Abstract
Sphingomyelin synthase-related protein (SMSr) synthesizes the sphingomyelin analog ceramide phosphoethanolamine (CPE) in cells. Previous cell studies indicated that SMSr is involved in ceramide homeostasis and is crucial for cell function. To further examine SMSr function in vivo, we generated Smsr KO mice that were fertile and had no obvious phenotypic alterations. Quantitative MS analyses of plasma, liver, and macrophages from the KO mice revealed only marginal changes in CPE and ceramide as well as other sphingolipid levels. Because SMS2 also has CPE synthase activity, we prepared Smsr/Sms2 double KO mice. We found that CPE levels were not significantly changed in macrophages, suggesting that CPE levels are not exclusively dependent on SMSr and SMS2 activities. We then measured CPE levels in Sms1 KO mice and found that Sms1 deficiency also reduced plasma CPE levels. Importantly, we found that expression of Sms1 or Sms2 in SF9 insect cells significantly increased not only SM but also CPE formation, indicating that SMS1 also has CPE synthase activity. Moreover, we measured CPE synthase Km and Vmax for SMS1, SMS2, and SMSr using different NBD ceramides. Our study reveals that all mouse SMS family members (SMSr, SMS1, and SMS2) have CPE synthase activity. However, neither CPE nor SMSr appears to be a critical regulator of ceramide levels in vivo.
Keywords: sphingomyelin synthase-related protein, sphingomyelin synthase 1, sphingomyelin synthase 2, mouse gene knockout
Ceramide is synthesized vectorially in cells. The initial step in de novo ceramide synthesis is condensation of serine and fatty acyl CoA to generate ceramide in the endoplasmic reticulum (ER) (1), which is then transported into the Golgi by ceramide transport protein (CERT) (2). Ceramide is then further metabolized to yield SM by sphingomyelin synthase 1 (SMS1) (3, 4) or glucosylceramide by glucosylceramide synthase (5) and then into monosialodihexosylgangliosides. SM in the Golgi is transported to the plasma membrane, where it forms lipid rafts together with cholesterol and glycosphingolipids (6). SMS2 contributes mainly to plasma membrane SMS activity (3, 4). We and others have shown that SMS1 and SMS2 expression positively correlates with cellular and membrane lipid raft SM levels (7–9).
SMS-related protein (SMSr), the third member of the SMS family, is conserved throughout the animal kingdom (3, 10). All three members share a similar membrane topology, with six transmembrane domains and cytoplasmic N and C termini (3, 10). Catalytic activity is based on a conserved triad of two histidines and one aspartate residue (3, 10, 11). Unlike SMS1 and SMS2, however, SMSr does not have SMS activity but instead catalyzes synthesis of trace amounts of the SM analog ceramide phosphoethanolamine (CPE) in the ER (12). SMS2 shows dual activity because it is also capable of synthesizing CPE (13).
Vacaru et al. (12) reported that siRNA-mediated knockdown of SMSr in cultured HeLa and Drosophila S2 cells can lead to a significant increase in ER ceramide levels and to a collapse of the early secretory pathway. Thus, they hypothesized that SMSr regulates ceramide synthesis. The same group recently reported that abnormal ceramide accumulation can lead to mislocalization of ceramide to mitochondria, triggering the mitochondrial apoptosis pathway, suggesting that SMSr might be a suppressor of apoptosis (14). However, it has been reported that siRNA depletion of Smsr in multiple cell lines was not observed to alter sphingolipid biosynthesis (15).
In the current study, we generated Smsr KO mice to investigate SMSr function in vivo. We were particularly interested in the impact of SMSr deficiency on plasma sphingolipids, which are closely related to the development of atherosclerosis (16–18). We also focused on the effect of Smsr deficiency on the liver because the tissue is a central organ for regulating whole-body sphingolipid metabolism and expresses relatively high levels of Smsr (observed in this study). Moreover, we were interested in the impact of Smsr deficiency in macrophages, which play an important role in lipid homeostasis.
MATERIALS AND METHODS
Smsr KO and Smsr/Sms2 double KO mouse preparation
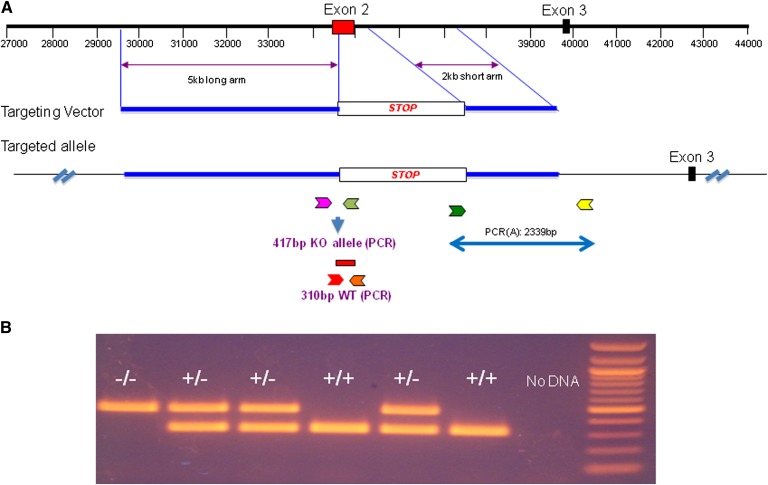
Mouse Smsr, also known as sterile α motif containing protein 8, is 48 kb in length and has six exons, with the translation start codon ATG in the first exon and the translation stop codon TGA in the sixth exon. We replaced the second exon with a STOP cassette from the pBIGT plasmid (Addgene; see Fig. 2A), which introduces a splicing acceptor, a PGK-NEO cassette, and a tri-phase translation stop codon, thus preventing any possible translation beyond this point. A 2,949-bp STOP cassette was inserted between nucleotides 34,351 and 35,063 to replace exon 2 and to generate the null Smsr allele. A gene-targeting vector was constructed that contained a 5-kb homology arm (5′ of the STOP cassette), the STOP cassette, and a 2-kb short homology arm (3′ of STOP cassette). The STOP cassette confers G418 resistance during gene targeting in PTL1 (129B6 hybrid) mouse embryonic stem cells. We crossed Smsr KO mice with our Sms2 KO (19) to obtain double KO mice.
