Abstract
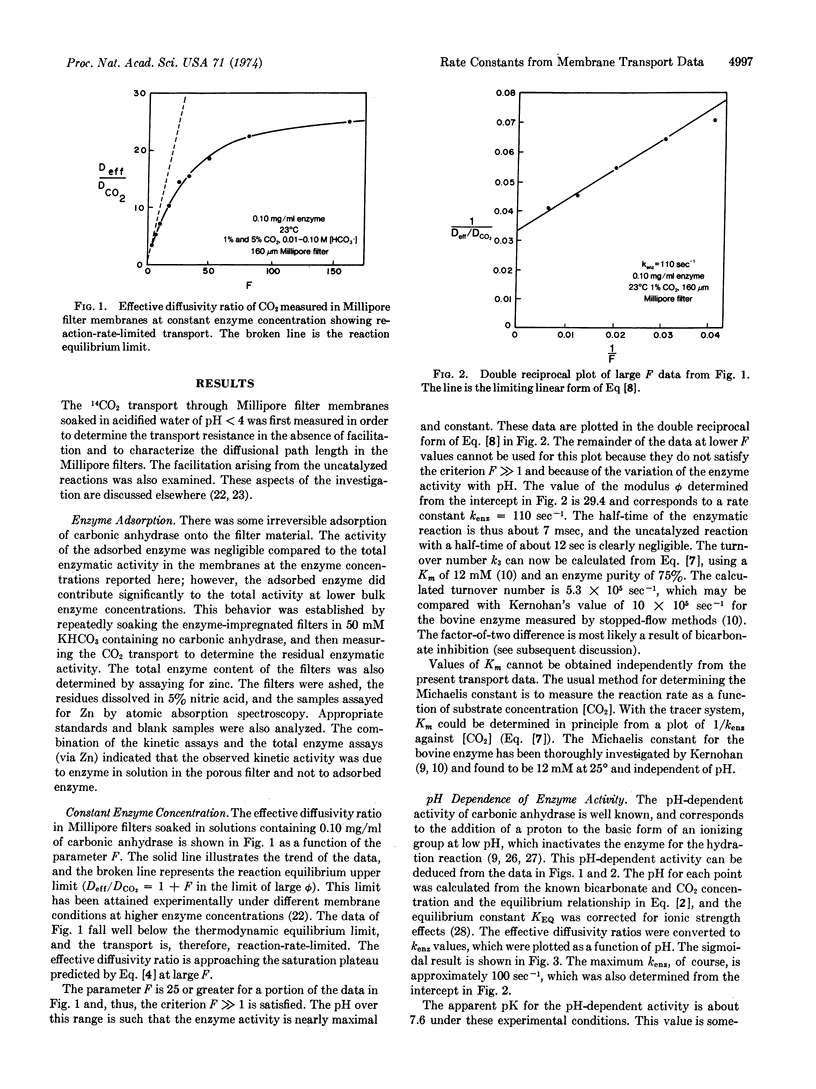
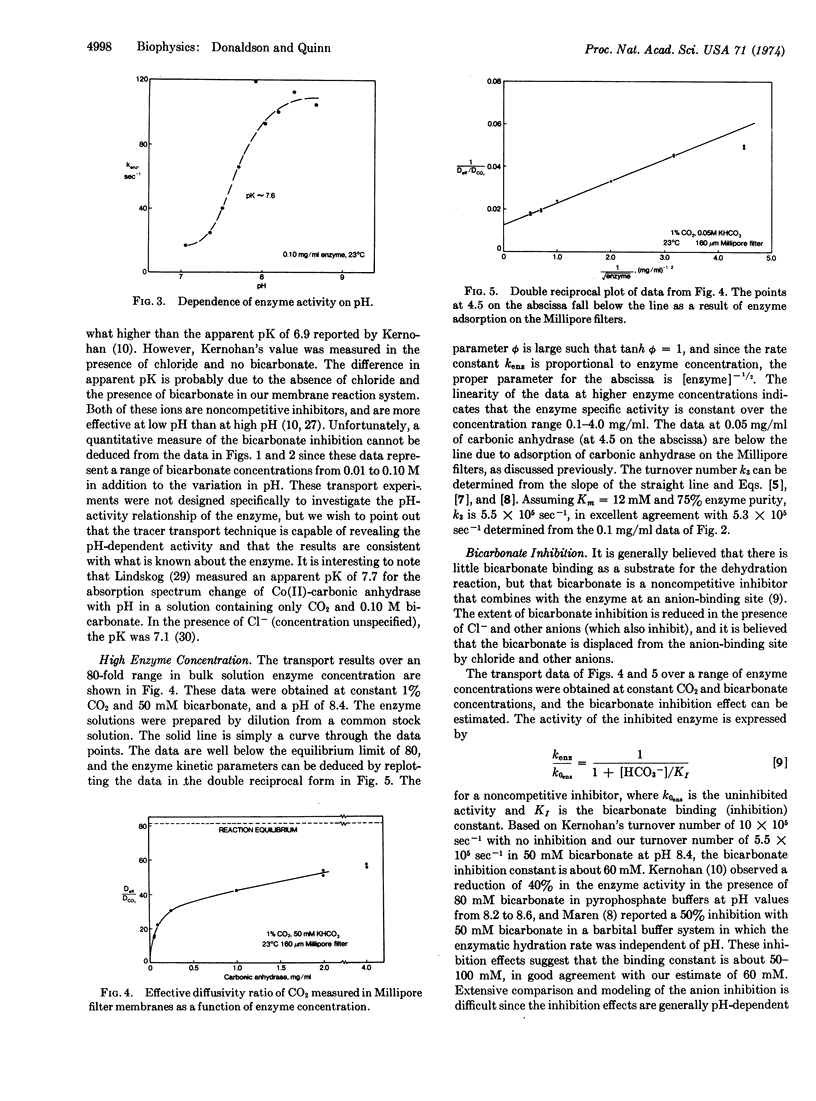
Facilitated diffusion rates can be used to determine kinetic constants for rapid reactions occurring within membranes and thin fluid layers. We have applied this technique to the study of the reversible CO2 hydration reactions catalyzed by carbonic anhydrase (EC 4.2.1.1; carbonate hydro-lyase). The experimental method entails the diffusion of tracer 14CO2 through Millipore filter membranes impregnated with aqueous bicarbonate solutions containing various concentrations of dissolved enzyme. A mathematical model of the simultaneous diffusion/reaction transport process is analyzed to predict the effective diffusion rate in terms of the relevant kinetic parameters. The solution to the mathematical model can be transformed to yield straight-line relations analogous to Lineweaver-Burk plots. The pseudo-first-order enzymatic rate constant for the hydration reaction can be determined from the slope or intercept of a plot of this straight-line relationship. Rate constants were accurately measured at high enzyme concentrations for reactions having half-times under a millisecond. The rate constants agree well with other reported kinetic constants for carbonic anhydrase, and the known pH-activity dependence and bicarbonate inhibition are quantitatively demonstrated. The specific activity is constant up to 4.0 mg/ml, which is believed to be the highest concentration at which the activity has been measured. The membrane transport technique has general applicability for other rapid reaction systems.
