1, O2, and the 3 PHDs: How Animal Cells Signal Hypoxia to the Nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 128.Matheus N, Mendoza C, Iceta R, et al. Regulation of serotonin transporter activity by adenosine in intestinal epithelial cells. Biochem Pharmacol. 2009;78:1198–1204. doi: 10.1016/j.bcp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 129.Damen R, Haugen M, Svejda B, et al. The stimulatory adenosine receptor ADORA2B regulates serotonin (5-HT) synthesis and release in oxygen-depleted EC cells in inflammatory bowel disease. PLoS One. 2013;8:e62607. doi: 10.1371/journal.pone.0062607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.LinanRico A, Wunderlich JE, Grants IS, et al. Purinergic Autocrine Regulation of Mechanosensitivity and Serotonin Release in a Human EC Model: ATP-gated P2X3 Channels in EC are Downregulated in Ulcerative Colitis. Inflamm Bowel Dis. 2013;19:2366–2379. doi: 10.1097/MIB.0b013e31829ecf4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kobie JJ, Shah PR, Yang L, et al. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. The Journal of Immunology. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 132.Odashima M, Bamias G, Rivera-Nieves J, et al. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129:26–33. doi: 10.1053/j.gastro.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 133.Alam MS, Kurtz CC, Wilson JM, et al. A2A adenosine receptor (AR) activation inhibits pro-inflammatory cytokine production by human CD4+ helper T cells and regulates Helicobacter-induced gastritis and bacterial persistence. Mucosal Immunol. 2009;2:232–242. doi: 10.1038/mi.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Orrú M, Quiroz C, Guitart X, et al. Pharmacological evidence for different populations of postsynaptic adenosine A2A receptors in the rat striatum. Neuropharmacology. 2011;61:967–974. doi: 10.1016/j.neuropharm.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Armentero MT, Pinna A, Ferré S, et al. Past, present and future of A2A adenosine receptor antagonists in the therapy of Parkinson’s disease. Pharmacol Ther. 2011;132:280–299. doi: 10.1016/j.pharmthera.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moriyama K, Sitkovsky MV. Adenosine A2A receptor is involved in cell surface expression of A2B receptor. J Biol Chem. 2010;285:39271–39288. doi: 10.1074/jbc.M109.098293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Canals M, Marcellino D, Fanelli F, et al. Adenosine A2A-dopamine D2 receptor-receptor heteromerization qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- 138.Ciruela F, Casadó V, Rodrigues RJ, et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. The Journal of neuroscience. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ferré S, Karcz-Kubicha M, Hope BT, et al. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proceedings of the National Academy of Sciences. 2002;99:11940–11945. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 141.Ivison SM, Himmel ME, Mayer M, et al. The stress signal extracellular ATP modulates antiflagellin immune responses in intestinal epithelial cells. Inflamm Bowel Dis. 2011;17:319–333. doi: 10.1002/ibd.21428. [DOI] [PubMed] [Google Scholar]
- 142.Souza CO, Santoro GF, Figliuolo VR, et al. Extracellular ATP induces cell death in human intestinal epithelial cells. Biochim Biophys Acta. 2012;1820:1867–1878. doi: 10.1016/j.bbagen.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 143.Grbic DM, Degagne E, Langlois C, et al. Intestinal inflammation increases the expression of the P2Y6 receptor on epithelial cells and the release of CXC chemokine ligand 8 by UDP. J Immunol. 2008;180:2659–2668. doi: 10.4049/jimmunol.180.4.2659. [DOI] [PubMed] [Google Scholar]
- 144.Degagne E, Turgeon N, Moore-Gagne J, et al. P2Y(2) receptor expression is regulated by C/EBPbeta during inflammation in intestinal epithelial cells. FEBS J. 2012;279:2957–2965. doi: 10.1111/j.1742-4658.2012.08676.x. [DOI] [PubMed] [Google Scholar]
