Abstract
The intestine plays a prominent role in the biosynthesis of triacylglycerol (triglyceride; TAG). Digested dietary TAG is repackaged in the intestine to form the hydrophobic core of chylomicrons, which deliver metabolic fuels, essential fatty acids, and other lipid-soluble nutrients to the peripheral tissues. By controlling the flux of dietary fat into the circulation, intestinal TAG synthesis can greatly impact systemic metabolism. Genes encoding many of the enzymes involved in TAG synthesis have been identified. Among TAG synthesis enzymes, acyl-CoA:monoacylglycerol acyltransferase 2 and acyl-CoA:diacylglycerol acyltransferase (DGAT)1 are highly expressed in the intestine. Their physiological functions have been examined in the context of whole organisms using genetically engineered mice and, in the case of DGAT1, specific inhibitors. An emerging theme from recent findings is that limiting the rate of TAG synthesis in the intestine can modulate gut hormone secretion, lipid metabolism, and systemic energy balance. The underlying mechanisms and their implications for humans are yet to be explored. Pharmacological inhibition of TAG hydrolysis in the intestinal lumen has been employed to combat obesity and associated disorders with modest efficacy and unwanted side effects. The therapeutic potential of inhibiting specific enzymes involved in intestinal TAG synthesis warrants further investigation.
Keywords: triglyceride, gut hormones, obesity, acyltransferases
Triacylglycerol (triglyceride; TAG) is an inert storage and transport molecule of fatty acids (1). Whereas fatty acids are crucial substrates for fueling metabolism and forming membrane phospholipids, they can trigger metabolic signaling and their detergent-like property can disrupt cell membranes. The biosynthesis of TAG thus serves many physiological functions. Because it is highly reduced and anhydrous, TAG is the primary energy substrate stored in adipose tissues to sustain animals during fasting. TAG is also synthesized in the liver for the assembly and secretion of VLDL to transport neutral lipids to other tissues, as well as in the mammary gland for the formation of milk fat globules to deliver fatty acids and other lipid-soluble nutrients to mammalian neonates.
In the intestine, TAG synthesis is prominent for its role in the absorption of dietary fat (2, 3). TAG forms the bulk of animal fats and plant oils. It accounts for approximately 95% of dietary fat, depending on the food sources, with the rest as phospholipids and trace amounts of sterols, lipid-soluble vitamins, and other lipophilic components. Because of its hydrophobicity, TAG does not traverse the cellular membrane. Its absorption involves hydrolysis to fatty acids and monoacylglycerol (MAG) in the intestinal lumen, lipid uptake by the enterocytes, resynthesis of TAG, and assembly and secretion of ApoB-containing chylomicrons for delivery of TAG and other lipid-soluble nutrients. Like with most nutrients, the absorption of TAG occurs mostly in the proximal half of the intestine and less so in the distal intestine, where specific compounds, such as bile acids and vitamin B12, are absorbed. The activity of TAG synthesis coincides with the absorption of dietary fat along the length of the intestine, and it is more active in the differentiated absorptive enterocytes populating the upper villi than in the progenitor cells in the crypts (4).
The assimilation of dietary TAG is remarkably efficient, partly because the absorption is near complete even when intake is high (5, 6). The metabolic costs of processing fat, including the conversion to body fat, are also low compared with carbohydrate and protein; thus, dietary fat has a lower thermic effect, the increase in energy expenditure after consumption, than the other two energy-yielding nutrients (7, 8). In addition, the hydrolysis products of dietary TAG trigger enteroendocrine and neural signals to coordinate systemic metabolism. Therefore, TAG synthesis is integral to the pivotal roles played by the intestine: the portal for dietary nutrients, a producer of TAG-rich lipoproteins, and an endocrine and neural organ that signals current nutritional status to regulate metabolism. Consequently, intestinal TAG synthesis can contribute to excessive accumulation of TAG in tissues, a hallmark of obesity and related pathologies including insulin resistance, diabetes, and hepatic steatosis.
Over the last two decades, genes encoding many of the enzymes involved in the synthesis of TAG have been identified, including acyl-CoA:monoacylglycerol acyltransferase (MGAT) and acyl-CoA:diacylglycerol acyltransferase (DGAT) (1, 9, 10). Studies manipulating these genes in animal models have generated many insights into their physiological functions. The goal of this review is to provide an overview of TAG synthesis in the intestine, focusing on recent findings supporting the idea that limiting the rate of TAG synthesis in the proximal intestine alters the kinetics of fat absorption and modulates systemic energy balance.
PROCESSING OF SUBSTRATES FOR INTESTINAL TAG SYNTHESIS
Intestinal TAG synthesis occurs mainly after meals, as the primary source of substrates for intestinal TAG synthesis are digested fats taken up from the apical membranes of enterocytes (2). After ingestion, partial digestion, and emulsification with bile, TAG is primarily hydrolyzed by pancreatic lipase in the proximal intestine. Pancreatic lipase, like other related lipases including lipoprotein lipase, hepatic lipase, and endothelial lipase, exhibits preference for ester bonds at the sn-1 and sn-3 positions, which leads to production of fatty acids and sn-2 MAG. After digestion in the intestinal lumen, TAG resynthesis occurs in the enterocytes. The rate of TAG synthesis fluctuates based on the availability of fatty acid substrates. In contrast, the biosynthesis of phospholipids is constitutive and tightly coordinated with the production of cellular membranes. In the intestine, phospholipid synthesis increases upon feeding to form the monolayer covering the surface of intracellular lipid droplets and secretory lipoproteins. In addition to dietary fatty acids taken up from the apical membranes, fatty acids can be de novo synthesized in enterocytes and plasma fatty acids can be taken up through basolateral membranes. Fatty acids from the latter two sources are used more for phospholipid membrane synthesis, oxidation as fuel, or TAG synthesis as part of relatively lipid-poor VLDL-sized particles secreted during fasting (11–17).
The uptake of fatty acids generated from digestion in the lumen is mediated through passive diffusion and protein-facilitated processes (18–20). The acidic microenvironment of the unstirred water layer coating the brush border membrane of enterocytes is conducive for taking up a large amount of fatty acids through “flip-flop” across the plasma membrane, which may be independent or be part of protein-facilitated fatty acid uptake (21). While the role of proteins in facilitating fatty acid uptake is well-documented, whether they function as classic transmembrane transporters is controversial. For example, fatty acid transport protein (FATP)4 is a member of the SCL27A gene family thought to transport long-chain fatty acids. It shares sequence homology with the acyl-CoA synthetase (ACSL) family and is expressed intracellularly. FATP4 likely facilitates fatty acid uptake by trapping fatty acids as fatty acyl-CoAs (22). In mice, FATP4 is crucial for lipid homeostasis in the skin but its role in intestinal lipid absorption is dispensable (23). Likewise, a fatty acid binding protein (FABP) associated with the plasma membrane on the microvilli of the intestine has been reported (24). The protein appears to be identical to the mitochondrial aspartate aminotransferase (25, 26), and its role in intestinal fatty acid uptake is not yet proven.
Cluster of differentiation (CD36 or fatty acid translocase), a transmembrane protein with a broad ligand specificity, is another protein facilitating fatty acid uptake and is highly expressed on the brush border membrane of villi in the proximal intestine (20, 27). Its role in facilitating fatty acid uptake and directing fatty acids for chylomicron production is well-established in animal and human studies (28). However, the mechanisms for its functions seem multifactorial. Recent studies suggest that CD36 is involved in sensing long-chain fatty acids and may regulate fat absorption at various stages, including the release of two gut hormones, secretin and cholecystokinin (29). Overexpression of CD36 facilitates fatty acid uptake by increasing TAG synthesis, but not fatty acid transport across the plasma membrane, in a cultured cell study (30). Thus, whether CD36 functions as a major fatty acid transporter in the intestine is questionable. CD36-mediated fatty acid uptake in adipocytes requires its localization to the lipid rafts on the plasma membrane (31). More recently, the lipid raft protein caveolin-1 has been shown to mediate intestinal fatty acid uptake using mice deficient in the protein (32). This series of observations supports the interesting hypothesis that lipid rafts on the brush border membranes and endocytotic vesicles mediate intestinal fatty acid uptake and transport them to the endoplasmic reticulum (ER), where TAG is synthesized.
The intestinal brush border membrane can take up MAG from digested fat through passive diffusion (33). A protein transporter for MAG has also been proposed, as MAG uptake can be saturated (34). The transporter, if it exists, is shared by MAG and fatty acids, because MAG (but not glycerol, DAG, or TAG) can competitively suppress fatty acid uptake (34, 35). The identity of such a transporter is not known. Interestingly, in genetically engineered mice expressing different levels of MGAT2 in the intestine, the levels of MGAT activity determine the rate of MAG, but not fatty acid, uptake from the lumen into enterocytes in a cell-autonomous manner (36, 37). These findings suggest that MGAT-mediated esterification may facilitate MAG uptake by trapping MAG for DAG/TAG synthesis, consistent with the idea that intracellular metabolism promotes substrate uptake. They also suggest that the uptake of MAG can be uncoupled from that of fatty acids.
After crossing the brush border membrane, the hydrophobic fatty acids and MAG are carried across the aqueous cytosol to the ER. Intracellular FABPs may serve as the carriers (38). Two FABPs are highly expressed in the proximal intestine: liver-type FABP (LFABP or FABP1), which is also expressed in the liver and kidney, and intestine-type FABP (IFABP or FABP2), which is more tissue-specific (39). In addition to their disparate expression patterns, they also have different structures and ligand binding properties (40). LFABP also binds other hydrophobic ligands, including MAG and acyl-CoA (41, 42), and can mediate the budding of prechylomicron transport vesicles from the ER (43). Studies using mice deficient in either FABP support the proposition that IFABP channels fatty acids for TAG synthesis, while LFABP channels MAG for TAG synthesis and may be involved in fatty acid oxidation (41). These mice do not show overt fat malabsorption, indicating that either these FABPs play minor roles in fat absorption or their functions in the process can be compensated for when they are absent. Upon high-fat feeding, mice lacking IFABP showed increased energy expenditure and less weight gain, while mice lacking LFABP showed a propensity to gain weight in a recent study (44). Other studies, however, found male mice lacking IFABP had greater liver mass and gained more weight under high-fat feeding than wild-type control mice did (45, 46), and mice lacking LFABP were protected against obesity and hepatic steatosis induced by diet without malabsorption of dietary fat (47). The strain and sex of mice, fatty acid profiles of the experimental diets, and other environmental factors could contribute to the discrepancies, but the differences in findings remain to be resolved. Nonetheless, these findings are consistent with the idea that FABPs have different physiological roles that are indispensable.
