Abstract
Cells produce two cholesteryl ester transfer protein (CETP) isoforms, full-length and a shorter variant produced by alternative splicing. Blocking synthesis of both isoforms disrupts lipid metabolism and storage. To further define the role of CETP in cellular lipid metabolism, we stably overexpressed full-length CETP in SW872 cells. These CETP+ cells had several-fold higher intracellular CETP and accumulated 50% less TG due to a 26% decrease in TG synthesis and 2.5-fold higher TG turnover rate. Reduced TG synthesis was due to decreased fatty acid uptake and impaired conversion of diglyceride to TG even though diacylglycerol acyltransferase activity was normal. Sterol-regulatory element binding protein 1 mRNA levels were normal, and although PPARγ expression was reduced, the expression of several of its target genes including adipocyte triglyceride lipase, FASN, and APOE was normal. CETP+ cells contained smaller lipid droplets, consistent with their higher levels of perilipin protein family (PLIN) 3 compared with PLIN1 and PLIN2. Intracellular CETP was mostly associated with the endoplasmic reticulum, although CETP near lipid droplets poorly colocalized with this membrane. A small pool of CETP resided in the cytoplasm, and a subfraction coisolated with lipid droplets. These data show that overexpression of full-length CETP disrupts lipid homeostasis resulting in the formation of smaller, more metabolically active lipid droplets.
Keywords: lipid metabolism, lipid turnover, lipid droplets, cellular localization, gene expression
In cells, multiple proteins transport phospholipids, sterols, and fatty acids between organelles (1, 2). However, little is known about the molecular mechanisms of cholesteryl ester (CE) and TG transport. A candidate protein for this function is cholesteryl ester transfer protein (CETP). CETP’s role in CE and TG transport in plasma and its impact on lipoprotein metabolism are well known (3–5). CETP is also present inside cells where two isoforms exist: full-length CETP, which is equivalent to plasma CETP, and exon 9-deleted CETP, a smaller form derived from alternatively spliced mRNA (6). The function of exon 9-deleted CETP is poorly understood. Overexpression studies suggest that exon 9-deleted CETP may bind to full-length CETP and hinder its secretion (6, 7), although this role was not supported by subsequent transgenic mouse studies (8).
Multiple studies suggest that intracellular CETP modulates lipid metabolism. Cell-associated CETP promotes CE uptake from HDL, stimulates the cell’s ability to efflux cholesterol, and influences the storage of CE in cells (9–12). Acute suppression of CETP biosynthesis by antisense oligonucleotides in SW872 cells reduces cholesterol synthesis and increases CE accumulation (13). SW872 cells chronically deficient in CETP manifest more profound alterations in lipid metabolism including reduced capacity to store TG (14). A role for CETP in cellular lipid storage is further supported by observations in transgenic mice. Adipose tissue-specific expression of human CETP in mice results in smaller adipocytes containing less TG and cholesterol and significantly reduces the expression of key lipogenic genes (15). In hypertriglyceridemic mice, CETP expression normalizes subcutaneous adipose depots and visceral adipocyte size (16). And in humans, a CETP gene variant that affects the coding sequence of both full-length and exon 9-deleted CETP is associated with increased adiposity following long-term overfeeding (17).
Our initial studies in intracellular CETP, which used a molecular approach that reduced both full-length and exon 9-deleted CETP expression, identified multiple aberrations in lipid metabolism and storage (13, 14). In that study, we developed an in vitro assay that measured the ability of cytosolic CETP to promote the transfer of CE and TG from an endoplasmic reticulum-enriched fraction to lipid droplets. We observed that cytosol from control cells promoted lipid transfer between these isolated organelles, whereas cytosol from CETP-deficient cells was ineffective in this activity. We recently determined that the interorganelle lipid transfer activity in control cytosol could be mediated by full-length CETP alone. This finding prompted us to investigate how overexpression of full-length CETP in SW872 cells affects their lipid metabolism.
We report here that an increase in intracellular full-length CETP levels also induces marked changes in lipid homeostasis, including a 2-fold reduction in TG storage due to reduced TG synthesis and enhanced turnover. Increased CETP synthesis also altered the expression of multiple genes involved in lipid metabolism and reduced the CE content of these cells despite an increase in its synthetic rate. Cellular localization studies show that a subfraction of CETP is in the cytoplasm and a portion of this pool coisolates with lipid droplets, further supporting a role for CETP in the formation of these dynamic structures.
EXPERIMENTAL PROCEDURES
Materials
[9,10(n)-3H]oleic acid was from Perkin-Elmer Life Sciences (Boston, MA). Stock 2.7 mM 3H-oleate/BSA (∼2,500 cpm/nmol oleate) and unlabeled oleate/BSA (7:1 mol ratio of oleate to BSA) were prepared as previously described (18). HDL was prepared from human plasma by sequential ultracentrifugation (19), dialyzed extensively versus 0.9% NaCl, 0.02% EDTA, then sterile filtered. The mouse monoclonal antibody against human CETP, TP2, was purchased from the Ottawa Heart Institute (Ottawa, Ontario, Canada). Penicillin, streptomycin, BSA, and sodium oleate were from Sigma-Aldrich Corp. (St. Louis, MO).
Preparation of cells overexpressing CETP
The human liposarcoma cell line, SW872, was purchased from American Type Culture Collection (Manassas, VA). Cells were grown in DMEM/Ham’s F-12 (1:1) containing 10% FBS (Atlas Biologicals, Fort Collins, CO) and 50 µg/ml penicillin/streptomycin in 5% CO2/95% air at 37°. Unless stated otherwise, cells were ∼90% confluent at the start of an experiment, at which time cells were further incubated as described below.
Full-length human CETP including the natural Kozak sequence and the stop codon was amplified by PCR from a cDNA construct (pCETP.11) purchased from American Type Culture Collection. The fragment (position -46 to +1,542, NM_000078) was inserted into pCDNA3 using BamHI and XbaI restriction sites. SW872 cells at 70% confluence were transfected with native pCDNA3 or pCDNA3-CETP plasmid using Lipofectamine 2000 transfection reagent following the manufacturer’s directions (Life Technologies, Grand Island, NY). Transfectants were selected using 500 µg/ml G418 (Life Technologies). After 10–15 days, clones were picked and subcultured under the same selection conditions. Both vector-transfected SW872 cells and native SW872 cells were used as control as indicated in the text and figure legends. No significant differences were noted between these cells for the end points reported here.
Clones were tested for CETP expression by measuring CETP activity in 48 h conditioned medium collected in the absence of serum (3, 13). Volumes of conditioned media assayed were normalized by the cell count per well. Assay aliquots typically reflected the amount of media conditioned by ∼6 × 105 cells. Normalized aliquots of conditioned media were also concentrated 8-fold by cold acetone precipitation, then applied to 4–20% SDS-PAGE gels (Lonza, Rockland, ME). Cells were lysed by passage through a 26-gauge needle, centrifuged at 1,000 g for 10 min, and the supernatant (25 µg protein) fractionated by electrophoresis. After transfer to polyvinylidene difluoride membranes, CETP was detected by TP2 antibody (13). Clones overexpressing CETP were designated as CETP+ cells. Trypsinized CETP+ cells adhered less efficiently when passaged but had the same growth rate as vector-transfected control cells after 2 days in culture.
CETP-deficient SW872 cells, prepared as previously described (14), were used in select experiments.
Oleate incorporation into TG and its precursors
Cells were washed with PBS and incubated in OptiMEM (Life Technologies) for 24 h before the addition of prewarmed 200 µM 3H-oleate/BSA in OptiMEM. At the indicated time, the media was removed and ice cold PBS was added to cells. Culture plates were kept on ice until cells were scraped. A zero time blank was used to correct for any metabolism that occurred during sample processing. Cellular lipids were extracted (20) and separated by thin layer chromatography. Initially, plates were developed halfway in a solvent system containing chloroform-acetone-methanol-acetic acid-water (60:80:20:20:10, v/v). After drying, chromatography continued in a second system of hexanes-diethyl ether-acetic acid (80:20:1, v/v). Lipid fractions were identified based on comigration with authentic lipid standards (Nu-chek Prep Inc., Waterville, MN; and Avanti Polar Lipids Inc., Alabaster, AL). Radioactivity was determined by scintillation counting.
TG and CE synthetic rates
Cells were cultured in growth media containing 200 µM unlabeled oleate/BSA for 48–72 h to initiate droplet formation. Cells were then washed with media and incubated for 0–4 h in the same media containing 200 µM 3H-oleate/BSA. Synthesis was stopped by removing the media and washing cells with cold PBS. Cells were kept on ice until released by trypsin. Cellular lipids were extracted (21) and separated by thin layer chromatography (hexanes-diethyl ether, 70:30, v/v). TG and CE bands were scraped and 3H quantified by scintillation counting.
To measure the transfer of newly synthesized TG to lipid droplets, cells were incubated as described above, washed, and then suspended in 500 µl cold hypotonic lysis buffer [10 mM Tris-HCl, pH 7.4, 1 mM EDTA, 10 mM sodium fluoride, 200 mM sucrose, and EDTA-free protease inhibitor cocktail (Roche Applied Science (Indianapolis, IN)] containing 300 µM diethylumberylferyl phosphate (Sigma-Aldrich Corp.). After 20 min, cells were homogenized by 10 strokes with a motor-driven Kontes pellet pestle (Sigma-Aldrich Corp.). Cell homogenates were centrifuged at 2,000 g for 10 min, and the resulting supernatant was centrifuged at 100,000 g for 1 h to yield a lipid droplet-rich fraction at the top and endoplasmic reticulum-enriched fraction at the bottom of the tube. Lipids in these fractions were extracted (21) and separated by thin layer chromatography as above.