- 145.Antonioli L, Fornai M, Colucci R, et al. Control of enteric neuromuscular functions by purinergic A(3) receptors in normal rat distal colon and experimental bowel inflammation. Br J Pharmacol. 2010;161:856–871. doi: 10.1111/j.1476-5381.2010.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bozarov A, Wang YZ, Yu JG, et al. Activation of adenosine low-affinity A3 receptors inhibits the enteric short interplexus neural circuit triggered by histamine. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1147–62. doi: 10.1152/ajpgi.00295.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Breunig E, Michel K, Zeller F, et al. Histamine excites neurones in the human submucous plexus through activation of H1, H2, H3 and H4 receptors. J Physiol (Lond) 2007;583:731–742. doi: 10.1113/jphysiol.2007.139352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chandrasekharan BP, Kolachala VL, Dalmasso G, et al. Adenosine 2B receptors (A(2B)AR) on enteric neurons regulate murine distal colonic motility. FASEB J. 2009;23:2727–2734. doi: 10.1096/fj.09-129544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Antonioli L, Fornai M, Colucci R, et al. Differential recruitment of high affinity A1 and A2A adenosine receptors in the control of colonic neuromuscular function in experimental colitis. Eur J Pharmacol. 2011;650:639–649. doi: 10.1016/j.ejphar.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 150.Fornai M, Antonioli L, Colucci R, et al. A1 and A2a receptors mediate inhibitory effects of adenosine on the motor activity of human colon. Neurogastroenterol Motil. 2009;21:451–466. doi: 10.1111/j.1365-2982.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 151.Cooke HJ, Wang Y, Liu CY, et al. Activation of neuronal adenosine A1 receptors suppresses secretory reflexes in the guinea pig colon. Am J Physiol. 1999;276:G451–62. doi: 10.1152/ajpgi.1999.276.2.G451. [DOI] [PubMed] [Google Scholar]
- 152.Kadowaki M, Nagakura Y, Tokita K, et al. Adenosine A1 receptor blockade reverses experimental postoperative ileus in rat colon. Eur J Pharmacol. 2003;458:197–200. doi: 10.1016/s0014-2999(02)02766-8. [DOI] [PubMed] [Google Scholar]
- 153.Wang L, Yao H, Yang Y, et al. Enhanced purinergic pathway occurs in postoperative ileus: reversal by orphanin FQ. 2004;126:A11–A11. [Google Scholar]
- 154.Strong DS, Cornbrooks CF, Roberts JA, et al. Purinergic neuromuscular transmission is selectively attenuated in ulcerated regions of inflamed guinea pig distal colon. J Physiol (Lond) 2010;588:847–859. doi: 10.1113/jphysiol.2009.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cosimelli B, Taliani S, Greco G, et al. Derivatives of benzimidazol-2-ylquinoline and benzimidazol-2-ylisoquinoline as selective A1 adenosine receptor antagonists with stimulant activity on human colon motility. ChemMedChem. 2011 Oct 4;6(10):1909–18. doi: 10.1002/cmdc.201100284. Epub 2011 Jul 27. [DOI] [PubMed] [Google Scholar]
- 156.Auli M, Martinez E, Gallego D, et al. Effects of excitatory and inhibitory neurotransmission on motor patterns of human sigmoid colon in vitro. Br J Pharmacol. 2008;155:1043–1055. doi: 10.1038/bjp.2008.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang G, Wang X, Hu H, et al. Inhibitory neuromuscular transmission mediated by the P2Y1 purinergic receptor in guinea pig small intestine. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2007;292:G1483–G1489. doi: 10.1152/ajpgi.00450.2006. [DOI] [PubMed] [Google Scholar]
- 158.Burnstock G. P2X receptors in the gut. Wiley Interdisciplinary Reviews: Membrane Transport and Signaling. 2012;1:269–279. doi: 10.1002/wmts.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136:1349–1358. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 160.Burnstock G. Targeting the visceral purinergic system for pain control. Curr Opin Pharmacol. 2012 Feb;12(1):80–6. doi: 10.1016/j.coph.2011.10.008. Epub 2011 Oct 28. [DOI] [PubMed] [Google Scholar]