Enzymes involved in major TAG synthesis pathways are dependent on long-chain acyl-CoA for acyl groups (22), not fatty acids. Thirteen ACSLs have been identified that activate long-chain fatty acids (16 or more carbons) by thioesterification of the carboxyl group with CoA. Among them, ACSL5 is most highly expressed in the intestinal mucosa. A recent study of mice deficient in ACSL5 found a reduction in total ACSL activity by 60% in the intestine. Surprisingly, no differences were found in fat absorption after a challenge of olive oil gavage, or in weight gain induced by high-fat feeding (48). The functions of ACSL5 likely can be compensated for by other ACSLs also expressed in the intestine.
ENZYMATIC PATHWAYS MEDIATING INTESTINAL TAG SYNTHESIS
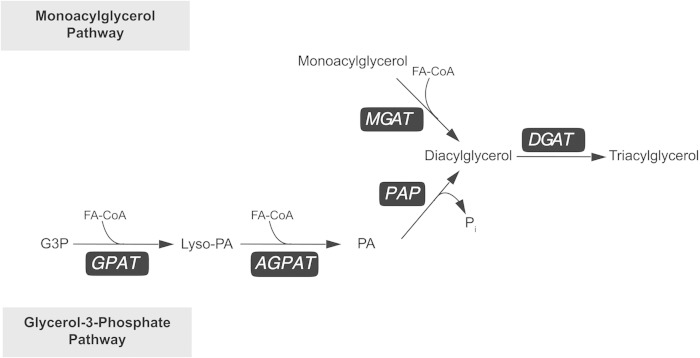
The enzymatic activities catalyzing the biosynthesis of TAG have been elucidated since the 1950s (Fig. 1) (49–52). Two major pathways for TAG synthesis in mammals use either glycerol-3-phosphate (G3P) or MAG as the initial acyl acceptor. Both pathways use fatty acyl-CoA thioesters as donors of acyl groups (53, 54), and they converge on the final acylation step (9). In most tissues, the biosynthesis of TAG is mainly mediated by the G3P pathway, in which DAG is produced from G3P after two acylation steps and the removal of phosphate. This series of reactions is catalyzed sequentially by three enzymes: G3P acyltransferase (GPAT), 1-acylglycerol-3-phosphate acyltransferase (AGPAT; also known as lysophosphatidic acid acyltransferase or LPAAT), and phosphatidic acid (PA) phosphatase (PAP or lipin). The G3P pathway also generates precursors for major glycerophospholipids (50), forming all cellular membranes. In the intestine with prolonged digestion, pancreatic lipase and several other lipases can convert MAG to fatty acids and glycerol. In the absence of MAG, these fatty acids are resynthesized through the G3P pathway.
Fig. 1.
Two major TAG synthesis pathways that use either MAG or G3P as the initial acyl acceptor.
The MAG pathway, using MAG for the biosynthesis of DAG and then TAG, is most prominent in the intestine and features the activity of MGAT (55–58). As MAG is mostly a hydrolysis product of TAG, MGAT activity is thought to be important only when recycling of degraded TAG is active, as is the case of intestinal fat absorption. During postprandial states when fatty acids and MAG are taken up by the enterocytes, the MAG pathway accounts for more than 75% of TAG synthesis in the intestine (59). The MAG pathway likely enables the enterocytes to process the massive influx of substrates after a fatty meal, contributing to the efficiency of fat absorption. MGAT may also facilitate the absorption of the fatty acyl group on the sn-2 position of MAG. For example, MGAT may be particularly important for the ability of human infants to absorb the long-chain saturated palmitate, which is mostly located at the sn-2 position of TAG in breast milk (60).
Shared by the G3P and MAG pathways, the final and committed step for TAG synthesis is catalyzed by DGAT, converting DAG generated from either pathway to TAG. Though the two TAG synthesis pathways have been considered separate, these pathways may connect at the level of 2-MAG through acylation/deacylation cycles, especially in hepatocytes and enterocytes, where TAG is synthesized for assembly and secretion of ApoB-containing lipoproteins (51, 61). In addition to DGAT, an acyl-CoA-independent DAG transacylase activity, in which two molecules of DAG are converted to TAG and MAG, has been reported in rat intestine (62). This represents a third TAG synthesis pathway. The gene coding for an intestinal DAG transacylase has not been identified.
GENES ENCODING INTESTINAL TAG SYNTHESIS ENZYMES
Many genes encoding enzymes involved in TAG synthesis pathways have been cloned and identified through their sequence similarity to known acyltransferases (1, 9). The identification of these genes greatly advanced the field. It is now evident that two or more enzymes that may or may not share sequence homology can catalyze each step of the TAG synthesis pathway. For example, three homologous genes have been identified as MGATs (63–65), while two unrelated genes code for the two known DGATs (66, 67). In the G3P pathway, four GPATs, three AGPATs, and three PAPs have been identified. These enzymes may have different catalytic properties, subcellular localization, and regulation. They are often expressed in many tissues, while the expression levels vary widely. On the other hand, many tissues, especially those active in TAG synthesis, often express more than one isoform of these enzymes. Many of these enzymes may be able to compensate for the absence of others to some extent. However, studies manipulating the expression of these genes in rodents have revealed that, in many cases, they are not completely redundant and may play indispensable roles in specific physiological processes.
MGAT2 MEDIATES THE INTESTINAL MAG PATHWAY
MGAT is best known for catalyzing intestinal TAG synthesis during the absorption of dietary fat, though the activity has also been characterized in the liver of neonatal rodents and in the adipose tissues of migrating birds (68, 69). The three known MGATs encoding genes, named MOGAT1, MOGAT2, and MOGAT3, were identified through their sequence homology with DGAT2 (note: the gene name MGAT has been used for an enzyme unrelated to TAG metabolism) (67). All three MGAT enzymes (MGAT1, -2, and -3) have been localized to the ER (63–65) using tagged and overexpressed enzymes. In cell free assays, they exhibit MGAT activity with broad substrate specificities toward long-chain fatty acyl-CoA and MAGs. However, they show varying degrees of DGAT activity; MGAT2 appears to be the most specific MGAT among the three, while MGAT3 exhibits more DGAT activity when MAG substrate concentration is low (64, 65, 70). They also differ in tissue expression patterns. In mice, MGAT1 expression is high in the stomach and kidney and is detectable in the liver, uterus, and adipose tissues but not in the intestine (63). A similar tissue expression pattern was found in humans but the levels seem very low. MGAT2, in contrast, is highly expressed in both mouse and human intestine. In mice, MGAT2 is almost intestine specific with low levels expressed in the kidney and adipose tissues. In humans, it is also highly expressed in the stomach, liver, and kidney, and has been found in mammary glands and adipose tissues (64, 71). Corresponding to the location of fat absorption, MGAT2 expression is higher in the proximal than in the distal intestine and higher in villi than in crypts (72). The expression is induced by high-fat feeding, further supporting its role in fat absorption. MGAT3 is expressed in human intestine, most highly in the ileum (70), but is a pseudogene and not expressed in mice (73).
MGAT2 appears to be a major murine intestinal MGAT. Mice with a global deletion of the coding gene (Mogat2−/−) and mice with an intestine-specific deletion (Mogat2IKO) exhibit significantly lower intestinal MGAT activity than their littermate controls by more than 60% in cell free assays (36). The ability of their intestines to esterify MAG injected into ligated intestinal pouches is also reduced by 90%, illustrating the predominant role of intestinal MGAT2 in catalyzing the esterification process. Reintroducing MGAT2 in the intestine of mice globally deficient in MGAT2 restores the ability to convert MAG to DAG and TAG (37). Interestingly, as discussed in the previous section, the ability to esterify MAG determines the amount of MAG, but not fatty acids, taken up by the intestinal mucosa, suggesting that MGAT may facilitate the uptake of MAG in a manner similar to how ACSL facilitates the uptake of fatty acids.
In addition to MGAT3, which is not expressed in mice, there are several candidates for additional intestinal MGATs. For example, both DGAT1 (as discussed below) as well as the lysophosphatidylglycerol acyltransferase, LPGAT1, exhibit MGAT activity in cell free assays and are highly expressed in the intestine in both humans and mice (74–76). However, at least in mice, they are insufficient to compensate for the physiological functions of MGAT2 as discussed in later sections.
DGAT1, A MAJOR INTESTINAL DGAT
Two unrelated DGAT enzymes are known. Despite catalyzing the same reaction, DGAT1 and DGAT2 do not share significant sequence homology (9). The mammalian DGAT1 gene has been identified by its similarity to the sequences of acyl-CoA:cholesterol acyltransferases (66). The DGATs are related to a larger membrane-bound O-acyltransferase (MBOAT) gene family of both lipid and protein acyltransferases, including the ghrelin activating enzyme, ghrelin-O-acyltransferase (77). DGAT2 belongs to a gene family that also includes MGATs and wax synthases (63, 64, 67, 70, 71, 78, 79). DGAT1 of humans or mice contains around 500 amino acids and several hydrophobic regions, three of which span membranes (80). The enzyme appears to form oligomers in the ER, exhibiting activity facing both the lumen and the cytosol (81, 82). In contrast, DGAT2 has 388 amino acids and two membrane-spanning domains (83), with most of the protein facing the cytosol.
Both DGATs exhibit DGAT activity, as expressed enzymes carry out the reaction in cell free assays and in cultured cells (9). However, DGAT1 also shows activity toward other acyl acceptors, including retinol, and may be involved in retinol absorption in the intestine and detoxification when vitamin A levels are high (75, 84). On the other hand, DGAT2 appears to be a potent and specific DGAT enzyme. The two DGATs have wide tissue expression patterns. Both are highly expressed in the adipose tissues, and they appear to account for all DGAT activity in adipocytes (85, 86). In addition, DGAT1 is highly expressed in the small intestine and mammary gland, while DGAT2 is most highly expressed in the liver.
While exhibiting broader substrate specificity for other acyl acceptors, DGAT1 is an important DGAT in the intestine. Like MGAT2, its expression is high in the villi of proximal intestine and is induced by high-fat feeding. In mice lacking DGAT1, intestinal DGAT activity is reduced by 90% (87), even though DGAT2 is expressed in the intestine of mice. Mice lacking DGAT2 die hours after birth due to skin defects and a severe deficiency in energy substrates (88); thus, the functional significance of DGAT2 in mouse intestine remains to be determined. In humans, only DGAT1 is detected in the intestine (67, 89). A congenital diarrheal disorder in two siblings, which was lethal in one case, has been linked to a null mutation of DGAT1, likely due to a severe deficiency of intestinal DGAT activity (89).