TG hydrolysis
To determine the rate of TG turnover, cells were incubated in growth media supplemented with 200 µM 3H-oleate/BSA for 24 h to label the cellular TG pool. The cells were washed with warm media, then either harvested (t = 0) or incubated for the indicated times in media containing 100 µg/ml HDL and 0.1% BSA in the presence of 10 µM triacsin C (Sigma-Aldrich Corp.), a fatty acyl-CoA synthetase inhibitor. Cells were harvested, and lipids extracted (21) and fractionated by thin layer chromatography as described above.
Fatty acid uptake
Cells were incubated in media containing 200 µM 3H-oleate/BSA for the indicated time. At each time point, the cells were washed with cold PBS, released from the dish by trypsin, washed three additional times with cold PBS, and solubilized in 0.1 N NaOH. The 3H-content was determined by scintillation counting.
Lipid droplet staining
Cells were cultured on glass cover slips in 12-well plates. At 70% confluence, 100 µM oleate/BSA was added, and the cells were incubated for 24 h. Cells were fixed using 4% formaldehyde, then stained for 15 min with 200 ng/ml BODIPY 558/568 C12 in PBS. The coverslips were applied using 4’,6-diamidino-2-phenylindole (DAPI)-containing mounting solution. Cells were visualized by confocal microscopy, and lipid droplet size and number determined by ImagePro software (Media Cybernetics, Silver Spring, MD).
Cellular localization of CETP
CETP+ cells were incubated in growth media containing 100 µM oleate/BSA to induce lipid droplet formation. Lipid-loaded cells were fixed for 30 min with 4% formaldehyde, permeabilized for 30 min with 0.01% digitonin (EMD Chemical Inc., San Diego, CA), and then blocked with PBS containing 5% horse serum for 1 h at room temperature. Subsequently, cells were reacted with mouse anti-human CETP antibody (TP2, 1/200) and rabbit anti-human calnexin (1/200) (Santa Cruz Biotechnology Inc., Santa Cruz, CA) for 24 h.
For CETP detection, antigen-antibody complexes were reacted with biotin-conjugated goat anti-mouse IgG (1/1,000) for 1 h and then with Alexa Fluor 488 streptavidin (1/1,000) for 15 min. For calnexin detection, cells were incubated with Alexa Fluor 647 chicken-anti rabbit IgG (1/1,000) for 1 h. After immunostaining, the cells were incubated with PBS containing 200 ng/ml of BODIPY 558/568 C12 for 15 min to stain lipid droplets. All fluorescent reagents were from Life Technologies. The cell were washed three times with PBS, then mounted on microscope slide using DAPI-containing mounting solution. Confocal images were obtained using a Leica TCS-NT confocal laser-scanning microscope.
The cellular distribution of CETP was also determined by Western blot of cell fractions. Lipid-loaded cells were lysed in hypotonic media as described above. The homogenate was centrifuged for 10 min at 2,000 g at 4°C. Supernatant aliquots containing equal protein (22) were centrifuged at 100,000 g for 1 h to yield lipid droplet (top), cytosolic (remaining supernatant), and endoplasmic reticulum-containing (pellet) fractions. Fractions were adjusted to the same volume using lysis buffer, and equal volumes were loaded on 4–20% SDS-PAGE gels. Western blots for CETP (TP2 antibody) and calreticulin (Sigma-Aldrich Corp.) were performed as previously described (13).
Perilipin Western blots
Confluent control and CETP+ cells were grown in media with or without 200 µM oleate/BSA for 48 h. The cells were washed three times with PBS, harvested in hypotonic lysis buffer, and incubated on ice for 30 min with frequent vortexing. Cells were passed 10 times through a 26-gauge needle, then spun at 1,000 g for 10 min. Supernatants were collected, their protein content was determined (22), and aliquots containing equal protein were spun at 100,000 g for 1 h. The entire supernatant containing cytosol and lipid droplets was collected. Equal volumes of each sample were cold acetone precipitated and separated on 4–20% SDS-PAGE gel. The levels of perilipin 1, 2, and 3 were determined using 1/2,000 dilutions of rabbit anti-human antibodies against perilipin protein family (PLIN) 1 (Sigma), PLIN2, or PLIN3 (ThermoFisher Scientific, Middletown, VA) followed by horseradish peroxidase-conjugated secondary antibody.
Diacylglycerol acyltransferase activity
Cells were lysed by sonication and centrifuged at 25,000 × g as previously described (23). Microsomes were isolated from the resultant supernatant as the 100,000 × g pellet. Diacylglycerol acyltransferase (DGAT) activity was measured using [14C]oleoyl-CoA (Perkin-Elmer Life Sciences) and sn-1,2-dioleoylglycerol (Avanti Polar Lipids) as substrates. Assays were performed as described by Ganji et al. (23) using 20 µg microsomal protein and an incubation time of 30 min. DGAT assays were stopped by lipid extraction (21). TG in the extract was isolated by thin layer chromatography in a developing system of hexanes:diethyl ether:acetic acid (70:30:1), and its 14C content determined by scintillation counting.
Real-time quantitative PCR
Total RNA was extracted from control and CETP+ cells using RNeasy Mini Kit (Qiagen, Germantown, MD) following the manufacturer’s protocol. cDNA was generated using a High Capacity RNA-to-cDNA Kit, and real-time quantitative PCR (qPCR) analysis was performed using TaqMan gene expression primer/probe sets and TaqMan Universal Master Mix from Life Technologies. Ribosomal 18S, judged to be the best reference gene among five candidate genes tested, was used to normalize gene expression data. Gene expression was calculated using the 2−ΔΔCT method (24) and reported relative to control cells.
Analytical methods
Protein was quantified by a modification of the Lowry et al. method (22) with BSA as standard. Cholesterol was determined by an enzymatic kit (ThermoFisher Scientific). TG was quantified by a fluorometric, enzymatic kit from Abcam (Cambridge, MA). Each sample was assayed with and without the addition of lipase; values in the absence of lipase were used as the assay blank for that sample. Lipid phosphorus was quantified by the method of Bartlett (25). Statistical analysis was performed by unpaired t-test.
RESULTS
Perturbed TG storage in CETP-overexpressing cells
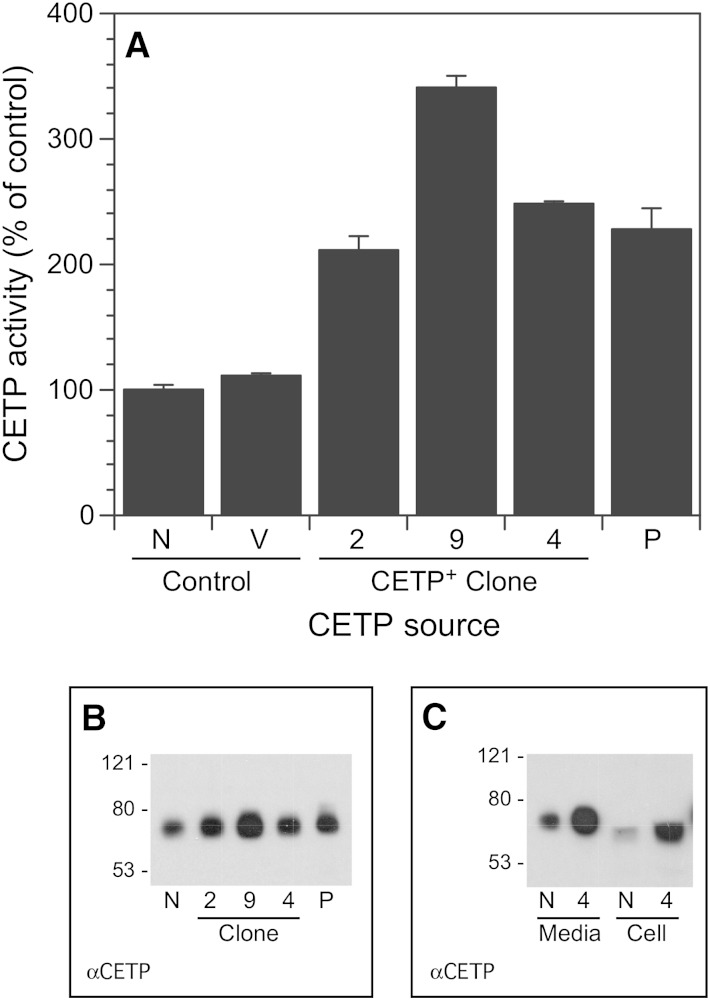
To examine the role of full-length CETP in cellular lipid metabolism, we created SW872 transfectants that stably overexpress this protein. Three clones secreting 2- to 3.4-fold higher CETP activity compared with control cells were isolated (Fig. 1A). Increases in media CETP activity closely mirrored CETP mass (Fig. 1B). The apparent molecular weight of CETP depends on its glycosylation status (6). CETP secreted by all cells was identical in molecular weight to CETP isolated from human plasma (Fig. 1B), indicating that CETP is properly synthesized even when overexpressed several fold. In addition to increased secretion of CETP, cells overexpressing CETP also have similar increments in intracellular CETP (Fig. 1C). Intracellular CETP had a slightly lower molecular weight, consistent with incomplete carbohydrate side-chain processing as previously reported (6).
Fig. 1.
CETP expression by CETP+ clones. A: CETP activity in media incubated with the indicated cells for 48 h. Lipid transfer activity in control cells was 19.7 ± 0.8%/ml (t = 16 h). B: CETP Western blot of the conditioned media assayed in A. In A and B, media aliquots were normalized to the number of cells per well. C: CETP Western blot of conditioned media and cell homogenates (25 µg protein). N, native SW872 cells; P, partially purified CETP from plasma; V, vector-transfected SW872 cells. Numerical designations refer to the CETP+ clone number.