- 161.Fan J, Yu LH, Zhang Y, et al. Estrogen altered visceromotor reflex and P2X(3) mRNA expression in a rat model of colitis. Steroids. 2009;74:956–962. doi: 10.1016/j.steroids.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 162.Yiangou Y, Facer P, Baecker PA, et al. ATP-gated ion channel P2X(3) is increased in human inflammatory bowel disease. Neurogastroenterol Motil. 2001;13:365–369. doi: 10.1046/j.1365-2982.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 163.Ford AP. In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal. 2012;8:3–26. doi: 10.1007/s11302-011-9271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rong W, Keating C, Sun B, et al. Purinergic contribution to small intestinal afferent hypersensitivity in a murine model of postinfectious bowel disease. Neurogastroenterology & Motility. 2009;21:665–671. e32. doi: 10.1111/j.1365-2982.2008.01259.x. [DOI] [PubMed] [Google Scholar]
- 165.Shinoda M, Feng B, Gebhart G. Peripheral and Central P2X3 Receptor Contributions to Colon Mechanosensitivity and Hypersensitivity in the Mouse. Gastroenterology. 2009;137:2096–2104. doi: 10.1053/j.gastro.2009.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu GY, Shenoy M, Winston JH, et al. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57:1230–1237. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]
- 167.Altomare A, Luca Guarino Sara Emerenziani MP, Cicala M, et al. Gastrointestinal sensitivity and gastroesophageal reflux disease. Ann N Y Acad Sci. 2013;1300:80–95. doi: 10.1111/nyas.12236. [DOI] [PubMed] [Google Scholar]
- 168.Cockayne DA, Hamilton SG, Zhu Q, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X 3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 169.Vlaskovska M, Kasakov L, Rong W, et al. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. The Journal of Neuroscience. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dang K, Lamb K, Cohen M, et al. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol. 2008;99:49–59. doi: 10.1152/jn.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fukuda K, Hayashida M, Fukunaga A, et al. Pain-relieving effects of intravenous ATP in chronic intractable orofacial pain: an open-label study. Journal of anesthesia. 2007;21:24–30. doi: 10.1007/s00540-006-0444-3. [DOI] [PubMed] [Google Scholar]
- 172.Handa T, Fukuda K, Hayashida M, et al. Effects of intravenous adenosine 5′-triphosphate on intraoperative hemodynamics and postoperative pain in patients undergoing major orofacial surgery: a double-blind placebo-controlled study. Journal of anesthesia. 2009;23:315–322. doi: 10.1007/s00540-009-0751-6. [DOI] [PubMed] [Google Scholar]
- 173.Habib AS, Minkowitz H, Osborn T, et al. Phase 2, double-blind, placebo-controlled, dose-response trial of intravenous adenosine for perioperative analgesia. Anesthesiology. 2008;109:1085–1091. doi: 10.1097/ALN.0b013e31818db88c. [DOI] [PubMed] [Google Scholar]
- 174.Gunosewoyo H, Kassiou M. P2X purinergic receptor ligands: recently patented compounds. Expert Opin Ther Pat. 2010;20:625–646. doi: 10.1517/13543771003702424. [DOI] [PubMed] [Google Scholar]
- 175.Gever JR, Soto R, Henningsen RA, et al. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol. 2010;160:1387–1398. doi: 10.1111/j.1476-5381.2010.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ballini E, Virginio C, Medhurst S, et al. Characterization of three diaminopyrimidines as potent and selective antagonists of P2X3 and P2X2/3 receptors with in vivo efficacy in a pain model. Br J Pharmacol. 2011;163:1315–1325. doi: 10.1111/j.1476-5381.2011.01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Beneng K, Yilmaz Z, Yiangou Y, et al. Sensory purinergic receptor P2X3 is elevated in burning mouth syndrome. Int J Oral Maxillofac Surg. 2010;39:815–819. doi: 10.1016/j.ijom.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 178.Arulkumaran N, Unwin RJ, Tam FW. A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert Opin Investig Drugs. 2011;20:897–915. doi: 10.1517/13543784.2011.578068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Harrison C. Patent Watch. Nat Rev Drug Discovery. 2008;7:553. doi: 10.1038/nrd2751. [DOI] [PubMed] [Google Scholar]