ENZYMES CATALYZING THE G3P PATHWAY IN THE INTESTINE
The G3P pathway has been reported in the intestine, but only accounts for 20–30% of TAG synthesis during fat absorption (59, 90). Most of its early characterization was performed in liver (1). As the pathway also generates phospholipid precursors, its activity is important for the fast turnover of intestinal epithelia. Many enzymes involved in the first three unique steps of the G3P pathway are found in the intestine. Whether any of them play an indispensable role in intestinal TAG synthesis is yet to be explored.
GPAT initiates the pathway by catalyzing the acylation of G3P to form 1-acyl-G3P (lysophosphatidic acid). Of the four isoforms identified, GPAT1 and -2 are localized to the mitochondrial membrane, whereas GPAT3 and -4 (originally named AGPAT8 and -6, respectively, due to sequence homology with AGPATs) are localized to the ER membrane. Both of the ER-associated GPATs are expressed in mouse and human intestine, with GPAT3 showing a higher mRNA copy number than GPAT4 in mice (91).
AGPAT catalyzes the second acylation to produce PA and has numerous candidate genes (92). APGATs 1–3 have been conclusively shown to have activity, and all are found in the intestine. PAP catalyzes the removal of phosphate to produce DAG (93). Three cytosolic mammalian PAPs (also named lipins) have been identified that can translocate to the ER to hydrolyze PA produced by AGPAT, and are not related to the plasma membrane-associated PA phosphatases. Among them, lipin 3 is most highly expressed in the intestine. Its role in intestinal TAG metabolism is yet to be explored.
DESTINATIONS OF TAG SYNTHESIZED IN THE INTESTINE
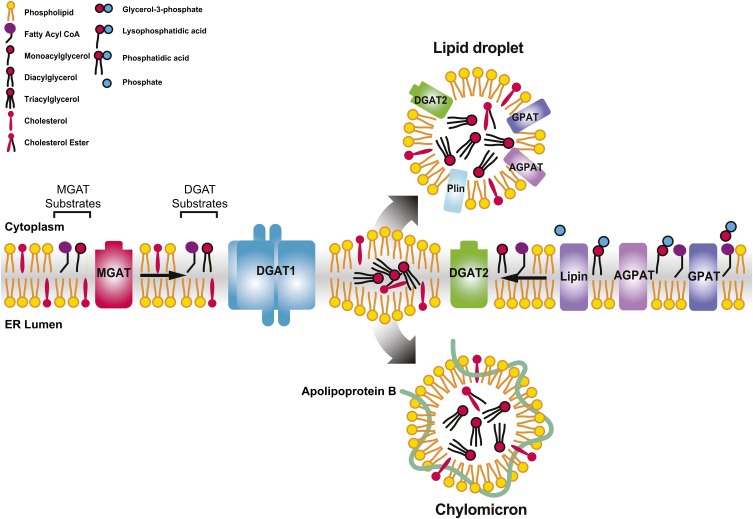
All of the TAG synthesis enzymes have been localized to the ER, where synthesis occurs (94). The end product is thought to release into the phospholipid bilayer (Fig. 2) (95). In most cells, the growing lens of TAG between ER membrane bilayers partitions into the cytosol. TAG forms the core of cytosolic lipid droplets, coated with a monolayer of phospholipids and associated proteins, such as perilipins, that may regulate TAG metabolism (96, 97). In enterocytes, TAG partitions into the lumen of the ER, where it is assembled into the neutral lipid core of chylomicrons, coated with polar lipids and apolipoproteins. The assembly of chylomicrons is likely through a similar two-step process described for VLDL assembly in hepatocytes (98). In the initial step, the microsomal TAG transfer protein (MTP) incorporates lipids into ApoB, which prevents the degradation of ApoB during its translation and forms the nascent prechylomicrons (20, 98). MTP also transfers TAG to form ApoB-free lipid droplets in the ER lumen (99). In the second step, prechylomicrons are transported from the ER to the Golgi apparatus and the rest of the secretory pathway (100). Chylomicrons mature after incorporating more neutral lipids and exchangeable apolipoproteins, such as ApoAIV (101). They are exported through the basolateral membrane and enter the lymphatic system and subsequently the blood stream, bypassing the liver. Chylomicrons deliver TAG for storage or for use as fuel in the peripheral tissues. Before uptake, TAG needs to undergo lipolysis within blood vessels. LPL anchored in the capillaries is the key regulator of this process (102), which in turn is subject to regulation by various factors modulating the flux of lipid nutrients. The remnants of chylomicrons deliver the remaining lipids to the liver, where dietary TAG contributes to hepatic lipid metabolism, along with fatty acids from other sources, and can be incorporated into VLDLs for redistribution to other tissues.
Fig. 2.
Hypothetical model illustrating the MAG and the G3P pathways in TAG synthesis in the ER of enterocytes. TAG products of the DGAT reaction may be channeled into the cores of cytosolic lipid droplets or TAG-rich chylomicrons for secretion. MGAT2 and DGAT1 with epitope tags have been shown as homotetramers (81, 115), and these two enzymes may form heterodimers when coexpressed in cultured cells (115). Several enzymes in the G3P pathway have also been located on the cytosolic lipid droplets. Plin, perilipin. Adapted from (9).
In enterocytes, the growing TAG core can also bud off the ER toward the cytosol and become cytosolic lipid droplets, which may play a dynamic and prominent role in fat absorption. The cytosolic lipid droplets appear to be the temporal reservoir, when TAG synthesis resulting from the influx of digested fatty acids and MAG overwhelms the capacity for chylomicron assembly and secretion (103). The levels of TAG accumulated in cytosolic lipid droplets correlate with the amount of dietary fat consumed, mostly in the proximal small intestine, and are cleared within 12 h (104).
The cytosolic lipid droplets may play a physiological role in fat absorption, as suggested by phenotypes of mice that fail to mobilize their TAG core (Table 1; discussed further in the next section). The process of mobilizing TAG in the cytosolic lipid droplets for secretion is likely mediated by a lipolysis/re-acylation cycle (105). Adipose TAG lipase (ATGL) and its activator, comparative gene identification-58 (CGI-58), appear to be important for intracellular TAG hydrolysis in enterocytes. In addition, mobilizing cytosolic TAG for secretion may involve proteins, such as cell death-inducing DFF45-like effector b (Cideb), that facilitate the movement of cytosolic TAG into the ER. Many of the proteins involved in the process, including ATGL, CGI-58, and Cideb, have recently been implicated in fat absorption (106–108).
TABLE 1.
Selected mouse models of intestinal TAG metabolism
| Gene | Tissues with High Expression | Genetic Manipulation | Phenotype | Reference |
| Mogat2 | Small intestine | Global knockout | Delayed fat absorption | (119, 122) |
| Increased energy expenditure | ||||
| Protection from obesity induced by diet or the agouti mutation | ||||
| Intestine-specific deletion | Delayed fat absorption | (36) | ||
| Partial protection from diet-induced obesity | ||||
| Intestine-specific expression | Restored fat absorption rate | (37) | ||
| Partial recovery of weight gain and metabolic efficiency when fed a high-fat diet | ||||
| Dgat1 | Small intestine, adipose tissue and mammary gland | Global knockout | Delayed fat absorption | (85, 87) |
| Increased energy expenditure | ||||
| Protection from obesity induced by diet or the agouti mutation | ||||
| Intestine-specific expression | Restored fat absorption rate | (113) | ||
| Recovery of weight gain and hepatic steatosis induced by high-fat feeding | ||||
| Dgat2 | Liver, adipose tissue and mammary gland | Global knockout | Death shortly after birth with severe deficiency in energy substrates and skin barrier defect | (88) |
| Intestine-specific over-expression | Increased TG secretion | (114) | ||
| Exacerbated hepatic steatosis | ||||
| MTP | Liver and intestine | Conditional intestine-specific deletion | Reduced chylomicron secretion | (120) |
| Large lipid droplets in enterocytes | ||||
| Steatorrhea | ||||
| ApoB | Liver and intestine | Intestine specific deletion | Defect in chylomicron production | (121) |
| Fat malabsorption | ||||
| ATGL | Adipose tissue | Intestine specific deletion | Increased cytosolic lipid droplets in enterocytes Increased fecal fat | (106) |
| CGI-58 | Adipose tissue and testes | Intestine specific deletion | Decreased fat absorption | (107) |
| Increased fecal fat | ||||
| TAG accumulation in enterocytes | ||||
| Cideb | Liver and intestine | Global knockout | Protection from diet-induced obesity | (108, 134) |
| Increased energy expenditure | ||||
| Reduced chylomicron secretion |
What determines the partitioning of newly synthesized TAG toward storage in the cytosolic lipid droplets versus secretion in the ER lumen is unclear. A previous model posits that DGAT1 accounts for the latent (luminal) DGAT activity producing TAG for secretion, while DGAT2 accounts for the overt (cytosolic) DGAT activity producing TAG for storage. The topology of these two enzymes, with the active site of DGAT2 facing the cytosol and DGAT1 facing both the cytosol as well as the ER lumen, supports the model (80, 83). However, the dichotomy is not supported by the observations that overexpression of either enzyme in cultured hepatocytes, livers, or intestines did not lead to increases in TAG-rich lipoproteins or cytosolic lipid droplets invariably (109–114).
Recently, MGAT2 has been shown to heterodimerize with DGAT1, but not DGAT2, when coexpressed in cells (115). This is consistent with the idea that enzymes involved in TAG synthesis form complexes, such as the TAG synthase complex isolated from rat intestine (116). Through physical interactions, lipid substrates can be metabolized through the pathway complex efficiently. We speculate that MGAT2 expressed in the intestine partners with DGAT1, and perhaps other proteins such as MTP, to channel the influx of absorbed fatty acids and MAG through the secretory pathway. In contrast, in addition to the ER, DGAT2, along with enzymes of the G3P pathway, has been located on cytosolic lipid droplets and shown to expand the TAG core (117, 118). While TAG produced from G3P can enter the secretory pathway on the ER, in the absence of the MGAT pathway, more substrates would be available for esterification by the G3P pathway on expanding cytosolic lipid droplets. Supporting the model, in mice deficient in MGAT2 or DGAT1 challenged with dietary fat, cytosolic lipid droplets accumulate in enterocytes while TAG secretion from the intestine is delayed (87, 119).