Cells deficient in CETP have impaired TG storage capacity (14). To examine the impact of CETP overexpression on TG storage, cells were incubated with 200 µM 3H-oleate/BSA for 48 h. Surprisingly, all clones overexpressing CETP had reduced ability to accumulate TG. This was observed whether CETP+ clones were compared with a nonclonal population (pool) of vector control cells or individual control clones (Table 1). Similar reductions in TG accumulation were noted in all clones regardless of their extent of CETP overexpression.
TABLE 1.
Effect of CETP overexpression on cellular TG content
| Cell | TG (nmol/mg Protein) | % of Vector Pool |
| Control pool | 80.4 ± 9.5 | 100 |
| Control clone 1 | 85.8 ± 17.3 | 106.7 |
| Control clone 2 | 80.0 ± 2.0 | 99.5 |
| Control clone 3 | 86.5 ± 10.4 | 107.6 |
| CETP+ clone 2 | 47.2 ± 3.0 | 58.7 |
| CETP+ clone 4 | 48.9 ± 6.8 | 60.8 |
| CETP+ clone 9 | 55.3 ± 9.0 | 68.8 |
Vector-transfected SW872 cells (pool and individual clones) and three CETP+ clones were incubated with 200 µM 3H-oleate/BSA for 48 h to uniformly label the TG pool. TG was extracted, purified by thin layer chromatography, and quantified by scintillation counting. Values are mean ± SD, n = 3. The TG content of all CETP+ clones differed significantly from control (P < 0.05).
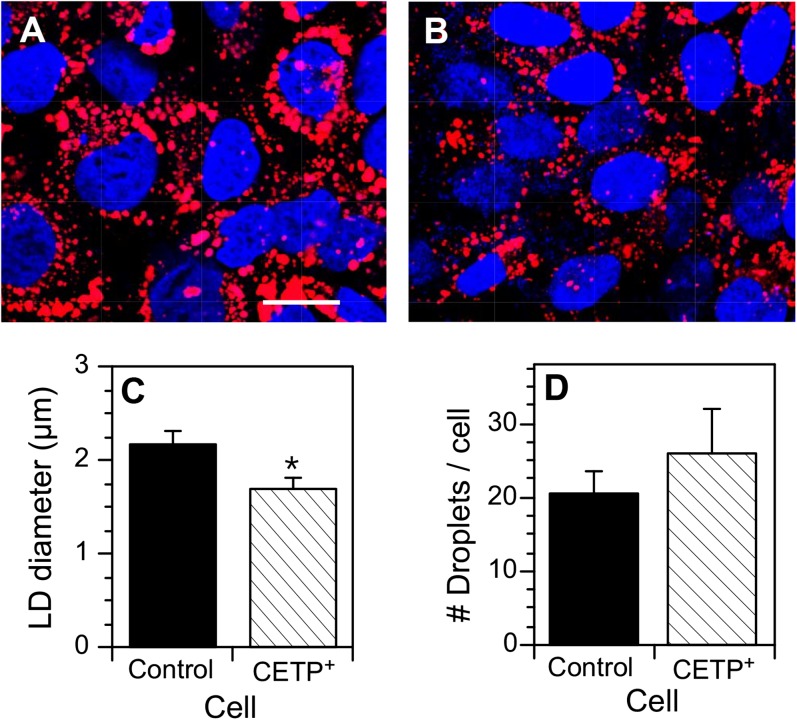
Clone 4 cells were selected for further study to investigate the basis for the decreased TG content. When incubated with oleate/BSA to induce lipid storage droplet formation, these CETP+ cells had an aberrant lipid droplet phenotype (Fig. 2A, B). Compared with control cells, lipid droplets in CETP+ cells were significantly smaller in size (Fig. 2C). CETP+ cells tended to contain more lipid droplets per cell, but this difference did not reach statistical significance (Fig. 2D). Assuming a spherical lipid droplet shape and considering the number of droplets per cell, the lipid droplet volume in CETP+ cells was 60% of that in vector control cells (66.0 ± 4.3 µm3/cell vs. 109.1 ± 7.5 µm3/cell, respectively). This compares very well to that determined by 3H-oleate incorporation (Table 1). By direct enzymatic assay of TG mass, CETP+ cells contained 48 ± 8% less TG than control. The agreement between 3H-oleate incorporation and enzymatic assay values on the magnitude of the TG decrease in CETP+ cells indicates that the 48 h labeling period is sufficient to reach isotopic equilibrium in the TG pool, just as we have previously reported for CE (13).
Fig. 2.
Lipid droplets in vector-transfected SW872 and CETP+ cells. Cells at 70% confluence were incubated with 100 µM oleate/BSA for 24 h to stimulate the development of lipid droplets. Cells were fixed, stained with BODIPY 558/568 C12 (lipid) and DAPI (nucleus), and viewed by confocal microscopy. The number and size of BODIPY 558/568 C12 stained lipid droplets were quantified by ImagePro. A: Vector-transfected SW872 cells. Bar = 10 µm. B: CETP+ cells. C: Quantification of lipid droplet (LD) diameter. D: Number of lipid droplets per cell. Data in C and D (mean ± SD) were derived from analyzing three fields of both cell types, each field containing 83–105 cells. * P < 0.01 versus control cells.
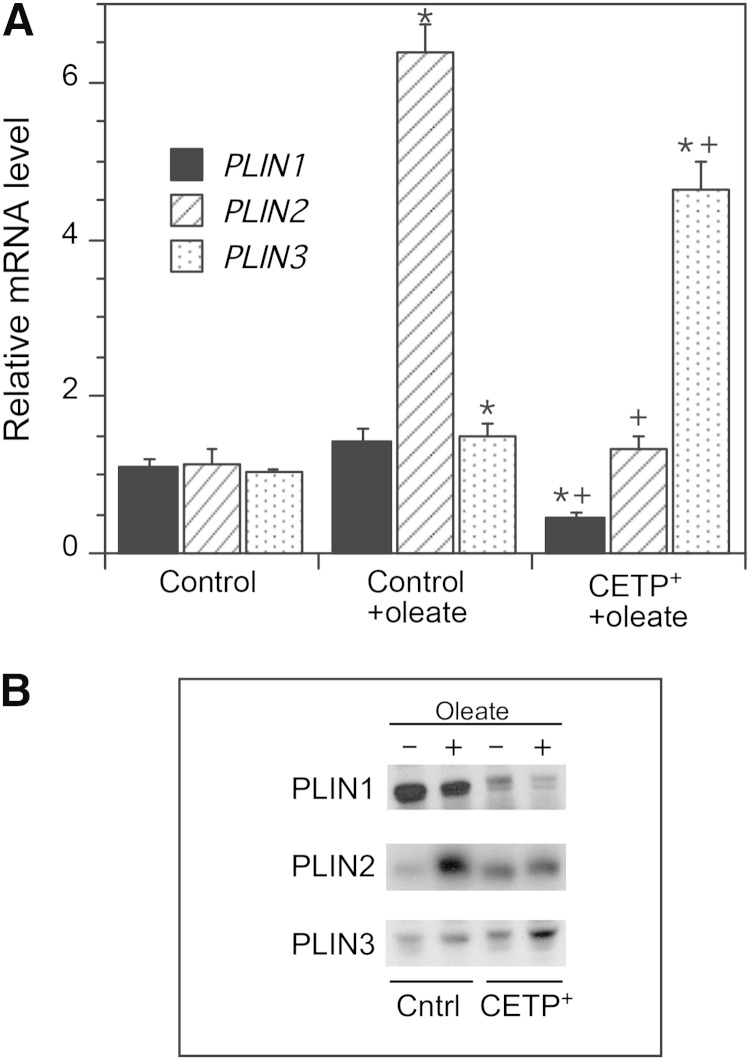
To determine whether the smaller lipid droplets in CETP+ cells were associated with changes in the expression of lipid droplet protein genes, we measured the mRNA levels of PLIN1, 2, and 3. Oleate treatment of control cells caused a small increase in PLIN1 and PLIN3 mRNA, but a 6-fold increase in PLIN2 expression (Fig. 3A). In contrast, in oleate-treated CETP+ cells PLIN1 and PLIN2 expression was much lower than that in oleate-treated control cells, but PLIN3 expression was increased more than 4-fold. These mRNA levels also correlate with the cellular levels of PLIN proteins. In the presence of oleate to stimulate lipid droplet formation, only PLIN2 increased significantly in control cells, whereas only PLIN3 was increased by oleate in CETP+ cells (Fig. 3B). Oleate-treated CETP+ cells contained markedly less PLIN1 and PLIN2 than oleate-treated control cells, but more PLIN3.
Fig. 3.
Perilipin gene expression and protein levels. A: Native SW872 and CETP+ cells were grown in 200 µM oleate for 48 h to induce lipid droplet formation. mRNA levels determined by qPCR are shown for PLIN1, PLIN2, and PLIN3. SW872 cells grown in the absence of added oleate were used as reference. Values are the mean ± SD of the values determined on RNA isolated from two to three separate cell cultures. * P < 0.05 versus control; + P < 0.05 versus oleate-treated control. B: Western blot of perilipins in the 100,000 g supernatant isolated from vector-transfected SW872 (Cntrl) and CETP+ cells. Cells were grown with or without oleate as described in A.