- 180.Malin SA, Molliver DC. Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain. 2010;6:21–8069-6-21. doi: 10.1186/1744-8069-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fernando SL, Saunders BM, Sluyter R, et al. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Respir Crit Care Med. 2007;175:360–366. doi: 10.1164/rccm.200607-970OC. [DOI] [PubMed] [Google Scholar]
- 182.Niño-Moreno P, Portales-Pérez D, Hernández-Castro B, et al. P2X7 and NRAMP1/SLC11 A1 gene polymorphisms in Mexican mestizo patients with pulmonary tuberculosis. Clinical & Experimental Immunology. 2007;148:469–477. doi: 10.1111/j.1365-2249.2007.03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chessell IP, Hatcher JP, Bountra C, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 184.McGaraughty S, Chu K, Namovic M, et al. P2X7-related modulation of pathological nociception in rats. Neuroscience. 2007;146:1817–1828. doi: 10.1016/j.neuroscience.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 185.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 186.Ferrari D, Pizzirani C, Adinolfi E, et al. The P2X7 receptor: a key player in IL-1 processing and release. The Journal of Immunology. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 187.Stock TC, Bloom BJ, Wei N, et al. Efficacy and safety of CE-224,535, an antagonist of P2X7 receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate. J Rheumatol. 2012;39:720–727. doi: 10.3899/jrheum.110874. [DOI] [PubMed] [Google Scholar]
- 188.von Arnim U, Peitz U, Vinson B, et al. STW 5, a phytopharmacon for patients with functional dyspepsia: results of a multicenter, placebo-controlled double-blind study. Am J Gastroenterol. 2007;102:1268–1275. doi: 10.1111/j.1572-0241.2006.01183.x. [DOI] [PubMed] [Google Scholar]
- 189.Madisch A, Holtmann G, Mayr G, et al. Treatment of functional dyspepsia with a herbal preparation. A double-blind, randomized, placebo-controlled, multicenter trial. Digestion. 2004;69:45–52. doi: 10.1159/000076546. [DOI] [PubMed] [Google Scholar]
- 190.Madisch A, Holtmann G, Plein K, et al. Treatment of irritable bowel syndrome with herbal preparations: results of a double-blind, randomized, placebo-controlled, multi-centre trial. Aliment Pharmacol Ther. 2004;19:271–279. doi: 10.1111/j.1365-2036.2004.01859.x. [DOI] [PubMed] [Google Scholar]
- 191.Michael S, Abdel-Aziz H, Weiser D, et al. Adenosine A2A receptor contributes to the anti-inflammatory effect of the fixed herbal combination STW 5 (Iberogast(R)) in rat small intestinal preparations. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:411–421. doi: 10.1007/s00210-011-0714-y. [DOI] [PubMed] [Google Scholar]
- 192.Soares-Bezerra RJ, Calheiros AS, da Silva Ferreira NC, et al. Natural Products as a Source for New Anti-Inflammatory and Analgesic Compounds through the Inhibition of Purinergic P2X Receptors. Pharmaceuticals (Basel) 2013;6:650–658. doi: 10.3390/ph6050650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang X, Chen H, Li Z, et al. Analgesic effect of paeoniflorin in rats with neonatal maternal separation-induced visceral hyperalgesia is mediated through adenosine A1 receptor by inhibiting the extracellular signal-regulated protein kinase (ERK) pathway. Pharmacology, Biochemistry & Behavior. 2009;94:88–97. doi: 10.1016/j.pbb.