INTESTINAL TAG SYNTHESIS, FAT ABSORPTION, AND SYSTEMIC ENERGY BALANCE
Studies using genetically engineered mice have advanced the understanding of the physiological roles of individual genes. For example, mice deficient in MTP or ApoB, required for chylomicron assembly, showed severe malabsorption of dietary fat as observed in human patients with similar mutations (120, 121). TAG synthesis is an essential step forming the hydrophobic core of chylomicrons. However, deletion of individual enzymes involved in TAG synthesis does not lead to severe fat malabsorption in mice, reflecting redundancy and adaptability of the process. Nonetheless, limiting TAG synthesis in the intestine often alters the timing and location of fat absorption, as best demonstrated in mice deficient in enzymes of the MAG pathway, MGAT2 or DGAT1. Like mice deficient in other TAG synthesis enzymes that change the flux of lipid substrates, mice deficient in MGAT2 or DGAT1, globally or tissue-specifically, exhibit changes in systemic energy balance. Key phenotypes observed in some of the discussed models are summarized in Table 1.
MGAT2
Mice deficient in MGAT2 (Mogat2−/−) grow without apparent abnormalities (119). Consistent with the purported role of MGAT2 in fat absorption, these mice gain less weight than their wild-type littermates when fed a high-fat diet. Mogat2−/− mice are also protected from glucose intolerance, hypercholesterolemia, and hepatic steatosis induced by high-fat feeding. However, Mogat2−/− mice consume and absorb a similarly high percentage of dietary fat like wild-type littermates, even when fat accounts for 60% of caloric intake. Their fecal fat levels are not increased, and their tissue stores of vitamin A and E are normal, suggesting MGAT2 is dispensable for absorbing a normal quantity of fat. However, deficiency of MGAT2 impairs intestinal TAG synthesis, slows gastric emptying, and alters the kinetics of fat absorption. In Mogat2−/− mice, the entry of dietary fat into the circulation is delayed and the normal rise in plasma TAG in response to an oral oil bolus is blunted. More cytosolic lipid droplets accumulate in the proximal intestine of Mogat2−/− mice, where normally MGAT2 is highly expressed, than accumulate in wild-type mice after a fatty meal. Following an intragastric bolus of radiolabeled TAG, less dietary fat is taken up in the proximal intestine and more fat reaches the distal intestine in Mogat2−/− mice (119).
The change in the kinetics of fat absorption seen in Mogat2−/− mice is associated with an increase in energy expenditure, which accounts for the decreases in metabolic efficiency and explains the resistance to obesity induced by high-fat feeding. Because of the established role of MGAT2 in fat absorption, high levels of dietary fat would have been expected to incite the effects of MGAT2 deficiency. Interestingly, even though the increase in energy expenditure is exacerbated when dietary fat content is high, the phenotype is not dependent on high-fat feeding (122). When fed low-fat diets, Mogat2−/− mice also exhibit increased energy expenditure. These mice increase their food intake to maintain energy balance. They lose weight when food intake is limited to the levels of wild-type littermates, indicating that the increase in food intake is an adaptive response to an obligatory increase in energy expenditure (122). Inactivation of MGAT2 decreases the propensity to gain weight when mice are fed a diet rich in refined carbohydrate and protects the hyperphagic Agouti yellow mouse from excess weight gain (122). These findings suggest that the role of MGAT2 in the efficient use and storage of metabolic energy is not limited to the calories from dietary fat.
Because of its relatively specific tissue expression pattern, MGAT2 in the intestine most likely mediates the effects on intestinal lipid processing and energy balance observed in mice. Indeed, expressing a human MGAT2 specifically in the intestine of Mogat2−/− mice restores the uptake and esterification of dietary MAG, as well as the rate at which dietary fat enters the circulation (37). This restoration of intestinal MGAT activity also improves the metabolic efficiency of Mogat2−/− mice. These mice, expressing MGAT2 only in the intestine, show levels of energy expenditure intermediate between those of Mogat2−/− and wild-type mice. They have an increased propensity to gain weight upon high-fat feeding as compared with Mogat2−/− mice, but not to the same extent as wild-type littermates (37).
Findings from intestine-specific deletion of MGAT2 largely confirm its role in the regulation of fat absorption and systemic energy balance. Like Mogat2−/− mice, mice with an intestine-specific deletion (Mogat2IKO) have impaired uptake and esterification of MAG in the proximal intestine, independent of gastric emptying (36). When these mice receive an intraluminal bolus of micelles containing FFAs and radiolabeled MAG, their enterocytes take up and incorporate fewer tracers into total lipid, which are mostly recovered as TAG. Accordingly, the secretion of TAG into the circulation is delayed. These Mogat2IKO mice also show increased energy expenditure and reduced weight gain, as compared with littermate controls, and are protected against obesity-associated comorbidities induced by high-fat feeding.
The mechanisms underlying the effects of intestinal MGAT2 are not clear. MGAT2 appears to couple TAG synthesis to the assembly and secretion of chylomicrons. Even though chylomicron-size lipoprotein particles are found and dietary fat is secreted as TAG in both Mogat2−/− and Mogat2IKO mice, the size of chylomicrons as well as the timing and amount of TAG entering the circulation have been altered, which may in turn have impacts on the delivery of TAG substrates and metabolism of peripheral tissues. In addition, the intestine initiates hormonal and neural signals to coordinate metabolism in response to the presence of nutrients (123). The change in the spatial distribution of fat absorption seen in Mogat2−/− and Mogat2IKO mice is associated with a change in the levels of gut hormones, most notably glucagon-like peptide 1 (GLP-1), in response to a fatty meal (36, 119). The change in gut hormone levels, like an increase in GLP-1, may lead to the transient reduction in food intake observed when Mogat2−/− and Mogat2IKO mice are first exposed to a diet rich in fat (36). Because MGAT2 exhibits high activity toward sn-2-arachidonoylglycerol, an endogenous ligand of the cannabinoid receptor, MGAT2 may also modulate energy balance through endocannabinoid signaling.
DGAT1
DGAT1 likely partners with MGAT2 and catalyzes the final step of intestinal TAG synthesis, as discussed above. In many aspects, the phenotypes of Mogat2−/− and Mogat2IKO mice resemble those of mice deficient in DGAT1 (Dgat1−/−). Dgat1−/− mice exhibit increased energy expenditure and are resistant to obesity, insulin resistance, and hepatic steatosis induced by high-fat feeding or the Agouti mutation (85, 124–126). Dgat1−/− mice can assemble and secrete chylomicrons as well as absorb normal quantities of dietary fat. However, in response to high dietary fat, they show slower gastric emptying, accumulation of cytosolic lipid droplets in enterocytes, and delayed entry of dietary fat into the circulation (87). These temporal and spatial changes in fat absorption are also associated with changes in postprandial gut hormone levels, including an increase in GLP-1 (127).
The phenotypes of Dgat1−/− mice are more pleiotropic than Mogat2−/− or Mogat2IKO mice. Female Dgat1−/− mice have defects in mammary gland development and do not produce milk (128). Both sexes of Dgat1−/− mice also show skin defects in reduced fur lipids, sebaceous gland atrophy, and hair loss (75, 129). These phenotypes may relate to the observation that DGAT1 catalyzes the esterification of several substrates, including retinol. DGAT1 may be involved in detoxification of vitamin A and modulating retinoic acid signaling by converting excess retinol to inert retinyl esters, as reducing dietary vitamin A corrects the hair follicle defects of Dgat1−/− mice (75).
Unlike MGAT2, DGAT1 is expressed ubiquitously with high expression in several tissues in addition to the small intestine. Indeed, the increases in energy expenditure and sensitivity to insulin and leptin have been attributed to its role in the adipose tissues, as transplanting white adipose tissue from Dgat1−/− mice confers wild-type mice resistance to diet-induced obesity (130). The causes for the increase in energy expenditure of Dgat1−/− mice are likely multifactorial. However, its role in intestinal TAG synthesis appears to be a major component. Reintroduction of DGAT1 into the intestine of Dgat1−/− mice reverses many of the fat absorption and energy balance phenotypes (113). Intestine-specific expression of DGAT1 clears the excess TAG storage in the cytosolic lipid droplets of enterocytes and restores the increases in plasma TAG in response to an oil bolus. More importantly, these mice with DGAT1 expressed only in the intestine are susceptible to obesity induced by a high-fat diet, like wild-type mice.
OTHER ENZYMES
The physiological roles of other TAG synthesis enzymes in the intestine have not been demonstrated. DGAT2 is also expressed in mouse intestine. However, deletion of DGAT2 is lethal and Dgat2−/− mice die shortly after birth (88), likely due to severe shortage of energy substrates and defects in skin barrier function. Many mouse models in which an enzyme catalyzing the G3P pathway is deleted also show energy metabolism phenotypes. For example, mice lacking GPAT3 showed a moderate reduction in weight gain when fed a high-fat diet, which has been attributed to changes in TAG synthesis in the adipose tissue (131). Likewise, mice lacking GPAT4 have reduced body weights. They show subdermal lipodystrophy and possibly heat loss, which in turn increases energy expenditure (132). However, the potential impacts of these enzyme deficiencies on fat absorption have not been reported.
On the other hand, several mouse models suggest that the mobilization of TAG synthesized in the enterocytes and stored in the cytosolic lipid droplets plays a physiological role in fat absorption and possibly systemic energy balance.
Intestine-specific inactivation of ATGL, which catalyzes the first step of TAG hydrolysis, increases cytosolic lipid droplets in enterocytes and increases fecal fat, even though the rate of fat absorption after an oral oil challenge remains normal (106). Intestine-specific deletion of its activator CGI-58 in mice reduces the rate of fat absorption and increases fecal fat (107). In contrast, intestine-specific overexpression of MAG lipase, the enzyme converting MAG to glycerol and fatty acid, leads to a decreased 2-arachidonoylglycerol level, hyperphagia, reduced metabolic rate, and obesity (133). Further, Cideb mobilizes cytosolic TAG to lipidate ApoB. Mice deficient in Cideb show reduced chylomicron secretion and increased TAG accumulation in the enterocytes, which is associated with resistance to high-fat diet-induced obesity (108, 134, 135). These findings further support the idea that TAG metabolism in the intestine regulates systemic energy balance.