Differentiation status of CETP+ cells
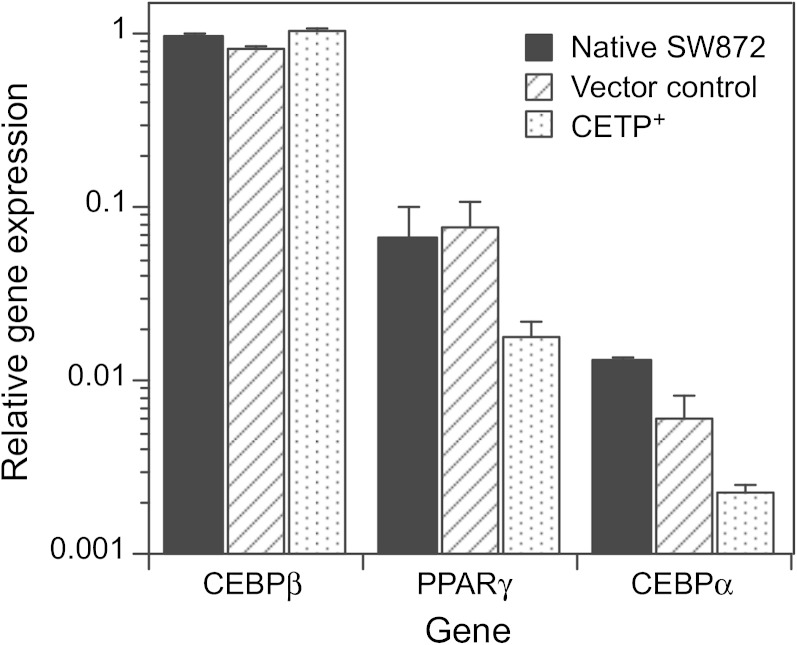
During adipogenesis, CCAAT/enhancer binding protein (CEBP)β, PPARγ, and CEBPα genes are expressed sequentially, leading to upregulation of pathways involved in TG synthesis and storage. The absolute levels of these transcription factors can vary >10-fold during differentiation, whereas the expression of these genes relative to each other is indicative of differentiation status (26, 27). We initially considered that the reduced TG content of CETP+ cells might occur because the differentiation status of these cells has been altered. Although we observed differences in the mRNA levels for some of genes, these differences were small compared with the natural variation of these genes during differentiation (Fig. 4). For native SW872, vector control, and CETP+ cells, the expression of these transcription factor genes followed the same order: CEBPβ > PPARγ > CEBPα. The expression of SREBF1, a master regulator of lipid synthesis, was unchanged, and the expression of PPARγ target genes such as PNPLA2, FASN, and APOE (28, 29) was not different in CETP+ cells (Table 2). SCL2A4 and FABP4 levels were reduced by about half. Overall, these data suggest that the differentiation status of CETP+ cells is very similar to that of control cells.
Fig. 4.
Expression of differentiation genes. Native SW872, vector-transfected SW872, and CETP+ cells were grown to 90% confluence and then harvested to determine the expression levels of genes associated with adipocyte differentiation. mRNA levels were determined by qPCR. Data are presented relative to the expression of CEBPβ in native SW872 cells.
TABLE 2.
Expression of adipocyte-related genes
| Gene | Control | CETP+ |
| SREBF1 | 1.08 ± 0.05 | 0.97 ± 0.29 |
| PNPLA2 | 1.06 ± 0.04 | 0.91 ± 0.06 |
| SCL2A4 | 1.16 ± 0.08 | 0.53 ± 0.34a |
| FABP4 | 1.01 ± 0.24 | 0.40 ± 0.15a |
| APOE | 1.00 ± 0.05 | 0.89 ± 0.21 |
| FASN | 1.13 ± 0.10 | 2.12 ± 1.10 |
The expression of the indicated genes was determined by qPCR. Data are the mean ± SD (n = 2–3 separate cell cultures). FABP4, fatty acid binding protein 4; PNPLA2, adipocyte triglyceride lipase; SCL2A4, glucose transporter type 4 (GLUT4); SREBF1, sterol regulatory element-binding protein 1.
P < 0.05 versus control.
TG metabolism in CETP+ cells
The reduced TG content of CETP+ cells is partly explained by reduced biosynthesis. TG synthetic rates were determined in cells incubated with 3H-oleate/BSA over a 4 h time course, during which TG synthesis was linear. In control cells, the TG synthetic rate was 15.8 ± 2.8 nmol/mg protein/h (n = 6). In CETP+ cells, this rate was 26% lower (11.6 ± 2.7 nmol/mg protein/h (n = 6), P = 0.03). We also determined whether CETP overexpression affected the movement of newly synthesized TG from the endoplasmic reticulum to lipid droplets during this 4 h period. For both cell types, after an initial 1 h lag, TG accumulation in lipid droplets was nearly linear (data not shown). There was no discernable difference between native control and CETP+ cells in this interorganelle transfer, showing that CETP overexpression did not appear to stimulate this process. In separate experiments, there was also no difference in the extent to which new TG was moved to lipid droplets in native SW872 versus vector-transfected SW872 cells (data not shown).
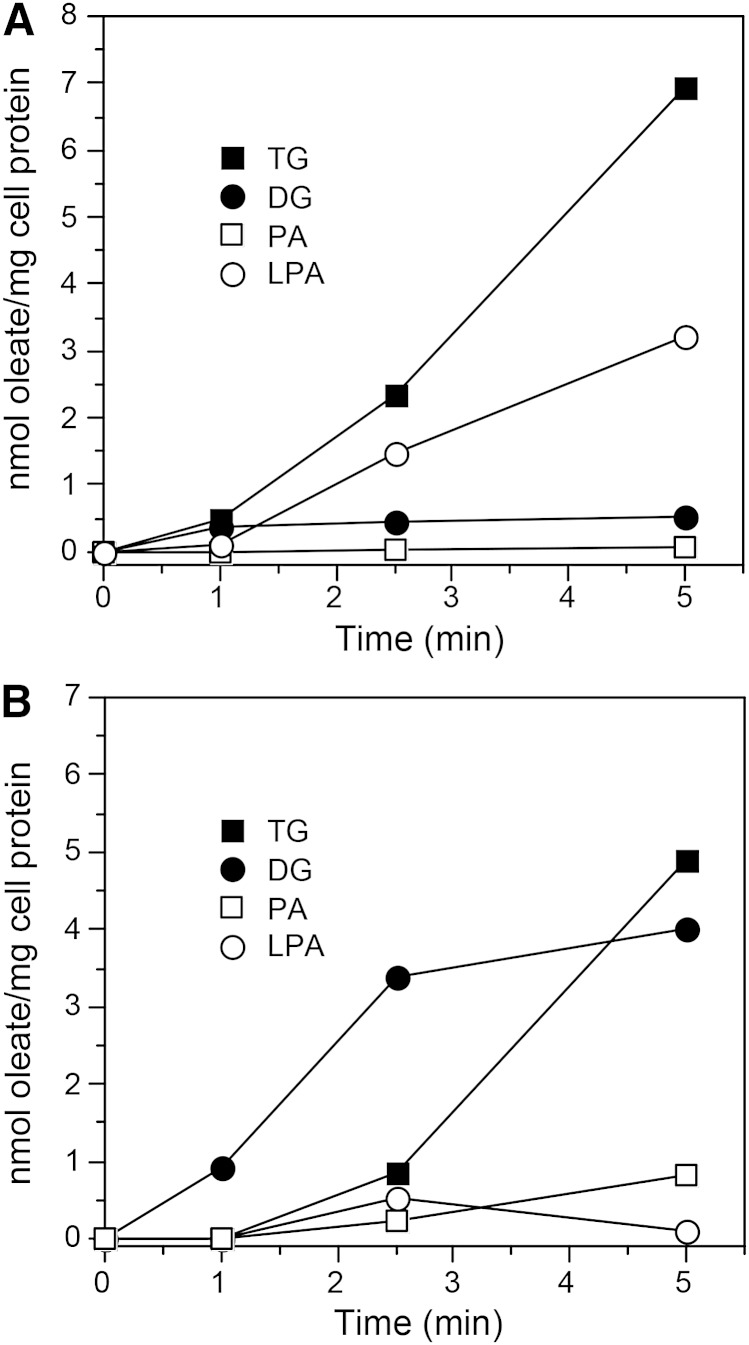
To investigate a mechanistic basis for the lower TG synthesis in CETP+ cells, we traced the initial movement of 3H-oleate through the TG biosynthetic pathway. In control cells, TG synthesis was nearly linear over the study time course (Fig. 5A). Diglyceride (DG) levels and phosphatidic acid levels were consistently low, although in some experiments DG levels were high at 1 min and declined quickly thereafter. Lysophosphatidic acid levels tended to increase over the time course, although in some experiments this increase was smaller than shown here. In contrast, in CETP+ cells the production of TG lagged initially and remained lower at the latest time point. On the other hand, DG levels were much higher than in control cells at all time points (Fig. 5B). In three experiments, at 5 min the DG/(DG + TG) ratio in CETP+ cells was 0.46 ± 0.05 compared with 0.17 ± 0.07 in native SW872 cells and 0.23 ± 0.09 in vector-transfected control cells.
Fig. 5.
Incorporation of 3H-oleate into TG and its precursors. Native SW872 (A) and CETP+ (B) cells were incubated with 200 µM 3H-oleate/BSA for the indicated times. Lipids were extracted, fractionated by thin layer chromatography, and 3H quantified by scintillation counting. PA, phosphatidic acid; LPA, lysophosphatidic acid. Results are representative of four experiments.
In addition to its conversion to TG, DG is also a substrate for neutral phospholipid synthesis. Oleate incorporation into the major membrane phospholipid, phosphatidylcholine, was 2-fold higher in CETP+ cells (0.59 ± 0.12 vs. 0.25 ± 0.07 nmol/mg protein at 5 min, n = 3, P = 0.014). Nonetheless, calculated from the sum of 3H-oleate incorporated into phosphatidylcholine, TG, and all TG precursors at 5 min, the flux of oleate through this biosynthetic pathway was diminished in cells overexpressing full-length CETP [8.31 ± 1.78 nmol/mg protein vs. 11.96 ± 0.40 nmol/mg protein in control cells (n = 3, P = 0.026)].