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 194.Sangkuhl K, Klein TE, Altman RB. Clopidogrel pathway. Pharmacogenet Genomics. 2010;20:463–465. doi: 10.1097/FPC.0b013e3283385420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Smecuol E, Bai J, Sugai E, et al. Acute gastrointestinal permeability responses to different non-steroidal anti-inflammatory drugs. Gut. 2001;49:650–655. doi: 10.1136/gut.49.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Whittle BJ. Mechanisms underlying intestinal injury induced by anti-inflammatory COX inhibitors. Eur J Pharmacol. 2004;500:427–439. doi: 10.1016/j.ejphar.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 197.Bours MJ, Troost FJ, Brummer RJ, et al. Local effect of adenosine 5′-triphosphate on indomethacin-induced permeability changes in the human small intestine. Eur J Gastroenterol Hepatol. 2007;19:245–250. doi: 10.1097/MEG.0b013e328011093c. [DOI] [PubMed] [Google Scholar]
- 198.Fukuda Y, Bamba H, Okui M, et al. Helicobacter pylori infection increases mucosal permeability of the stomach and intestine. Digestion. 2001;63:93–96. doi: 10.1159/000051918. [DOI] [PubMed] [Google Scholar]
- 199.Murphy M, Eastham E, Nelson R, et al. Intestinal permeability in Crohn’s disease. Arch Dis Child. 1989;64:321–325. doi: 10.1136/adc.64.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Welcker K, Martin A, Kolle P, et al. Increased intestinal permeability in patients with inflammatory bowel disease. Eur J Med Res. 2004;9:456–460. [PubMed] [Google Scholar]
- 201.Arnott I, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand J Gastroenterol. 2000;35:1163–1169. doi: 10.1080/003655200750056637. [DOI] [PubMed] [Google Scholar]
- 202.D’Incà R, Di Leo V, Corrao G, et al. Intestinal permeability test as a predictor of clinical course in Crohn’s disease. Am J Gastroenterol. 1999;94:2956–2960. doi: 10.1111/j.1572-0241.1999.01444.x. [DOI] [PubMed] [Google Scholar]
- 203.DeMeo MT, Mutlu EA, Keshavarzian A, et al. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34:385–396. doi: 10.1097/00004836-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 204.Barbara G, Cremon C, De Giorgio R, et al. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep. 2011;13:308–315. doi: 10.1007/s11894-011-0195-7. [DOI] [PubMed] [Google Scholar]
- 205.Wagner MCE. The Therapeutic Potential of Adenosine Triphosphate as an Immune Modulator in the Treatment of HIV/AIDS: A Combination Approach with HAART. Current HIV Research. 2011;9:209–222. doi: 10.2174/157016211796320289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Voss U, Turesson MF, Robaye B, et al. The enteric nervous system of P2Y receptor null mice is resistant against high-fat-diet- and palmitic-acid-induced neuronal loss. Purinergic Signal. 2014 doi: 10.1007/s11302-014-9408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Espada S, Ortega F, Molina-Jijon E, et al. The purinergic P2Y(13) receptor activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Free Radic Biol Med. 2010;49:416–426. doi: 10.1016/j.freeradbiomed.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 208.Adjei A, Morioka T, Ameho C, et al. Nucleoside-nucleotide free diet protects rat colonic mucosa from damage induced by trinitrobenzene sulphonic acid. Gut. 1996;39:428–433. doi: 10.1136/gut.39.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Adjei AA, Ameho CK, Harrison EK, et al. Nucleoside-nucleotide-free diet suppresses cytokine production and contact sensitivity responses in rats with trinitrobenzene sulphonic acid-induced colitis. Am J Med Sci. 1997;314:89–96. doi: 10.1097/00000441-199708000-00010. [DOI] [PubMed] [Google Scholar]
- 210.Somers GR, Hammet FM, Trute L, et al. Expression of the P2Y6 purinergic receptor in human T cells infiltrating inflammatory bowel disease. Lab Invest. 1998;78:1375–1383. [PubMed] [Google Scholar]