SPECIFIC INHIBITORS OF THE MAG PATHWAY
The interpretation of results from genetically engineered mice can be complicated by compensatory effects, such as developmental programming. Studies using specific inhibitors of these enzymes in animals and in humans complement findings from studies using these mouse models. Numerous specific inhibitors of DGAT1 have been developed as potential treatments for type 2 diabetes and obesity (136), based on the favorable metabolic phenotypes (enhanced insulin sensitivity and resistance to weight gain) observed in Dgat1−/− mice. Several have been tested in animals and recapitulated the favorable outcomes predicted by the genetically engineered mice, including delayed gastric emptying and fat absorption, blunted postprandial plasma TAG, increased GLP-1 levels, enhanced fatty acid oxidation, reduced liver TAG, improved insulin sensitivity, and weight loss (137–143).
In clinical trials, several DGAT1 inhibitors have shown efficacy in achieving favorable outcomes, such as reducing postprandial plasma TAG, increasing GLP-1 levels, normalizing insulin sensitivity, and inducing weight loss. However, they are often associated with intolerable gastrointestinal problems, including nausea, diarrhea, and vomiting. These side effects may result from the target; unlike in mice, DGAT1 appears to be the only intestinal DGAT in humans. Inhibiting the only DGAT enzyme in human intestine may block TAG synthesis fully and lead to severe fat malabsorption, as observed in patients with a null mutation in the DGAT1 gene (89, 144). Thus, the utility of DGAT1 inhibitors as a treatment for diabetes or obesity remains uncertain. Inhibiting MGAT2 will likely not cause as severe a deficiency in TAG synthesis, as the alternative G3P pathway is active in human intestine. However, unlike mice, humans also have MGAT3 expressed in the distal intestine. MGAT3 could compensate for MGAT2 and possibly dampen the therapeutic efficacy of MGAT2 inhibitors. Interestingly, MGAT3, as well as MGAT2, is highly expressed in the liver of human subjects with nonalcoholic fatty liver disease and their hepatic expression levels reduce significantly after weight loss (145). Thus, inhibiting both MGAT2 and MGAT3 may present a therapeutic opportunity for modulating hepatic lipid metabolism.
PERSPECTIVES
TAG is a condensed source of dietary calories that can be readily assimilated. TAG synthesis in the intestine, featuring the MAG pathway, plays an integral role for this efficiency. The process of TAG hydrolysis in the intestinal lumen has been a drug target for treating human obesity; an inhibitor to the intestinal lipases is currently one of the few medicines available, but with limited efficacy and some unwanted side effects (146). Findings from the inactivation of enzymes involved in the MAG pathway, MGAT2 and DGAT1, support the idea that intestinal TAG synthesis plays a crucial role in the regulation of fat metabolism and systemic energy balance. The inactivation of intestinal TAG synthesis leads to temporal and spatial changes in fat absorption, reduced postprandial triglyceridemia, changes in postprandial gut hormone levels, and resistance to diet-induced obesity without fat malabsorption in rodents. The physiological mechanism for obesity resistance in many of these models involves increased energy expenditure, but the underlying molecular mechanisms involved appear novel. What the mechanisms are and whether they also function in a similar fashion in humans are yet to be explored. The intestine is a major source of TAG-rich lipoprotein, contributing to postprandial hypertriglyceridemia, and it over produces the ApoB-containing lipoproteins under insulin resistant states in both animal models and humans (147, 148). The therapeutic potential of inhibiting specific enzymes involved in intestinal TAG synthesis for treating metabolic disorders associated with excess lipid accumulation warrants further investigation.
Acknowledgments
The authors appreciate the important contributions many investigators have made to advance the research field. They thank Drs. Charles Mansbach II, and Kimberley Buhman for insightful comments.
Footnotes
Abbreviations:
- ACSL
- acyl-CoA synthetase
- AGPAT
- 1-acylglycerol-3-phosphate acyltransferase
- ATGL
- adipose triglyceride lipase
- CD36
- cluster of differentiation
- CGI-58
- comparative gene identification-58
- Cideb
- cell death-inducing DNA fragmentation factor 45 (DFF45)-like effector b
- DAG
- diacylglycerol
- DGAT
- acyl-CoA:diacylglycerol acyltransferase
- ER
- endoplasmic reticulum
- FABP
- fatty acid binding protein
- GLP-1
- glucagon-like peptide 1
- GPAT
- glycerol-3-phosphate acyltransferase
- G3P
- glycerol-3-phosphate
- IFABP
- intestine-type fatty acid binding protein
- LFABP
- liver-type fatty acid binding protein
- MAG
- monoacylglycerol
- MBOAT
- membrane-bound O-acyltransferase
- MGAT
- acyl-CoA:monoacylglycerol acyltransferase
- MTP
- microsomal triacylglycerol transfer protein
- PA
- phosphatidic acid
- PAP
- phosphatidic acid phosphatase
- TAG
- triacylglycerol (triglyceride)
This work was funded by the National Institutes of Health Grant DK088210 and US Department of Agriculture Grant WIS01442.
REFERENCES
- 1.Coleman R. A., Mashek D. G. 2011. Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chem. Rev. 111: 6359–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phan C. T., Tso P. 2001. Intestinal lipid absorption and transport. Front. Biosci. 6: D299–D319. [DOI] [PubMed] [Google Scholar]
- 3.Kuksis A., Lehner R. 2001. Intestinal synthesis of triacylglycerols. In Intestinal Lipid Metabolism. C. M. Mansbach, P. Tso, and A. Kuksis, editors. Springer, New York. 185–213. [Google Scholar]
- 4.Shiau Y. F., Boyle J. T., Umstetter C., Koldovsky O. 1980. Apical distribution of fatty acid esterification capacity along the villus-crypt unit of rat jejunum. Gastroenterology. 79: 47–53. [PubMed] [Google Scholar]
- 5.Kasper H. 1970. Faecal fat excretion, diarrhea, and subjective complaints with highly dosed oral fat intake. Digestion. 3: 321–330. [DOI] [PubMed] [Google Scholar]
- 6.Mansbach C. M., Dowell R. 2000. Effect of increasing lipid loads on the ability of the endoplasmic reticulum to transport lipid to the Golgi. J. Lipid Res. 41: 605–612. [PubMed] [Google Scholar]
- 7.Swaminathan R., King R. F., Holmfield J., Siwek R. A., Baker M., Wales J. K. 1985. Thermic effect of feeding carbohydrate, fat, protein and mixed meal in lean and obese subjects. Am. J. Clin. Nutr. 42: 177–181. [DOI] [PubMed] [Google Scholar]
- 8.Maffeis C., Schutz Y., Grezzani A., Provera S., Piacentini G., Tato L. 2001. Meal-induced thermogenesis and obesity: is a fat meal a risk factor for fat gain in children? J. Clin. Endocrinol. Metab. 86: 214–219. [DOI] [PubMed] [Google Scholar]
- 9.Yen C. L., Stone S. J., Koliwad S., Harris C., Farese R. V., Jr 2008. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 49: 2283–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reue K., Brindley D. N. 2008. Glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J. Lipid Res. 49: 2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangl A., Ockner R. K. 1975. Intestinal metabolism of plasma free fatty acids. Intracellular compartmentation and mechanisms of control. J. Clin. Invest. 55: 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Storch J., Zhou Y. X., Lagakos W. S. 2008. Metabolism of apical versus basolateral sn-2-monoacylglycerol and fatty acids in rodent small intestine. J. Lipid Res. 49: 1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson A. B. R., Dietschy J. M. 1981. Intestinal lipid absorption: Major extracellular and intracellular events. In Physiology of the Gastrointestinal Tract. L. R. Johnson, editor. Raven Press, New York. 1147–1220. [Google Scholar]
- 14.Mansbach C. M., 2nd, Parthasarathy S. 1982. A re-examination of the fate of glyceride-glycerol in neutral lipid absorption and transport. J. Lipid Res. 23: 1009–1019. [PubMed] [Google Scholar]
- 15.Goodridge A. G. 1991. Fatty acid synthesis in encaryotes. In Biochemistry of Lipids, Lipoproteins and Membranes. D. E. Vance and J. Vance, editors. Elsevier Science Publishing, Amsterdam. 141–169. [Google Scholar]
- 16.Mahley R. W., Bennett B. D., Morre D. J., Gray M. E., Thistlethwaite W., LeQuire V. S. 1971. Lipoproteins associated with the Golgi apparatus isolated from epithelial cells of rat small intestine. Lab. Invest. 25: 435–444. [PubMed] [Google Scholar]
- 17.Shiau Y. F., Popper D. A., Reed M., Umstetter C., Capuzzi D., Levine G. M. 1985. Intestinal triglycerides are derived from both endogenous and exogenous sources. Am. J. Physiol. 248: G164–G169. [DOI] [PubMed] [Google Scholar]
- 18.Stahl A., Hirsch D. J., Gimeno R. E., Punreddy S., Ge P., Watson N., Patel S., Kotler M., Raimondi A., Tartaglia L. A., et al. 1999. Identification of the major intestinal fatty acid transport protein. Mol. Cell. 4: 299–308. [DOI] [PubMed] [Google Scholar]
- 19.Mashek D. G., Coleman R. A. 2006. Cellular fatty acid uptake: the contribution of metabolism. Curr. Opin. Lipidol. 17: 274–278. [DOI] [PubMed] [Google Scholar]
- 20.Abumrad N. A., Davidson N. O. 2012. Role of the gut in lipid homeostasis. Physiol. Rev. 92: 1061–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niot I., Poirier H., Tran T. T., Besnard P. 2009. Intestinal absorption of long-chain fatty acids: evidence and uncertainties. Prog. Lipid Res. 48: 101–115. [DOI] [PubMed] [Google Scholar]