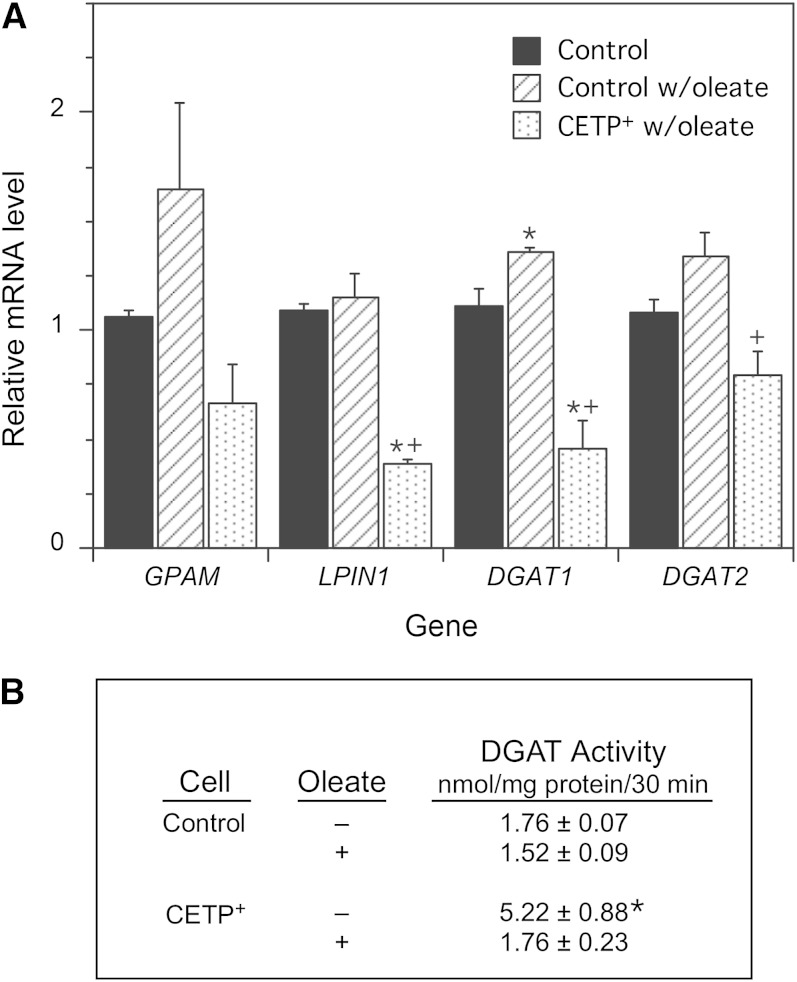
Expression levels of genes involved in the TG biosynthetic pathway were determined to better understand the cause of this aberrant TG synthesis. Incubation of control cells with oleate induced slight increases in the four genes examined, but only the DGAT1 increase reached statistical significance (Fig. 6A). In CETP+ cells, the levels of GPAM, whose product mediates the initial and rate-limiting step in TG biosynthesis, were reduced, but this did not quite reach statistical significance (P = 0.08). However, mRNA levels of DGAT2 were reduced by 40%, and LPIN1 and DGAT1 were decreased by 65% compared with oleate-treated control cells. LPIN1’s gene product, phosphatidate phosphohydrolase, produces DG, and DGAT 1 and 2 mediate the conversion of DG to TG. The impaired conversion of DG into TG and the reduced levels of DGAT1 and DGAT2 mRNA suggest that DGAT activity might be reduced in CETP+ cells. However, this was not the case. In the absence of oleate, DGAT activity in the microsomal fraction from CETP+ cells was increased >2-fold, but under conditions where TG deposition is stimulated (+oleate), DGAT activity in CETP+ cells was not different from control (Fig. 6B).
Fig. 6.
mRNA levels of TG biosynthetic pathway enzymes and DGAT activity. A: Control and CETP+ cells were incubated without or with 200 µM oleate for 48 h, then mRNA levels of GPAM (glycerol-3-phosphate acyltransferase 1, mitochondrial), LPIN1 (phosphatidate phosphatase), and DGAT1 and 2 were measured by qPCR. Data are expressed relative to the gene levels in SW872 cells incubated without oleate. Values are the mean ± SE of the values determined on RNA isolated from two to three separate cell cultures. * P < 0.05 versus control; + P < 0.05 versus oleate-treated control. B: DGAT activity in isolated microsomes. Data are the mean ± SD of values determined on two separate microsomal preparations for each cell type, each assayed in triplicate. * P < 0.05 versus all other values.
Fatty acid uptake
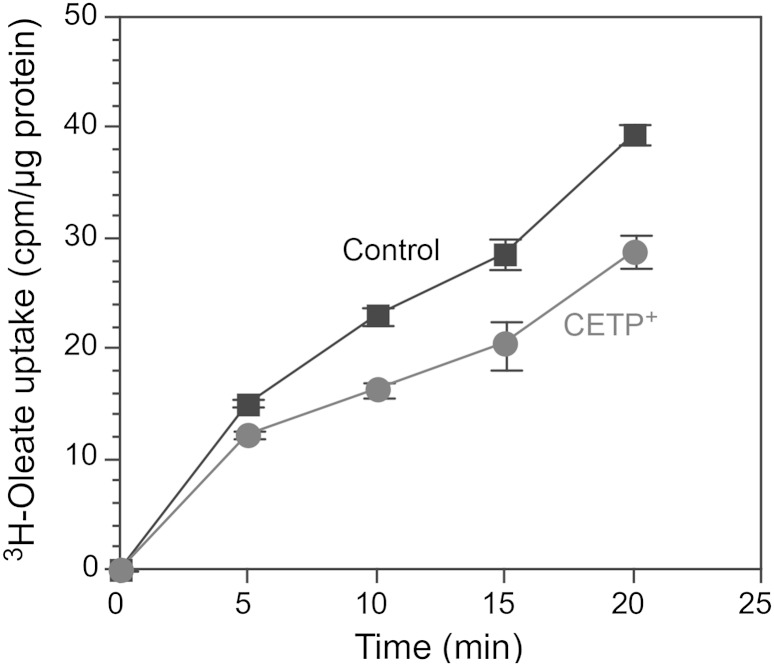
Another potential mechanism for the reduced TG content of CETP+ cells is impaired fatty acid uptake. When quantified, we found that oleate uptake by CETP+ cells was, on average, 24% lower than in control cells (Fig. 7). One mechanism for fatty acid uptake is through the CD36 scavenger receptor (30). Knockout of the CD36 gene markedly impairs the accumulation of TG in adipose (31). We observed that mRNA levels for CD36 were reduced by 60% in CETP+ cells (0.40 ± 0.20 vs. 1.12 ± 0.07, n = 3, P < 0.05). The incubation of control cells in oleate-containing media for 48 h to promote TG accumulation increased CD36 expression 2.8-fold (2.78 ± 0.51, P < 0.05). In contrast, CD36 expression in CETP+ cells was not responsive to oleate treatment (0.29 ± 0.06), causing CD36 expression in CETP+ to be about 10% of control values under conditions where TG synthesis is stimulated. Reduced fatty acid uptake could contribute to the decreased TG content of CETP+ cells.
Fig. 7.
Oleate uptake. Native SW872 and CETP+ cells were incubated with 200 µM 3H-oleate/BSA for the indicated time, and then the cellular content of 3H was determined. Values are the mean ± SD of triplicate determinations.
Cellular localization of CETP
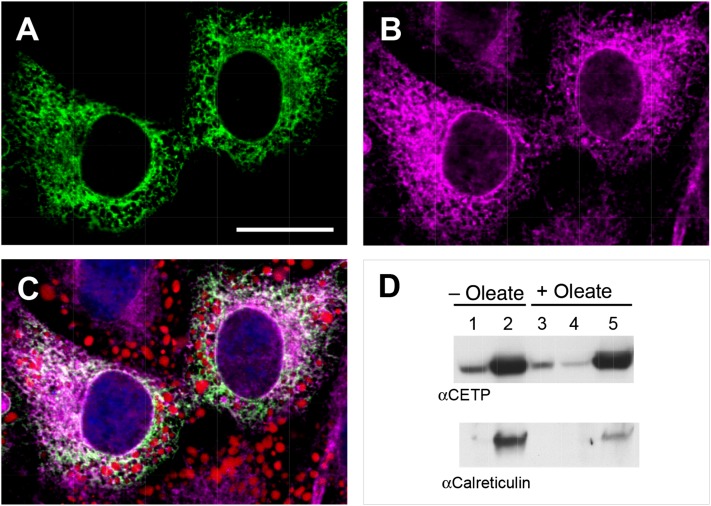
We previously suggested that one of the functions of intracellular CETP might be the interorganelle transfer of lipids, giving it a possible direct role in lipid droplet formation (14). Such a function requires that at least a portion of cellular CETP be in the cytoplasm where it can perform this transfer function, instead of being in the endoplasmic reticulum lumen, the expected location for a protein in the secretory pathway. We took advantage of the higher intracellular CETP levels in CETP+ cells to investigate the location of CETP in cells. Confocal imaging showed that CETP is widely distributed within cells (Fig. 8A) with a cellular distribution similar to that of the endoplasmic reticulum protein calnexin (Fig. 8B). While there is considerable colocalization of CETP with the endoplasmic reticulum, in peripheral areas where lipid droplets are abundant CETP immunofluorescence greatly exceeded that of the endoplasmic reticulum marker (Fig. 8C). This suggests that some CETP may not be associated with endoplasmic reticulum in this region.
Fig. 8.
Immunolocalization of CETP. CETP+ cells were grown in the presence of 100 µM oleate/BSA for 48 h to induce lipid droplet formation. A, B: Cells were fixed and immunostained for CETP (A) and calnexin (B). Bar = 20 µm. C: Merge of CETP (green), calnexin (magenta), and BODIPY lipid stain (red). Confocal microscopy images are shown. D: CETP+ cells grown without or with 500 µM oleate were lysed and cellular components fractionated by centrifugation into cytosol (lanes 1 and 4), endoplasmic reticulum-containing membrane (lanes 2 and 5), and lipid droplet (lane 3) fractions. CETP and calreticulin were detected by Western blot.