Keywords: enzyme, diffusion-reaction coupling, CO2 hydration
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger K. L., Balko B., Borcherdt W., Friauff W. High speed optical stopped-flow apparatus. Rev Sci Instrum. 1968 Apr;39(4):486–493. doi: 10.1063/1.1683414. [DOI] [PubMed] [Google Scholar]
- Enns T. Facilitation by carbonic anhydrase of carbon dioxide transport. Science. 1967 Jan 6;155(3758):44–47. doi: 10.1126/science.155.3758.44. [DOI] [PubMed] [Google Scholar]
- Goldman R., Kedem O., Katchalski E. Papain--collodion membranes. II. Analysis of the kinetic behavior of enzymes immobilized in artificial membranes. Biochemistry. 1968 Dec;7(12):4518–4532. doi: 10.1021/bi00852a048. [DOI] [PubMed] [Google Scholar]
- Goldman R., Kedem O., Silman I. H., Caplan S. R., Katchalski E. Papain-collodion membranes. I. Preparation and properties. Biochemistry. 1968 Feb;7(2):486–500. [PubMed] [Google Scholar]
- KERNOHAN J. C., FORREST W. W., ROUGHTON F. J. The activity of concentrated solutions of carbonic anhydrase. Biochim Biophys Acta. 1963 Jan 8;67:31–41. doi: 10.1016/0006-3002(63)91794-3. [DOI] [PubMed] [Google Scholar]
- KERNOHAN J. C. THE PH-ACTIVITY CURVE OF BOVINE CARBONIC ANHYDRASE AND ITS RELATIONSHIP TO THE INHIBITION OF THE ENZYME BY ANIONS. Biochim Biophys Acta. 1965 Feb 22;96:304–317. [PubMed] [Google Scholar]
- Kernohan J. C., Roughton F. J. Thermal studies of the rates of the reactions of carbon dioxide in concentrated haemoglobin solutions and in red blood cells. A. The reactions catalysed by carbonic anhydrase. B. The carbamino reactions of oxygenated and deoxygenated haemoglobin. J Physiol. 1968 Jul;197(2):345–361. doi: 10.1113/jphysiol.1968.sp008563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDSKOG S. Effects of pH and inhibitors on some properties related to metal binding in bovine carbonic anhydrase. J Biol Chem. 1963 Mar;238:945–951. [PubMed] [Google Scholar]
- Lindskog S. Interaction of cobalt(II)--carbonic anhydrase with anions. Biochemistry. 1966 Aug;5(8):2641–2646. doi: 10.1021/bi00872a023. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Forster R. E. Diffusion of Carbon Monoxide through Thin Layers of Hemoglobin Solution. Science. 1962 Nov 23;138(3543):897–898. doi: 10.1126/science.138.3543.897. [DOI] [PubMed] [Google Scholar]
- ROUGHTON F. J., RUPP J. C. Problems concerning the kinetics of the reactions of oxygen, carbon monoxide and carbon dioxide in the intact red cell. Ann N Y Acad Sci. 1958 Oct 13;75(1):156–166. doi: 10.1111/j.1749-6632.1958.tb36861.x. [DOI] [PubMed] [Google Scholar]
- Roughton F. J., Booth V. H. The effect of substrate concentration, pH and other factors upon the activity of carbonic anhydrase. Biochem J. 1946;40(2):319–330. doi: 10.1042/bj0400319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Broun G., Sélégny E. Monoenzymatic model membranes: diffusion-reaction kinetics and phenomena. Biochimie. 1972;54(2):229–244. doi: 10.1016/s0300-9084(72)80108-1. [DOI] [PubMed] [Google Scholar]
- Ward W. J., 3rd, Robb W. L. Carbon dioxide--oxygen separation: facilitated transport of carbon dioxide across a liquid film. Science. 1967 Jun 16;156(3781):1481–1484. doi: 10.1126/science.156.3781.1481. [DOI] [PubMed] [Google Scholar]