- 22.Grevengoed T. J., Klett E. L., Coleman R. A. 2014. Acyl-CoA metabolism and partitioning. Annu. Rev. Nutr. 34: 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shim J., Moulson C. L., Newberry E. P., Lin M. H., Xie Y., Kennedy S. M., Miner J. H., Davidson N. O. 2009. Fatty acid transport protein 4 is dispensable for intestinal lipid absorption in mice. J. Lipid Res. 50: 491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stremmel W., Lotz G., Strohmeyer G., Berk P. D. 1985. Identification, isolation, and partial characterization of a fatty acid binding protein from rat jejunal microvillous membranes. J. Clin. Invest. 75: 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berk P. D., Wada H., Horio Y., Potter B. J., Sorrentino D., Zhou S. L., Isola L. M., Stump D., Kiang C. L., Thung S. 1990. Plasma membrane fatty acid-binding protein and mitochondrial glutamic-oxaloacetic transaminase of rat liver are related. Proc. Natl. Acad. Sci. USA. 87: 3484–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stump D. D., Zhou S. L., Berk P. D. 1993. Comparison of plasma membrane FABP and mitochondrial isoform of aspartate aminotransferase from rat liver. Am. J. Physiol. 265: G894–G902. [DOI] [PubMed] [Google Scholar]
- 27.Chen M., Yang Y., Braunstein E., Georgeson K. E., Harmon C. M. 2001. Gut expression and regulation of FAT/CD36: possible role in fatty acid transport in rat enterocytes. Am. J. Physiol. Endocrinol. Metab. 281: E916–E923. [DOI] [PubMed] [Google Scholar]
- 28.Pepino M. Y., Kuda O., Samovski D., Abumrad N. A. 2014. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 34: 281–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundaresan S., Shahid R., Riehl T. E., Chandra R., Nassir F., Stenson W. F., Liddle R. A., Abumrad N. A. 2013. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB J. 27: 1191–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu S., Jay A., Brunaldi K., Huang N., Hamilton J. A. 2013. CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry. 52: 7254–7261. [DOI] [PubMed] [Google Scholar]
- 31.Pohl J., Ring A., Korkmaz U., Ehehalt R., Stremmel W. 2005. FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol. Biol. Cell. 16: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiqi S., Sheth A., Patel F., Barnes M., Mansbach C. M., 2nd 2013. Intestinal caveolin-1 is important for dietary fatty acid absorption. Biochim. Biophys. Acta. 1831: 1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulthess G., Lipka G., Compassi S., Boffelli D., Weber F. E., Paltauf F., Hauser H. 1994. Absorption of monoacylglycerols by small intestinal brush border membrane. Biochemistry. 33: 4500–4508. [DOI] [PubMed] [Google Scholar]
- 34.Ho S. Y., Storch J. 2001. Common mechanisms of monoacylglycerol and fatty acid uptake by human intestinal Caco-2 cells. Am. J. Physiol. Cell Physiol. 281: C1106–C1117. [DOI] [PubMed] [Google Scholar]
- 35.Murota K., Matsui N., Kawada T., Takahashi N., Fushuki T. 2001. Inhibitory effect of monoacylglycerol on fatty acid uptake into rat intestinal epithelial cells. Biosci. Biotechnol. Biochem. 65: 1441–1443. [DOI] [PubMed] [Google Scholar]
- 36.Nelson D. W., Gao Y., Yen M. I., Yen C. L. 2014. Intestine-specific Deletion of acyl-CoA:monoacylglycerol acyltransferase (MGAT) 2 protects mice from diet-induced obesity and glucose intolerance. J. Biol. Chem. 289: 17338–17349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Y., Nelson D. W., Banh T., Yen M. I., Yen C. L. 2013. Intestine-specific expression of MOGAT2 partially restores metabolic efficiency in Mogat2-deficient mice. J. Lipid Res. 54: 1644–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storch J., Corsico B. 2008. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu. Rev. Nutr. 28: 73–95. [DOI] [PubMed] [Google Scholar]
- 39.Storch J., McDermott L. 2009. Structural and functional analysis of fatty acid-binding proteins. J. Lipid Res. 50(Suppl): S126–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson J., Ory J., Reese-Wagoner A., Banaszak L. 1999. The liver fatty acid binding protein–comparison of cavity properties of intracellular lipid-binding proteins. Mol. Cell. Biochem. 192: 9–16. [PubMed] [Google Scholar]
- 41.Lagakos W. S., Guan X., Ho S. Y., Sawicki L. R., Corsico B., Kodukula S., Murota K., Stark R. E., Storch J. 2013. Liver fatty acid-binding protein binds monoacylglycerol in vitro and in mouse liver cytosol. J. Biol. Chem. 288: 19805–19815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storch J., Thumser A. E. 2000. The fatty acid transport function of fatty acid-binding proteins. Biochim. Biophys. Acta. 1486: 28–44. [DOI] [PubMed] [Google Scholar]
- 43.Neeli I., Siddiqi S. A., Siddiqi S., Mahan J., Lagakos W. S., Binas B., Gheyi T., Storch J., Mansbach C. M., 2nd 2007. Liver fatty acid-binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J. Biol. Chem. 282: 17974–17984. [DOI] [PubMed] [Google Scholar]
- 44.Gajda A. M., Zhou Y. X., Agellon L. B., Fried S. K., Kodukula S., Fortson W., Patel K., Storch J. 2013. Direct comparison of mice null for liver or intestinal fatty acid-binding proteins reveals highly divergent phenotypic responses to high fat feeding. J. Biol. Chem. 288: 30330–30344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agellon L. B., Drozdowski L., Li L., Iordache C., Luong L., Clandinin M. T., Uwiera R. R., Toth M. J., Thomson A. B. 2007. Loss of intestinal fatty acid binding protein increases the susceptibility of male mice to high fat diet-induced fatty liver. Biochim. Biophys. Acta. 1771: 1283–1288. [DOI] [PubMed] [Google Scholar]
- 46.Vassileva G., Huwyler L., Poirier K., Agellon L. B., Toth M. J. 2000. The intestinal fatty acid binding protein is not essential for dietary fat absorption in mice. FASEB J. 14: 2040–2046. [DOI] [PubMed] [Google Scholar]
- 47.Newberry E. P., Xie Y., Kennedy S., Han X., Buhman K. K., Luo J., Gross R. W., Davidson N. O. 2003. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J. Biol. Chem. 278: 51664–51672. [DOI] [PubMed] [Google Scholar]
- 48.Meller N., Morgan M. E., Wong W. P., Altemus J. B., Sehayek E. 2013. Targeting of acyl-CoA synthetase 5 decreases jejunal fatty acid activation with no effect on dietary long-chain fatty acid absorption. Lipids Health Dis. 12: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kennedy E. P. 1957. Metabolism of lipides. Annu. Rev. Biochem. 26: 119–148. [DOI] [PubMed] [Google Scholar]
- 50.Bell R. M., Coleman R. A. 1980. Enzymes of glycerolipid synthesis in eukaryotes. Annu. Rev. Biochem. 49: 459–487. [DOI] [PubMed] [Google Scholar]
- 51.Lehner R., Kuksis A. 1996. Biosynthesis of triacylglycerols. Prog. Lipid Res. 35: 169–201. [DOI] [PubMed] [Google Scholar]
- 52.Coleman R. A., Lee D. P. 2004. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 43: 134–176. [DOI] [PubMed] [Google Scholar]
- 53.Ellis J. M., Frahm J. L., Li L. O., Coleman R. A. 2010. Acyl-coenzyme A synthetases in metabolic control. Curr. Opin. Lipidol. 21: 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coleman R. A., Lewin T. M., Van Horn C. G., Gonzalez-Baro M. R. 2002. Do long-chain acyl-CoA synthetases regulate fatty acid entry into synthetic versus degradative pathways? J. Nutr. 132: 2123–2126. [DOI] [PubMed] [Google Scholar]
- 55.Skipski V. P., Morehouse M. G., Deuel H. J., Jr 1959. The absorption in the rat of a 1,3-dioleyl-2-deuteriostearyl glyceride-C14 and a 1-monodeuteriostearyl glyceride-C14. Arch. Biochem. Biophys. 81: 93–104. [DOI] [PubMed] [Google Scholar]
- 56.Kayden H. J., Senior J. R., Mattson F. H. 1967. The monoglyceride pathway of fat absorption in man. J. Clin. Invest. 46: 1695–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodgers J. B., Jr 1969. Assay of acyl-CoA:monoglyceride acyltransferase from rat small intestine using continuous recording spectrophotometry. J. Lipid Res. 10: 427–432. [PubMed] [Google Scholar]
- 58.Grigor M. R., Bell R. M. 1982. Separate monoacylglycerol and diacylglycerol acyltransferases function in intestinal triacylglycerol synthesis. Biochim. Biophys. Acta. 712: 464–472. [DOI] [PubMed] [Google Scholar]
- 59.Johnston J. M., Rao G. A. 1967. Intestinal absorption of fat. Protoplasma. 63: 40–44. [DOI] [PubMed] [Google Scholar]
- 60.Innis S. M. 2011. Dietary triacylglycerol structure and its role in infant nutrition. Adv. Nutr. 2: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lehner R., Kuksis A. 1992. Utilization of 2-monoacylglycerols for phosphatidylcholine biosynthesis in the intestine. Biochim. Biophys. Acta. 1125: 171–179. [DOI] [PubMed] [Google Scholar]
- 62.Lehner R., Kuksis A. 1993. Triacylglycerol synthesis by an sn-1,2(2,3)-diacylglycerol transacylase from rat intestinal microsomes. J. Biol. Chem. 268: 8781–8786. [PubMed] [Google Scholar]
- 63.Yen C. L., Stone S. J., Cases S., Zhou P., Farese R. V., Jr 2002. Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proc. Natl. Acad. Sci. USA. 99: 8512–8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yen C. L., Farese R. V., Jr 2003. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 278: 18532–18537. [DOI] [PubMed] [Google Scholar]
- 65.Cao J., Cheng L., Shi Y. 2007. Catalytic properties of MGAT3, a putative triacylgycerol synthase. J. Lipid Res. 48: 583–591. [DOI] [PubMed] [Google Scholar]
- 66.Cases S., Smith S. J., Zheng Y. W., Myers H. M., Lear S. R., Sande E., Novak S., Collins C., Welch C. B., Lusis A. J., et al. 1998. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA. 95: 13018–13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cases S., Stone S. J., Zhou P., Yen E., Tow B., Lardizabal K. D., Voelker T., Farese R. V., Jr 2001. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 276: 38870–38876. [DOI] [PubMed] [Google Scholar]
- 68.Coleman R. A., Haynes E. B. 1984. Hepatic monoacylglycerol acyltransferase. Characterization of an activity associated with the suckling period in rats. J. Biol. Chem. 259: 8934–8938. [PubMed] [Google Scholar]
- 69.Mostafa N., Bhat B. G., Coleman R. A. 1994. Adipose monoacylglycerol:acyl-coenzyme A acyltransferase activity in the white-throated sparrow (Zonotrichia albicollis): characterization and function in a migratory bird. Lipids. 29: 785–791. [DOI] [PubMed] [Google Scholar]