In plasma, almost all CETP is bound to lipoproteins (32, 33) even though during lipid transfer it carries lipids between lipoproteins (34, 35). This suggests that any CETP released from the endoplasmic reticulum into the cytoplasm might be membrane bound. Because lipoprotein-bound CETP is dissociated by centrifugation (36), we reasoned that CETP not contained within the endoplasmic reticulum lumen can be identified by cell fractionation studies. Lysed cells were centrifuged to yield an endoplasmic reticulum-containing membrane fraction, cytosol, and lipid droplets. Calreticulin, a soluble endoplasmic reticulum luminal protein, was used to verify the integrity of the endoplasmic reticulum. Regardless of oleate treatment, most CETP was associated with the endoplasmic reticulum membrane fraction (Fig. 8D). However, a subfraction of CETP was found in the cytosolic fraction under conditions where calreticulin was detected only in the membrane-rich fraction. Moreover, when cells were pretreated with oleate to stimulate lipid droplet formation, a small CETP pool was also found in the lipid droplet fraction. Collectively, these immunolocalization and cell fractionation studies show that most cellular CETP is associated with the endoplasmic reticulum, but a portion of intracellular CETP is in the cytoplasm and may associate with lipid droplets.
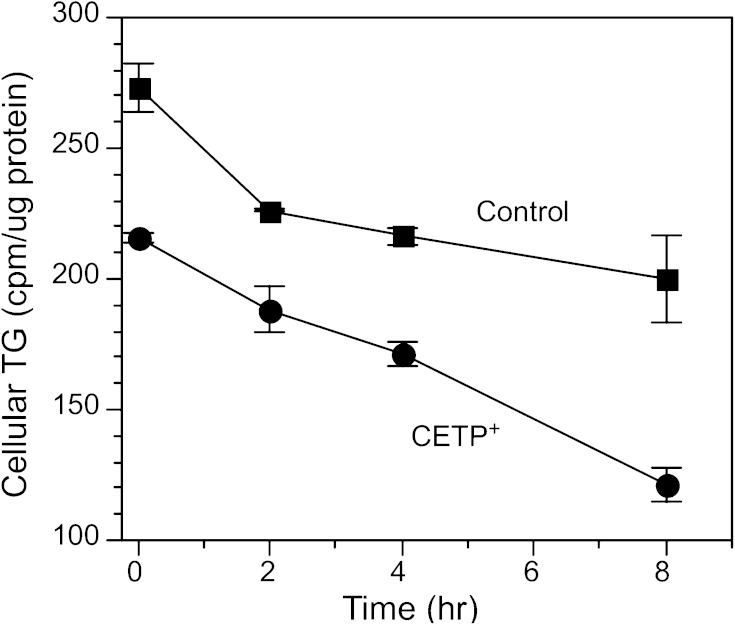
Turnover of TG in CETP-overexpressing cells
In CETP+ cells, the decrease in TG synthesis accounted for about half of the decline in TG content, suggesting that other mechanisms contribute to the reduced capacity of these cells to store TG. We assessed the role of TG hydrolysis in the reduced TG content of CETP+ cells by preincubating cells with 3H-oleate to label TG pools, then placing them in oleate-free chase media containing albumin and a fatty acyl-CoA synthetase inhibitor to prevent reincorporation of 3H-oleate once cleaved from TG. Although control cells had a more rapid initial loss of 3H-TG in the first 2 h, the loss of 3H from the TG pool was nearly linear for both cell types thereafter (Fig. 9). Over the 2–8 h period, 3H-TG hydrolysis in CETP+ cells was 2.7-fold higher than control cells (11.4 vs. 4.3 cpm/µg protein/h, respectively). Even including the initial 2 h period, CETP+ cells lost 44% of their TG pool over 8 h, whereas control cells hydrolyzed only 27%. These results show that the absolute and fractional hydrolysis rates of lipid droplet TG are elevated in cells overexpressing CETP.
Fig. 9.
TG hydrolysis. Native SW872 and CETP+ cells were incubated in 200 µM 3H-oleate/BSA for 24 h. After washing in oleate-free media, cells were incubated in media containing 100 µg/ml HDL, 0.01% BSA, and 10 µM Triacsin C. Values are the mean ± SD of triplicate determinations.
Effect of CETP overexpression on cholesterol metabolism
The reduced TG content of cells overexpressing CETP mirrors the decreased TG content of cells with low CETP expression (14). Because CETP-deficient cells also have significant alterations in cholesterol metabolism, we investigated the effect of CETP overexpression on CE levels and CE synthetic rates in oleate-treated cells. CETP+ cells had 31% lower CE content compared with control cells even though the CE synthetic rate was 50% higher (Table 3). This paradoxical observation is explained by the markedly elevated hydrolysis of the CE pool in CETP+ cells (Table 3). Thus, CETP+ cells have elevated CE synthesis but also increased degradation leading to reduced CE content. This differs from CETP-deficient cells, which have elevated CE content (13, 14), decreased CE synthesis [0.12 ± 0.05 nmol/mg protein/h (n = 4), P = 0.04 vs. control (Table 3)], and impaired CE hydrolysis (14).
TABLE 3.
Sterol metabolism in native and CETP+ cells
| Relative mRNA level | |||||
| Cell | CE content | CE synthesis | CE hydrolysis | HMGCR | ABCG1 |
| Control | 2.81 ± 0.41 | 0.22 ± 0.07 | 23.0 ± 1.0 | 1.07 ± 0.08 | 1.06 ± 0.07 |
| CETP+ | 1.93 ± 0.31a | 0.33 ± 0.03b | 59.1 ± 10.3b | 2.59 ± 0.35b | 0.01 ± 0.01b |
CE content was determined from the steady-state level of 3H-CE after 48 h incubation with 200 µM 3H-oleate/BSA. CE synthesis rates were determined from the slope of the 3H-CE accumulation curve over 4 h. To measure CE hydrolysis, after cells were incubated with 3H-oleate/BSA for 48 h, the decay in the 3H-CE pool was measured after oleate removal and the addition of HDL and BSA to the media. Gene expression for HMGCR (HMG-CoA reductase) and ABCG1 was measured by qPCR. Units are CE content, nmol/mg protein (n = 3); CE synthetic rate, nmol/mg protein/h (n = 6); CE hydrolysis, % decrease in CE pool over 8 h period (n = 2–3).
P < 0.05 versus control.
P < 0.01 versus control.
In addition to altering CE levels and the dynamics of this lipid pool, CETP overexpression also altered genes involved in sterol homeostasis. Compared with control cells, HMGCR mRNA levels were increased more than 2-fold in CETP+ cells (Table 3) but unchanged in CETP-deficient cells (0.90 ± 0.18 relative units). However, mRNA levels for the intracellular cholesterol transporter ABCG1 (37) were profoundly decreased in both CETP+ (Table 3) and CETP-deficient cells (0.14 ± 0.04 relative units). Together, these findings show that CETP overexpression induces a unique cholesterol phenotype.
DISCUSSION
We previously reported that chronic inhibition of both CETP isoforms in SW872 cells disrupted cholesterol homeostasis, increased CE content, and decreased TG content. These changes were associated with decreased hydrolysis and release of these lipids from lipid droplets under conditions stimulating efflux and the inefficient transport of CE and TG from the endoplasmic reticulum to lipid droplets (14). In vitro, we demonstrated that CETP can promote this interorganelle lipid movement, suggesting that the inefficient transport of CE and TG in CETP-deficient cells was directly related to reduced CETP levels.
To further understand the involvement of CETP in cellular lipid metabolism, we investigated the effects of overexpressing one of the two CETP isoforms present in cells. Stable cell clones synthesizing 2- to 3.4-fold more full-length CETP than control were produced. These elevated levels are within the range of enhanced CETP secretion that occurs when SW872 and Caco2 cells are treated with acetylated LDL (13) or when animals consume cholesterol-enriched diets (38). All three clones isolated were defective in their ability to store TG, accumulating ∼40% less TG regardless of CETP overexpression level. Lipid droplets that accumulated in cells overexpressing CETP were much smaller but as numerous as those in control cells. Reduced TG storage and smaller lipid droplets are also seen in vivo in the adipocytes from CETP transgenic mice expressing the transgene under control of an adipose tissue-specific promoter (15). Collectively, these data suggest that decreased TG content is a common phenotype induced when CETP expression is increased.
Our data suggest two likely causes for the reduced TG content of cells overexpressing CETP. First, the flux of fatty acid through the TG biosynthetic pathway is reduced. Although phospholipid synthesis was increased in CETP+ cells, which diverts DG for phospholipid instead of TG synthesis, this pathway is quantitatively small and, therefore, does not explain the reduction in TG synthesis. Further, CETP+ cells have impaired conversion of DG to TG. mRNA levels for the enzymes responsible for this conversion, DGAT1 and DGAT2, are decreased up to 60%; however, direct assay of total cellular DGAT activity did not reveal any decrease. CD36 is a plasma membrane receptor that binds multiple ligands including fatty acids (30, 39). Recent studies have suggested that CD36 does not directly transport fatty acids across the plasma membrane, but rather through signaling events or direct interaction with enzymes such as acyl-CoA synthetase it enhances fatty acid uptake and promotes TG synthesis (40). CD36 mRNA levels were reduced by 90% in CETP+ cells grown in oleate-containing media, suggesting that reduced fatty acid uptake may explain the reduced flux of fatty acids through the TG biosynthetic pathway. Indeed, we determined that oleate uptake by CETP+ cells was reduced by 24%. This value is nearly identical to the reduction in fatty acid flux though the TG synthetic pathway determined in short-term assays and to the TG synthetic rate measured in longer-term assays. Additionally, because reduced CD36 expression has been shown to impair the conversion of DG to TG (31), the very low expression of CD36 in CETP+ cells may explain the unusual DG kinetics observed in these cells. Overall, these data suggest that reduced fatty acid uptake, perhaps secondary to aberrant CD36 expression, contribute to the deficits noted in TG synthesis.