- 211.Müller CE, Jacobson KA. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2011;1808:1290–1308. doi: 10.1016/j.bbamem.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Keating C, Pelegrin P, Martínez CM, et al. P2X7 receptor-dependent intestinal afferent hypersensitivity in a mouse model of postinfectious irritable bowel syndrome. The Journal of Immunology. 2011;187:1467–1474. doi: 10.4049/jimmunol.1100423. [DOI] [PubMed] [Google Scholar]
- 213.Bynoe MS, Waickman AT, Mahamed DA, et al. CD73 is critical for the resolution of murine colonic inflammation. J Biomed Biotechnol. 2012;2012:260983. doi: 10.1155/2012/260983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gil V, Martinez-Cutillas M, Mane N, et al. P2Y(1) knockout mice lack purinergic neuromuscular transmission in the antrum and cecum. Neurogastroenterol Motil. 2013;25:e170–82. doi: 10.1111/nmo.12060. [DOI] [PubMed] [Google Scholar]
- 215.Shinoda M, La JH, Bielefeldt K, et al. Altered purinergic signaling in colorectal dorsal root ganglion neurons contributes to colorectal hypersensitivity. J Neurophysiol. 2010;104:3113–3123. doi: 10.1152/jn.00560.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Guo X, Chen J, Lu Y, et al. Electroacupuncture at He-Mu points reduces P2X4 receptor expression in visceral hypersensitivity***. Neural Regeneration Research. 2013;8:2069–2077. doi: 10.3969/j.issn.1673-5374.2013.22.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Migita K, Moriyama T, Koguchi M, et al. Modulation of P2X receptors in dorsal root ganglion neurons of streptozotocin-induced diabetic neuropathy. Neurosci Lett. 2009;452:200–203. doi: 10.1016/j.neulet.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 218.Xu GY, Li G, Liu N, et al. Mechanisms underlying purinergic P2X3 receptor-mediated mechanical allodynia induced in diabetic rats. Mol Pain. 2011;7:60–8069-7-60. doi: 10.1186/1744-8069-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shi L, Zhang HH, Hu J, et al. Purinergic P2X receptors and diabetic neuropathic pain. Sheng Li Xue Bao. 2012;64:531–542. [PubMed] [Google Scholar]
- 220.O’Donnell AM, Puri P. Deficiency of purinergic P2Y receptors in aganglionic intestine in Hirschsprung’s disease. Pediatr Surg Int. 2008;24:77–80. doi: 10.1007/s00383-007-2044-1. [DOI] [PubMed] [Google Scholar]
- 221.De Man JG, Seerden TC, De Winter BY, et al. Alteration of the purinergic modulation of enteric neurotransmission in the mouse ileum during chronic intestinal inflammation. Br J Pharmacol. 2003;139:172–184. doi: 10.1038/sj.bjp.0705218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Degagne E, Grbic DM, Dupuis AA, et al. P2Y2 receptor transcription is increased by NF-kappa B and stimulates cyclooxygenase-2 expression and PGE2 released by intestinal epithelial cells. J Immunol. 2009;183:4521–4529. doi: 10.4049/jimmunol.0803977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Roberts JA, Durnin L, Sharkey KA, et al. Oxidative stress disrupts purinergic neuromuscular transmission in the inflamed colon. J Physiol (Lond) 2013;591:3725–3737. doi: 10.1113/jphysiol.2013.254136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Antonioli L, Fornai M, Colucci R, et al. A2a receptors mediate inhibitory effects of adenosine on colonic motility in the presence of experimental colitis. Inflamm Bowel Dis. 2006;12:117–122. doi: 10.1097/01.MIB.0000198535.13822.a9. [DOI] [PubMed] [Google Scholar]
- 225.Lomax AE, Mawe GM, Sharkey KA. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J Physiol (Lond) 2005;564:863–875. doi: 10.1113/jphysiol.2005.084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pretorius J, Malan SF, Castagnoli N, et al. Dual inhibition of monoamine oxidase B and antagonism of the adenosine A2A receptor by (E, E)-8-(4-phenylbutadien-1-yl) caffeine analogues. Bioorg Med Chem. 2008;16:8676–8684. doi: 10.1016/j.bmc.2008.07.088. [DOI] [PubMed] [Google Scholar]