- 70.Cheng D., Nelson T. C., Chen J., Walker S. G., Wardwell-Swanson J., Meegalla R., Taub R., Billheimer J. T., Ramaker M., Feder J. N. 2003. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J. Biol. Chem. 278: 13611–13614. [DOI] [PubMed] [Google Scholar]
- 71.Cao J., Lockwood J., Burn P., Shi Y. 2003. Cloning and functional characterization of a mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J. Biol. Chem. 278: 13860–13866. [DOI] [PubMed] [Google Scholar]
- 72.Cao J., Hawkins E., Brozinick J., Liu X., Zhang H., Burn P., Shi Y. 2004. A predominant role of acyl-CoA: Monoacylglycerol acyltransferase-2 in dietary fat absorption implicated by tissue distribution, subcellular localization, and up-regulation by high fat diet. J. Biol. Chem. 279: 18878–18886. [DOI] [PubMed] [Google Scholar]
- 73.Yue Y. G., Chen Y. Q., Zhang Y., Wang H., Qian Y. W., Arnold J. S., Calley J. N., Li S. D., Perry W. L., 3rd, Zhang H. Y., et al. 2011. The acyl coenzymeA:monoacylglycerol acyltransferase 3 (MGAT3) gene is a pseudogene in mice but encodes a functional enzyme in rats. Lipids. 46: 513–520. [DOI] [PubMed] [Google Scholar]
- 74.Yen C. L., Monetti M., Burri B. J., Farese R. V., Jr 2005. The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J. Lipid Res. 46: 1502–1511. [DOI] [PubMed] [Google Scholar]
- 75.Shih M. Y., Kane M. A., Zhou P., Yen C. L., Streeper R. S., Napoli J. L., Farese R. V., Jr 2009. Retinol esterification by DGAT1 is essential for retinoid homeostasis in murine skin. J. Biol. Chem. 284: 4292–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hiramine Y., Emoto H., Takasuga S., Hiramatsu R. 2010. Novel acyl-coenzyme A:monoacylglycerol acyltransferase plays an important role in hepatic triacylglycerol secretion. J. Lipid Res. 51: 1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang J., Brown M. S., Liang G., Grishin N. V., Goldstein J. L. 2008. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 132: 387–396. [DOI] [PubMed] [Google Scholar]
- 78.Yen C. L., Brown C. H. t., Monetti M., Farese R. V., Jr 2005. A human skin multifunctional O-acyltransferase that catalyzes the synthesis of acylglycerols, waxes, and retinyl esters. J. Lipid Res. 46: 2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng J. B., Russell D. W. 2004. Mammalian wax biosynthesis. II. Expression cloning of wax synthase cDNAs encoding a member of the acyltransferase enzyme family. J. Biol. Chem. 279: 37798–37807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McFie P. J., Stone S. L., Banman S. L., Stone S. J. 2010. Topological orientation of acyl-CoA:diacylglycerol acyltransferase-1 (DGAT1) and identification of a putative active site histidine and the role of the n terminus in dimer/tetramer formation. J. Biol. Chem. 285: 37377–37387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng D., Meegalla R. L., He B., Cromley D. A., Billheimer J. T., Young P. R. 2001. Human acyl-CoA:diacylglycerol acyltransferase is a tetrameric protein. Biochem. J. 359: 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wurie H. R., Buckett L., Zammit V. A. 2011. Evidence that diacylglycerol acyltransferase 1 (DGAT1) has dual membrane topology in the endoplasmic reticulum of HepG2 cells. J. Biol. Chem. 286: 36238–36247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stone S. J., Levin M. C., Farese R. V., Jr 2006. Membrane topology and identification of key functional amino acid residues of murine acyl-CoA:diacylglycerol acyltransferase-2. J. Biol. Chem. 281: 40273–40282. [DOI] [PubMed] [Google Scholar]
- 84.Wongsiriroj N., Piantedosi R., Palczewski K., Goldberg I. J., Johnston T. P., Li E., Blaner W. S. 2008. The molecular basis of retinoid absorption: a genetic dissection. J. Biol. Chem. 283: 13510–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith S. J., Cases S., Jensen D. R., Chen H. C., Sande E., Tow B., Sanan D. A., Raber J., Eckel R. H., Farese R. V., Jr 2000. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat. Genet. 25: 87–90. [DOI] [PubMed] [Google Scholar]
- 86.Harris C. A., Haas J. T., Streeper R. S., Stone S. J., Kumari M., Yang K., Han X., Brownell N., Gross R. W., Zechner R., et al. 2011. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J. Lipid Res. 52: 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buhman K. K., Smith S. J., Stone S. J., Repa J. J., Wong J. S., Knapp F. F., Jr, Burri B. J., Hamilton R. L., Abumrad N. A., Farese R. V., Jr 2002. DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J. Biol. Chem. 277: 25474–25479. [DOI] [PubMed] [Google Scholar]
- 88.Stone S. J., Myers H. M., Watkins S. M., Brown B. E., Feingold K. R., Elias P. M., Farese R. V., Jr 2004. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J. Biol. Chem. 279: 11767–11776. [DOI] [PubMed] [Google Scholar]
- 89.Haas J. T., Winter H. S., Lim E., Kirby A., Blumenstiel B., DeFelice M., Gabriel S., Jalas C., Branski D., Grueter C. A., et al. 2012. DGAT1 mutation is linked to a congenital diarrheal disorder. J. Clin. Invest. 122: 4680–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnston J. M., Rao G. A., Lowe P. A. 1967. The separation of the alpha-glycerophosphate and monoglyceride pathways in the intestinal biosynthesis of triglycerides. Biochim. Biophys. Acta. 137: 578–580. [DOI] [PubMed] [Google Scholar]
- 91.Shan D., Li J. L., Wu L., Li D., Hurov J., Tobin J. F., Gimeno R. E., Cao J. 2010. GPAT3 and GPAT4 are regulated by insulin-stimulated phosphorylation and play distinct roles in adipogenesis. J. Lipid Res. 51: 1971–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takeuchi K., Reue K. 2009. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 296: E1195–E1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Csaki L. S., Dwyer J. R., Fong L. G., Tontonoz P., Young S. G., Reue K. 2013. Lipins, lipinopathies, and the modulation of cellular lipid storage and signaling. Prog. Lipid Res. 52: 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mansbach C. M. 2001. Triacylglycerol movement in enterocytes. In Intestinal Lipid Metabolism. C. M. Mansbach, P. Tso, and A. Kuksis, editors. Springer, New York. 215–233. [Google Scholar]
- 95.Wilfling F., Haas J. T., Walther T. C., Farese R. V., Jr 2014. Lipid droplet biogenesis. Curr. Opin. Cell Biol. 29C: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Greenberg A. S., Egan J. J., Wek S. A., Garty N. B., Blanchette-Mackie E. J., Londos C. 1991. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 266: 11341–11346. [PubMed] [Google Scholar]
- 97.Brasaemle D. L. 2007. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48: 2547–2559. [DOI] [PubMed] [Google Scholar]
- 98.Davidson N. O., Shelness G. S. 2000. Apolipoprotein B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu. Rev. Nutr. 20: 169–193. [DOI] [PubMed] [Google Scholar]
- 99.Lehner R., Lian J., Quiroga A. D. 2012. Lumenal lipid metabolism: implications for lipoprotein assembly. Arterioscler. Thromb. Vasc. Biol. 32: 1087–1093. [DOI] [PubMed] [Google Scholar]
- 100.Mansbach C. M., Siddiqi S. A. 2010. The biogenesis of chylomicrons. Annu. Rev. Physiol. 72: 315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tso P., Liu M., Kalogeris T. J., Thomson A. B. 2001. The role of apolipoprotein A-IV in the regulation of food intake. Annu. Rev. Nutr. 21: 231–254. [DOI] [PubMed] [Google Scholar]
- 102.Young S. G., Zechner R. 2013. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 27: 459–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nevin P., Koelsch D., Mansbach C. M., 2nd 1995. Intestinal triacylglycerol storage pool size changes under differing physiological conditions. J. Lipid Res. 36: 2405–2412. [PubMed] [Google Scholar]
- 104.Zhu J., Lee B., Buhman K. K., Cheng J. X. 2009. A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging. J. Lipid Res. 50: 1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang L. Y., Kuksis A., Myher J. J., Steiner G. 1995. Origin of triacylglycerol moiety of plasma very-low-density lipoproteins in the rat - structural studies. J. Lipid Res. 36: 125–136. [PubMed] [Google Scholar]
- 106.Obrowsky S., Chandak P. G., Patankar J. V., Povoden S., Schlager S., Kershaw E. E., Bogner-Strauss J. G., Hoefler G., Levak-Frank S., Kratky D. 2013. Adipose triglyceride lipase is a TG hydrolase of the small intestine and regulates intestinal PPARalpha signaling. J. Lipid Res. 54: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xie P., Guo F., Ma Y., Zhu H., Wang F., Xue B., Shi H., Yang J., Yu L. 2014. Intestinal Cgi-58 deficiency reduces postprandial lipid absorption. PLoS ONE. 9: e91652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang L. J., Wang C., Yuan Y., Wang H., Wu J., Liu F., Li L., Gao X., Zhao Y. L., Hu P. Z., et al. 2014. Cideb facilitates the lipidation of chylomicrons in the small intestine. J. Lipid Res. 55: 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liang J. J., Oelkers P., Guo C., Chu P. C., Dixon J. L., Ginsberg H. N., Sturley S. L. 2004. Overexpression of human diacylglycerol acyltransferase 1, acyl-CoA:cholesterol acyltransferase 1, or acyl-CoA:cholesterol acyltransferase 2 stimulates secretion of apolipoprotein B-containing lipoproteins in McA-RH7777 cells. J. Biol. Chem. 279: 44938–44944. [DOI] [PubMed] [Google Scholar]
- 110.Yamazaki T., Sasaki E., Kakinuma C., Yano T., Miura S., Ezaki O. 2005. Increased very low density lipoprotein secretion and gonadal fat mass in mice overexpressing liver DGAT1. J. Biol. Chem. 280: 21506–21514. [DOI] [PubMed] [Google Scholar]
- 111.Millar J. S., Stone S. J., Tietge U. J., Tow B., Billheimer J. T., Wong J. S., Hamilton R. L., Farese R. V., Jr, Rader D. J. 2006. Short-term overexpression of DGAT1 or DGAT2 increases hepatic triglyceride but not VLDL triglyceride or apoB production. J. Lipid Res. 47: 2297–2305. [DOI] [PubMed] [Google Scholar]