The second mechanism identified as causative in the low TG content of CETP+ cells is elevated catabolism of this lipid. The turnover of TG stored in lipid droplets was more than 2.5-fold higher in cells overexpressing CETP. It seems likely that this elevated breakdown of stored TG is related to changes in the nature of lipid droplets that accumulate in CETP+ cells. CETP+ cells synthesize normal numbers of lipid droplets; however, these droplets are significantly smaller in size. Quantification of PLIN mRNAs and direct assessment of perilipin protein levels in the 100,000 g supernatant of cells show that the small lipid droplet phenotype correlates with aberrantly high levels of PLIN3 and low PLIN 1 and PLIN2 levels. This PLIN protein profile is characteristic of immature lipid droplets (41). Higher turnover rates for small lipid droplets are commonly observed in other systems (42, 43). In this context, the elevated phosphatidylcholine synthesis observed in CETP+ cells may also contribute to the formation of small-sized lipid droplets in these cells. Phosphatidylcholine synthesis stabilizes the lipid droplet surface and decreases lipid droplet growth by fusion, resulting in smaller lipid droplets (44). Collectively, these results suggest that full-length CETP overexpression does not interfere with mechanisms involved in lipid droplet initiation, but it does interfere with their maturation to larger lipid-filled structures. Whether enhanced TG catabolism causes lipid droplets to be small, or whether TG catabolism is elevated because small lipid droplets are inherently more metabolically active remains to be determined.
Although both full-length CETP overexpression and the simultaneous suppression of full-length and exon 9-deleted CETP synthesis (14) cause cells to accumulate less TG, the effect of these manipulations on CE metabolism were different. Cell with reduced CETP expression contain high CE levels, which resulted from elevated CE synthesis and reduced CE hydrolysis (14). Conversely, we observed that cells overexpressing full-length CETP contain less CE. This reduction is largely due to a 2.5-fold increase in the catabolism of CE. Thus, the effects of CETP over- or underexpression on CE levels are opposite and largely explained by the different effects these two conditions have on CE hydrolysis rates.
Subsequent to synthesis, TG and CE must move from the endoplasmic reticulum to the lipid droplet for storage. Several mechanisms for this process have been proposed (43). According to a prevailing model, nascent lipid droplets form by budding of the cytoplasmic face of the endoplasmic reticulum membrane and then mature in the cytoplasm either by fusion of small droplets or by the transfer of lipids into existing nascent droplets (41, 45–48). The relative contribution of these two processes to lipid droplet growth is unknown, but there are instances where lipid transfer predominates (41, 42, 47). Multiple proteins appear to be involved in the recruitment of TG into lipid droplets in adipocytes, such as fat storage-inducing transmembrane 2 and fat specific protein 27 (49, 50). We propose in CETP-expressing species that CETP also participates in this process, and our previous studies in CETP-deficient cells support this hypothesis (14). Our findings here show that CETP overexpression did not enhance the rate of TG transfer to lipid droplets, suggesting that endogenous CETP levels are sufficient for this process to occur. We also found that intracellular CETP is distributed throughout the cell. Much of this CETP appears to associate with the endoplasmic reticulum, consistent with a previous observation that intracellular CETP is susceptible to endoglycosidase H digestion (6). However, a CETP pool not associated with the endoplasmic reticulum is present in the cellular region where lipid droplets accumulate. This may be the pool of CETP we determined to be cytosolic in cell fractionation studies. Although the mechanism for CETP release into the cytoplasm is unknown, the escape of other endoplasmic reticulum luminal proteins to the cytoplasm has been reported (51–53). Notably, just like CETP, several of these proteins bind to plasma lipoproteins. Perhaps their amphipathic nature is involved in the release mechanism.
CETP expression is upregulated early in adipocyte development (54, 55). Additionally, the relative abundance of full-length and exon 9-deleted CETP mRNAs is variable and tissue specific (6). These data suggest that the level of CETP and the ratio of its isoforms reflect specific cellular needs. The data reported here show that overexpression of full-length CETP in SW872 adipocyte-like cells produces a unique lipid metabolic phenotype where TG and CE levels are reduced and cells accumulate small lipid droplets. The reduction in TG results from at least two factors, reduced synthesis, most likely from impaired fatty acid uptake, and enhanced turnover of lipid droplet TG. We hypothesize that overexpressing full-length CETP interferes with normal processes that couple TG homeostasis with the maturation of nascent lipid droplets. As a result, these cells continue to produce nascent lipid droplets normally, but these droplets are underfilled due to underlying disruptions in TG synthesis and degradation. It is notable that both the overexpression of full-length CETP reported here and the suppression of both full-length and exon 9-deleted CETP isoforms (14) result in the same low TG phenotype. In contrast to CETP+ cells, the low TG content of CETP-deficient cells is caused by reduced TG synthesis and inefficient transport of newly synthesized TG out of the endoplasmic reticulum, which appears to lead to its premature degradation before incorporation into lipid droplets (D. J. Greene, L. Izem, and R. E. Morton, unpublished observations). It remains to be determined whether the low TG in these two cell models arises from variations in the absolute levels of full-length CETP per se or to a disruption in the cellular levels and the relative amounts of the two CETP isoforms. Overall, these data provide further evidence that endogenous CETP plays a fundamental role in cellular lipid homeostasis in cells naturally expressing this protein.
Note added in proof
During proof stage, the authors informed the Journal that an author, Katarzyna Bialkowska, was inadvertently omitted from the first published version of their manuscript.
Footnotes
Abbreviations:
- CE
- cholesteryl ester
- CETP
- cholesteryl ester transfer protein
- CETP+ cells
- cells overexpressing full-length CETP
- DG
- diglyceride
- DGAT
- diacylglycerol acyltransferase
- FABP4
- fatty acid binding protein 4
- HMGCR
- HMG-CoA reductase
- LPIN1
- phosphatidate phosphatase
- PLIN
- perilipin protein family
- PNPLA2
- adipocyte triglyceride lipase
- qPCR
- real-time quantitative PCR
- SCL2A4
- glucose transporter type 4 (GLUT4)
- SREBF1
- sterol regulatory element-binding protein 1
This work was supported in part by National Institutes of Health National Heart, Lung and Blood Institute Grant HL60934.
REFERENCES
- 1.Wirtz K. W. A. 1997. Phospholipid transfer proteins revisited. Biochem. J. 324: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soccio R. E., Adams R. M., Romanowski M. J., Sehayek E., Burley S. K., Breslow J. L. 2002. The cholesterol-regulated StarD4 gene encodes a StAR-related lipid transfer protein with two closely related homologues, StarD5 and StarD6. Proc. Natl. Acad. Sci. USA. 99: 6943–6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton R. E., Zilversmit D. B. 1983. Inter-relationship of lipids transferred by the lipid-transfer protein isolated from human lipoprotein-deficient plasma. J. Biol. Chem. 258: 11751–11757. [PubMed] [Google Scholar]
- 4.Tall A. 1995. Plasma lipid transfer proteins. Annu. Rev. Biochem. 64: 235–257. [DOI] [PubMed] [Google Scholar]
- 5.Barter P. J., Brewer H. B., Jr, Chapman M. J., Hennekens C. H., Rader D. J., Tall A. R. 2003. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23: 160–167. [DOI] [PubMed] [Google Scholar]
- 6.Inazu A., Quinet E. M., Wang S., Brown M. L., Stevenson S., Barr M. L., Moulin P., Tall A. R. 1992. Alternative splicing of the mRNA encoding the human cholesteryl ester transfer protein. Biochemistry. 31: 2352–2358. [DOI] [PubMed] [Google Scholar]
- 7.Quinet E., Yang T. P., Marinos C., Tall A. 1993. Inhibition of the cellular secretion of cholesteryl ester transfer protein by a variant protein formed by alternative splicing of mRNA. J. Biol. Chem. 268: 16891–16894. [PubMed] [Google Scholar]
- 8.Yang T. P., Agellon L. B., Walsh A., Breslow J. L., Tall A. R. 1996. Alternative splicing of the human cholesteryl ester transfer protein gene in transgenic mice. Exon exclusion modulates gene expression in response to dietary or developmental change. J. Biol. Chem. 271: 12603–12609. [DOI] [PubMed] [Google Scholar]
- 9.Gauthier A., Lau P., Zha X., Milne R., McPherson R. 2005. Cholesteryl ester transfer protein directly mediates selective uptake of high density lipoprotein cholesteryl esters by the liver. Arterioscler. Thromb. Vasc. Biol. 25: 2177–2184. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z., Yamashita S., Hirano K., Nakagawa-Toyama Y., Matsuyama A., Nishida M., Sakai N., Fukasawa M., Arai H., Miyagawa J., et al. 2001. Expression of cholesteryl ester transfer protein in human atherosclerotic lesions and its implication in reverse cholesterol transport. Atherosclerosis. 159: 67–75. [DOI] [PubMed] [Google Scholar]