- 227.Mitrovic V, Seferovic P, Dodic S, et al. Cardio-renal effects of the A1 adenosine receptor antagonist SLV320 in patients with heart failure. Circ Heart Fail. 2009;2:523–531. doi: 10.1161/CIRCHEARTFAILURE.108.798389. [DOI] [PubMed] [Google Scholar]
- 228.Pang PS, Mehra M, Maggioni AP, et al. Rationale, design, and results from RENO-DEFEND 1: a randomized, dose-finding study of the selective A1 adenosine antagonist SLV320 in patients hospitalized with acute heart failure. Am Heart J. 2011;161:1012–23. e3. doi: 10.1016/j.ahj.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 229.Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14:645–658. doi: 10.1016/j.nuclcard.2007.06.114. [DOI] [PubMed] [Google Scholar]
- 230.Prenner BM, Bukofzer S, Behm S, et al. A randomized, double-blind, placebo-controlled study assessing the safety and tolerability of regadenoson in subjects with asthma or chronic obstructive pulmonary disease. J Nucl Cardiol. 2012;19:681–692. doi: 10.1007/s12350-012-9547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Moeller FG, Steinberg JL, Lane SD, et al. Increased Orbitofrontal Brain Activation after Administration of a Selective Adenosine A(2A) Antagonist in Cocaine Dependent Subjects. Front Psychiatry. 2012;3:44. doi: 10.3389/fpsyt.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jakobsen O, Naesheim T, Aas KN, et al. Adenosine instead of supranormal potassium in cardioplegia: it is safe, efficient, and reduces the incidence of postoperative atrial fibrillation. A randomized clinical trial. J Thorac Cardiovasc Surg. 2013;145:812–818. doi: 10.1016/j.jtcvs.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 233.El Tahan MR. Effects of aminophylline on cognitive recovery after sevoflurane anesthesia. J Anesth. 2011;25:648–656. doi: 10.1007/s00540-011-1190-8. [DOI] [PubMed] [Google Scholar]
- 234.http://www.astrazenecaclinicaltrials.com/Submission/View?id=1884
- 235.Keystone EC, Wang MM, Layton M, et al. Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann Rheum Dis. 2012;71:1630–1635. doi: 10.1136/annrheumdis-2011-143578. [DOI] [PubMed] [Google Scholar]
- 236.Ali Z, Laurijssens B, Ostenfeld T, et al. Pharmacokinetic and pharmacodynamic profiling of a P2X7 receptor allosteric modulator GSK1482160 in healthy human subjects. Br J Clin Pharmacol. 2013;75:197–207. doi: 10.1111/j.1365-2125.2012.04320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Erlinge D, Gurbel PA, James S, et al. Prasugrel 5 mg in the very elderly attenuates platelet inhibition but maintains noninferiority to prasugrel 10 mg in nonelderly patients: the GENERATIONS trial, a pharmacodynamic and pharmacokinetic study in stable coronary artery disease patients. J Am Coll Cardiol. 2013;62:577–583. doi: 10.1016/j.jacc.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 238.Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909–1917. doi: 10.1056/NEJMoa1007964. [DOI] [PubMed] [Google Scholar]
- 239.Spiel AO, Derhaschnig U, Schwameis M, et al. Effects of prasugrel on platelet inhibition during systemic endotoxaemia: a randomized controlled trial. Clin Sci (Lond) 2012;123:591–600. doi: 10.1042/CS20120194. [DOI] [PubMed] [Google Scholar]
- 240.Jarvis MF, Burgard EC, McGaraughty S, et al. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.McGaraughty S, Wismer CT, Zhu CZ, et al. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol. 2003;140:1381–1388. doi: 10.1038/sj.bjp.0705574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gessi S, Merighi S, Varani K, et al. The A3 adenosine receptor: An enigmatic player in cell biology. Pharmacol Ther. 2008;117:123–140. doi: 10.1016/j.pharmthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 243.Brown JB, Lee G, Grimm GR, et al. Therapeutic benefit of pentostatin in severe IL-10−/− Colitis. Inflamm Bowel Dis. 2008;14:880–887. doi: 10.1002/ibd.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]