- 112.Monetti M., Levin M. C., Watt M. J., Sajan M. P., Marmor S., Hubbard B. K., Stevens R. D., Bain J. R., Newgard C. B., Farese R. V., Sr, et al. 2007. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 6: 69–78. [DOI] [PubMed] [Google Scholar]
- 113.Lee B., Fast A. M., Zhu J., Cheng J. X., Buhman K. K. 2010. Intestine-specific expression of acyl CoA:diacylglycerol acyltransferase 1 reverses resistance to diet-induced hepatic steatosis and obesity in Dgat1-/- mice. J. Lipid Res. 51: 1770–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Uchida A., Slipchenko M. N., Eustaquio T., Leary J. F., Cheng J. X., Buhman K. K. 2013. Intestinal acyl-CoA:diacylglycerol acyltransferase 2 overexpression enhances postprandial triglyceridemic response and exacerbates high fat diet-induced hepatic triacylglycerol storage. Biochim. Biophys. Acta. 1831: 1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang J., Xu D., Nie J., Cao J., Zhai Y., Tong D., Shi Y. 2014. Monoacylglycerol acyltransferase-2 is a tetrameric enzyme that selectively heterodimerizes with diacylglycerol acyltransferase-1. J. Biol. Chem. 289: 10909–10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lehner R., Kuksis A. 1995. Triacylglycerol synthesis by purified triacylglycerol synthetase of rat intestinal mucosa. Role of acyl-CoA acyltransferase. J. Biol. Chem. 270: 13630–13636. [DOI] [PubMed] [Google Scholar]
- 117.Kuerschner L., Moessinger C., Thiele C. 2008. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic. 9: 338–352. [DOI] [PubMed] [Google Scholar]
- 118.Wilfling F., Wang H., Haas J. T., Krahmer N., Gould T. J., Uchida A., Cheng J. X., Graham M., Christiano R., Frohlich F., et al. 2013. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev. Cell. 24: 384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yen C. L., Cheong M. L., Grueter C., Zhou P., Moriwaki J., Wong J. S., Hubbard B., Marmor S., Farese R. V., Jr 2009. Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat. Med. 15: 442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xie Y., Newberry E. P., Young S. G., Robine S., Hamilton R. L., Wong J. S., Luo J., Kennedy S., Davidson N. O. 2006. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J. Biol. Chem. 281: 4075–4086. [DOI] [PubMed] [Google Scholar]
- 121.Young S. G., Cham C. M., Pitas R. E., Burri B. J., Connolly A., Flynn L., Pappu A. S., Wong J. S., Hamilton R. L., Farese R. V., Jr 1995. A genetic model for absent chylomicron formation: mice producing apolipoprotein B in the liver, but not in the intestine. J. Clin. Invest. 96: 2932–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nelson D. W., Gao Y., Spencer N. M., Banh T., Yen C. L. 2011. Deficiency of MGAT2 increases energy expenditure without high-fat feeding and protects genetically obese mice from excessive weight gain. J. Lipid Res. 52: 1723–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Woods S. C., Seeley R. J., Porte D., Jr, Schwartz M. W. 1998. Signals that regulate food intake and energy homeostasis. Science. 280: 1378–1383. [DOI] [PubMed] [Google Scholar]
- 124.Chen H. C., Smith S. J., Ladha Z., Jensen D. R., Ferreira L. D., Pulawa L. K., McGuire J. G., Pitas R. E., Eckel R. H., Farese R. V., Jr 2002. Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J. Clin. Invest. 109: 1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen H. C., Ladha Z., Farese R. V., Jr 2002. Deficiency of acyl coenzyme a:diacylglycerol acyltransferase 1 increases leptin sensitivity in murine obesity models. Endocrinology. 143: 2893–2898. [DOI] [PubMed] [Google Scholar]
- 126.Chen H. C., Farese R. V., Jr 2005. Inhibition of triglyceride synthesis as a treatment strategy for obesity: lessons from DGAT1-deficient mice. Arterioscler. Thromb. Vasc. Biol. 25: 482–486. [DOI] [PubMed] [Google Scholar]
- 127.Okawa M., Fujii K., Ohbuchi K., Okumoto M., Aragane K., Sato H., Tamai Y., Seo T., Itoh Y., Yoshimoto R. 2009. Role of MGAT2 and DGAT1 in the release of gut peptides after triglyceride ingestion. Biochem. Biophys. Res. Commun. 390: 377–381. [DOI] [PubMed] [Google Scholar]
- 128.Cases S., Zhou P., Shillingford J. M., Wiseman B. S., Fish J. D., Angle C. S., Hennighausen L., Werb Z., Farese R. V. 2004. Development of the mammary gland requires DGAT1 expression in stromal and epithelial tissues. Development. 131: 3047–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen H. C., Smith S. J., Tow B., Elias P. M., Farese R. V., Jr 2002. Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J. Clin. Invest. 109: 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen H. C., Jensen D. R., Myers H. M., Eckel R. H., Farese R. V., Jr 2003. Obesity resistance and enhanced glucose metabolism in mice transplanted with white adipose tissue lacking acyl CoA:diacylglycerol acyltransferase 1. J. Clin. Invest. 111: 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cao J., Perez S., Goodwin B., Lin Q., Peng H., Qadri A., Zhou Y., Clark R. W., Perreault M., Tobin J. F., et al. 2014. Mice deleted for GPAT3 have reduced GPAT activity in white adipose tissue and altered energy and cholesterol homeostasis in diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 306: E1176–E1187. [DOI] [PubMed] [Google Scholar]
- 132.Vergnes L., Beigneux A. P., Davis R., Watkins S. M., Young S. G., Reue K. 2006. Agpat6 deficiency causes subdermal lipodystrophy and resistance to obesity. J. Lipid Res. 47: 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chon S. H., Douglass J. D., Zhou Y. X., Malik N., Dixon J. L., Brinker A., Quadro L., Storch J. 2012. Over-expression of monoacylglycerol lipase (MGL) in small intestine alters endocannabinoid levels and whole body energy balance, resulting in obesity. PLoS ONE. 7: e43962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li J. Z., Ye J., Xue B., Qi J., Zhang J., Zhou Z., Li Q., Wen Z., Li P. 2007. Cideb regulates diet-induced obesity, liver steatosis, and insulin sensitivity by controlling lipogenesis and fatty acid oxidation. Diabetes. 56: 2523–2532. [DOI] [PubMed] [Google Scholar]
- 135.Ye J., Li J. Z., Liu Y., Li X., Yang T., Ma X., Li Q., Yao Z., Li P. 2009. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 9: 177–190. [DOI] [PubMed] [Google Scholar]
- 136.DeVita R. J., Pinto S. 2013. Current status of the research and development of diacylglycerol O-acyltransferase 1 (DGAT1) inhibitors. J. Med. Chem. 56: 9820–9825. [DOI] [PubMed] [Google Scholar]
- 137.Ables G. P., Yang K. J., Vogel S., Hernandez-Ono A., Yu S., Yuen J. J., Birtles S., Buckett L. K., Turnbull A. V., Goldberg I. J., et al. 2012. Intestinal DGAT1 deficiency reduces postprandial triglyceride and retinyl ester excursions by inhibiting chylomicron secretion and delaying gastric emptying. J. Lipid Res. 53: 2364–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cao J., Zhou Y., Peng H., Huang X., Stahler S., Suri V., Qadri A., Gareski T., Jones J., Hahm S., et al. 2011. Targeting acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) with small molecule inhibitors for the treatment of metabolic diseases. J. Biol. Chem. 286: 41838–41851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schober G., Arnold M., Birtles S., Buckett L. K., Pacheco-Lopez G., Turnbull A. V., Langhans W., Mansouri A. 2013. Diacylglycerol acyltransferase-1 inhibition enhances intestinal fatty acid oxidation and reduces energy intake in rats. J. Lipid Res. 54: 1369–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dow R. L., Li J. C., Pence M. P., Gibbs E. M., LaPerle J. L., Litchfield J., Piotrowski D. W., Munchhof M. J., Manion T. B., Zavadoski W. J., et al. 2011. Discovery of PF-04620110, a potent, selective, and orally bioavailable inhibitor of DGAT-1. ACS Med. Chem. Lett. 2: 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yamamoto T., Yamaguchi H., Miki H., Kitamura S., Nakada Y., Aicher T. D., Pratt S. A., Kato K. 2011. A novel coenzyme A:diacylglycerol acyltransferase 1 inhibitor stimulates lipid metabolism in muscle and lowers weight in animal models of obesity. Eur. J. Pharmacol. 650: 663–672. [DOI] [PubMed] [Google Scholar]
- 142.Zhao G., Souers A. J., Voorbach M., Falls H. D., Droz B., Brodjian S., Lau Y. Y., Iyengar R. R., Gao J., Judd A. S., et al. 2008. Validation of diacyl glycerolacyltransferase I as a novel target for the treatment of obesity and dyslipidemia using a potent and selective small molecule inhibitor. J. Med. Chem. 51: 380–383. [DOI] [PubMed] [Google Scholar]
- 143.Cheng D., Iqbal J., Devenny J., Chu C. H., Chen L., Dong J., Seethala R., Keim W. J., Azzara A. V., Lawrence R. M., et al. 2008. Acylation of acylglycerols by acyl coenzyme A:diacylglycerol acyltransferase 1 (DGAT1). Functional importance of DGAT1 in the intestinal fat absorption. J. Biol. Chem. 283: 29802–29811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Denison H., Nilsson C., Lofgren L., Himmelmann A., Martensson G., Knutsson M., Al-Shurbaji A., Tornqvist H., Eriksson J. W. 2014. Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes Obes. Metab. 16: 334–343. [DOI] [PubMed] [Google Scholar]
- 145.Hall A. M., Kou K., Chen Z., Pietka T. A., Kumar M., Korenblat K. M., Lee K., Ahn K., Fabbrini E., Klein S., et al. 2012. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. J. Lipid Res. 53: 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yanovski S. Z., Yanovski J. A. 2014. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 311: 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Adeli K., Lewis G. F. 2008. Intestinal lipoprotein overproduction in insulin-resistant states. Curr. Opin. Lipidol. 19: 221–228. [DOI] [PubMed] [Google Scholar]
- 148.Xiao C., Dash S., Morgantini C., Lewis G. F. 2014. New and emerging regulators of intestinal lipoprotein secretion. Atherosclerosis. 233: 608–615. [DOI] [PubMed] [Google Scholar]