- 11.Sawada S., Sugano M., Makino N., Okamoto H., Tsuchida K. 1999. Secretion of preβ HDL increases with the suppression of cholesteryl ester transfer protein in Hep G2 cells. Atherosclerosis. 146: 291–298. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z., Inazu A., Kawashiri M. A., Nohara A., Higashikata T., Mabuchi H. 2003. Dual effects on HDL metabolism by cholesteryl ester transfer protein inhibition in HepG2 cells. Am. J. Physiol. Endocrinol. Metab. 284: E1210–E1219. [DOI] [PubMed] [Google Scholar]
- 13.Izem L., Morton R. E. 2001. Cholesteryl ester transfer protein biosynthesis and cellular cholesterol homeostasis are tightly interconnected. J. Biol. Chem. 276: 26534–26541. [DOI] [PubMed] [Google Scholar]
- 14.Izem L., Morton R. E. 2007. Possible role for intracellular cholesteryl ester transfer protein in adipocyte lipid metabolism and storage. J. Biol. Chem. 282: 21856–21865. [DOI] [PubMed] [Google Scholar]
- 15.Zhou H., Li Z., Hojjati M. R., Jang D., Beyer T. P., Cao G., Tall A. R., Jiang X. C. 2006. Adipose tissue-specific CETP expression in mice: impact on plasma lipoprotein metabolism. J. Lipid Res. 47: 2011–2019. [DOI] [PubMed] [Google Scholar]
- 16.Salerno A. G., Silva T. R., Amaral M. E., Alberici L. C., Bonfleur M. L., Patricio P. R., Francesconi E. P., Grassi-Kassisse D. M., Vercesi A. M., Boschero A. C., et al. 2007. Overexpression of apolipoprotein CIII increases and CETP reverses diet-induced obesity in transgenic mice. Int. J. Obes. (Lond.). 31: 1586–1595. [DOI] [PubMed] [Google Scholar]
- 17.Terán-García M., Després J. P., Tremblay A., Bouchard C. 2008. Effects of cholesterol ester transfer protein (CETP) gene on adipocity in response to long-term overfeeding. Atherosclerosis. 196: 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clevidence B. A., Morton R. E., West G., Dusek D. M., Hoff H. F. 1984. Cholesterol esterification in macrophages: stimulation by lipoproteins containing apo B isolated from human aortas. Arteriosclerosis. 4: 196–207. [DOI] [PubMed] [Google Scholar]
- 19.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 21.Thompson J. N., Erdody P., Brien R., Murray T. K. 1971. Fluorometric determination of vitamin A in human blood and liver. Biochem. Med. 5: 67–89. [DOI] [PubMed] [Google Scholar]
- 22.Peterson G. L. 1977. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 83: 346–356. [DOI] [PubMed] [Google Scholar]
- 23.Ganji S. H., Tavintharan S., Zhu D., Xing Y., Kamanna V. S., Kashyap M. L. 2004. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J. Lipid Res. 45: 1835–1845. [DOI] [PubMed] [Google Scholar]
- 24.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta deltaC(T) method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 25.Bartlett G. R. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234: 466–468. [PubMed] [Google Scholar]
- 26.Wu Z., Xie Y., Morrison R. F., Bucher N. L. R., Farmer S. R. 1998. PPARγ induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPα during the conversion of 3T3 fibroblasts into adipocytes. J. Clin. Invest. 101: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J. B., Spiegelman B. M. 1996. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 10: 1096–1107. [DOI] [PubMed] [Google Scholar]
- 28.Rogue A., Spire C., Brun M., Claude N., Guillouzo A. 2010. Gene expression changes induced by PPAR gamma agonists in animals and human liver. PPAR Res. 2010: 325183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigamonti E., Chinnetti-Gbaguidi G., Staels B. 2008. Regulation of macrophage functions by PPAR-α, PPAR-γ and LXRs in mice and men. Arterioscler. Thromb. Vasc. Biol. 28: 1050–1059. [DOI] [PubMed] [Google Scholar]
- 30.Febbraio M., Hajjar D. P., Silverstein R. L. 2001. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J. Clin. Invest. 108: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coburn C. T., Knapp F. F., Jr, Febbraio M., Beets A. L., Silverstein R. L., Abumrad N. A. 2000. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 275: 32523–32529. [DOI] [PubMed] [Google Scholar]
- 32.Morton R. E. 1985. Binding of plasma-derived lipid transfer protein to lipoprotein substrates: the role of binding in the lipid transfer process. J. Biol. Chem. 260: 12593–12599. [PubMed] [Google Scholar]
- 33.Marcel Y. L., McPherson R., Hogue M., Czarnecka H., Zawadzki Z., Weech P. K., Whitlock M. E., Tall A. R., Milne R. W. 1990. Distribution and concentration of cholesteryl ester transfer protein in plasma of normolipemic subjects. J. Clin. Invest. 85: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu X., Mistry A., Ammirati M., Chrunyk B., Clark R., Cong Y., Culp J., Danley D., Freeman T., Geoghegan K., et al. 2007. Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat. Struct. Mol. Biol. 14: 106–113. [DOI] [PubMed] [Google Scholar]
- 35.Koivuniemi A., Vuorela T., Kovanen P. T., Vattulainen I., Hyvönen M. T. 2012. Lipid exchange mechanism of the cholesteryl ester transfer protein clarified by atomistic and coarse-grained simulations. PLoS Comput. Biol. 8: e1002299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morton R. E., Zilversmit D. B. 1982. Purification and characterization of lipid transfer protein(s) from human lipoprotein-deficient plasma. J. Lipid Res. 23: 1058–1067. [PubMed] [Google Scholar]
- 37.Tarling E. J., Edwards P. A. 2011. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc. Natl. Acad. Sci. USA. 108: 19719–19724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Son Y. S. C., Zilversmit D. B. 1986. Increased lipid transfer activities in hyperlipidemic rabbit plasma. Arteriosclerosis. 6: 345–351. [PubMed] [Google Scholar]
- 39.Febbraio M., Guy E., Coburn C., Knapp F. F., Jr, Beets A. L., Abumrad N. A., Silverstein R. L. 2002. The impact of overexpression and deficiency of fatty acid translocase (FAT)/CD36. Mol. Cell. Biochem. 239: 193–197. [PubMed] [Google Scholar]
- 40.Xu S., Jay A., Brunaldi K., Huang N., Hamilton J. A. 2013. CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry. 52: 7254–7261. [DOI] [PubMed] [Google Scholar]
- 41.Wolins N. E., Quaynor B. K., Skinner J. R., Schoenfish M. J., Tzekov A., Bickel P. E. 2005. S3-12, adipophilin, and TIP47 package lipid in adipocytes. J. Biol. Chem. 280: 19146–19155. [DOI] [PubMed] [Google Scholar]
- 42.Paar M., Jüngst C., Steiner N. A., Magnes C., Sinner F., Kolb D., Lass A., Zimmerman R., Zumbusch A., Kohlwein S. D., et al. 2012. Remodeling of lipid droplets during lipolysis and growth in adipocytes. J. Biol. Chem. 287: 11164–11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srivastava R. A. K. 2011. Evaluation of anti-atherosclerotic activities of PPAR-α, PPAR-γ, and LXR agonists in hyperlipidemic atherosclerosis-susceptible F1B hamsters. Atherosclerosis. 214: 86–93. [DOI] [PubMed] [Google Scholar]
- 44.Ohtani H., Hayashi K., Hirata Y., Dojo S., Nakashima K., Nishio E., Kurushima H., Saeki M., Kajiyama G. 1990. Effects of dietary cholesterol and fatty acids on plasma cholesterol level and hepatic lipoprotein metabolism. J. Lipid Res. 31: 1413–1422. [PubMed] [Google Scholar]
- 45.Murphy D. J., Vance J. 1999. Mechanisms of lipid-body formation. Trends Biochem. Sci. 24: 109–115. [DOI] [PubMed] [Google Scholar]
- 46.Ducharme N. A., Bickel P. E. 2008. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 149: 942–949. [DOI] [PubMed] [Google Scholar]
- 47.Kellner-Weibel G., McHendry-Rindle B., Haynes M. P., Adelman S. 2001. Evidence that newly synthesized esterified cholesterol is deposited in existing cytoplasmic lipid inclusions. J. Lipid Res. 42: 768–777. [PubMed] [Google Scholar]
- 48.Kuerschner L., Moessinger C., Thiel C. 2008. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic. 9: 338–352. [DOI] [PubMed] [Google Scholar]
- 49.Gong J., Sun Z., Wu L., Xu W., Schieber N., Xu D., Shui G., Yang H., Parton R. G., Li P. 2011. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol. 195: 953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gross D. A., Zhan C., Silver D. L. 2011. Direct binding of triglyceride to fat storage-inducing transmembrane proteins 1 and 2 is important for lipid droplet formation. Proc. Natl. Acad. Sci. USA. 108: 19581–19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quinn C. M., Kagedal K., Terman A., Stroikin U., Brunk U. T., Jessup W., Garner B. 2004. Induction of fibroblast apolipoprotein E expression during apoptosis, starvation-induced growth arrest and mitosis. Biochem. J. 378: 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shu X., Chan J., Ryan R. O., Forte T. M. 2007. Apolipoprotein A-V association with intracellular lipid droplets. J. Lipid Res. 48: 1445–1450. [DOI] [PubMed] [Google Scholar]
- 53.Lamant M., Smih F., Harmancey R., Philip-Cauderc P., Pathak A., Roncalli J., Galinier M., Collet X., Massabuau P., Senard J-M., et al. 2006. ApoO, a novel apolipoprotein, is an original glycoprotein up-regulated by diabetes in human heart. J. Biol. Chem. 281: 36289–36302. [DOI] [PubMed] [Google Scholar]
- 54.Radeau T., Lau P., Robb M., McDonnell M., Ailhaud G., McPherson R. 1995. Cholesteryl ester transfer protein (CETP) mRNA abundance in human adipose tissue: relationship to cell size and membrane cholesterol content. J. Lipid Res. 36: 2552–2561. [PubMed] [Google Scholar]
- 55.Radeau T., Robb M., McDonnell M., McPherson R. 1998. Preferential expression of cholesteryl ester transfer protein mRNA by stromal-vascular cells of human adipose tissue. Biochim. Biophys. Acta. 1392: 245–253. [DOI] [PubMed] [Google Scholar]