Fig. 2.
Smsr KO mouse preparation. A: Strategy for generating Smsr KO in mice. Exon 2 of Smsr was replaced with a STOP cassette to generate the null Smsr allele, which was then introduced into mice with a gene-targeting vector. The small color arrows indicate the PCR primers for genotyping. B: PCR confirmation of the KO (417 bp) and WT (310 bp) alleles in genomic DNA from the mice with the primers indicated in A. Homozygous Smsr KO (−/−), heterozygous Smsr KO (+/−), and WT (+/+) mice were identified. The negative control lacked DNA, and a 100 bp ladder was used as the size markers.
Liver, macrophages, and kidney CPE synthase assays
Liver, macrophage, and kidney samples from 10-week-old mice were homogenized in buffer (20 mM HEPES-KOH [pH 7.2], 15 mM KCl, 5 mM NaCl, and 250 mM sucrose) containing a protease inhibitor cocktail (Roche Diagnostics). Protein concentrations of the homogenates were determined using the BCA assay kit (Pierce). An aliquot of the homogenate (100 μg protein) was incubated with [14C]C6-ceramide (0.1 μCi) (American Radiolabeled Chemicals), phosphoethanolamine (1 mg) (Sigma-Aldrich), and the buffer (final volume 1 ml) at 37°C with continuous shaking for 2 h. The reaction was stopped by addition of 1 ml methanol and 0.5 ml chloroform and vigorous vortexing. The lipids were then extracted and dried. For analysis, the dried lipid film was redissolved in a 25 μl of chloroform:methanol (2:1 v/v) and applied to a TLC plate (silica gel) (Whatman). The [14C]ceramide and [14C]CPE were separated using a basic eluent [chloroform:methanol:ammonia (aqueous), 50:25:6 v/v/v]. After thoroughly drying the TLC plate, 14C-lipid species were visualized on a PhosphorImager (GE Healthcare), and images were contrast adjusted using the Imagequant software and then exported to tiff format.
CPE synthase and sphingolyelin synthase assays of transfected SF9 cells
The nucleotide sequences encoding full-length SMS1 and SMS2 were inserted into the pFastBac1 vector (Invitrogen) with a C-terminal Flag tag. Recombinant baculovirus shuttle vectors were created by transforming DH10Bac cells and isolating DNA from white colonies with the Bac-to-Bac Baculovirus Expression System (Invitrogen). SF9 cells were transfected with the constructs in 6-well plates at 0.9 × 106 cells/well using CellFectin (Invitrogen). Passage 0 viruses were collected at 72 h post-transfection and used to infect SF9 cells in suspension at 100 μl of virus per 50 ml of cells at 1.5 × 106 cells/ml. Passage 1 viruses were collected after 96 h. For protein production, 1 liter of 1.5 × 106 SF9 cells/ml was infected with 5 ml of passage 1 virus, and the cells were harvested after 48 h. The SMS1 and SMS2 SF9 cell pellets were resuspended in ice-cold buffer containing 20 mM Tris-HCl (pH 7.5), 250 mM sucrose, 1 mM EDTA, and a protease inhibitor cocktail (Roche Diagnostics) and were then homogenized three times on ice for 30 s. Each resulting lysate was centrifuged for 10 min at 1,000 g at 4°C, and the supernatant was transferred to a new tube and centrifuged again at 100,000 g for 1 h. The pellets, representing the membrane fraction, were resuspended in the same buffer and frozen at −80°C until use. Protein concentrations were determined with a BCA Protein Assay kit (Pierce) using BSA as a standard.
CPE and SM synthase assay reactions (total volume of 100 μl) contained 50 mM Tris-HCl (pH 7.4), 25 mM KCl, 0.5 mM EDTA, 20 µM C8-ceramide, 20 μM dioleoyl phosphocholine, and 20 μM dioleoyl phosphatidylethanolamine or 20 μM dioleoyl phosphatidylcholine. Reactions were initiated by the addition of the membrane proteins (5 μg), incubated for 2 h at room temperature, and stopped by adding 100 μl of 1 M HCl in methanol. We then added 25 μl of chloroform, followed by vigorous mixing and centrifugation. The lower layer was then sampled for LC/MS analysis. Conditions for HPLC (Model 1100; Agilent Technologies) were as follows: 2.1 × 30 mm C18 XBridge column; mobile phase A, 55:5:40 methanol:chloroform:water + 5 mM ammonium acetate; mobile phase B, 57:29:14 isopropanol:methanol:chloroform + 5 mM ammonium acetate; flow rate 800 μl/min; 100% A for 0.3 min to 100% B at 1 min, hold until 2 min, return to 100% A at 2.1 min, and reequilibrate until 4 min. MS was carried out with an ABSciex 4000 using positive turbo ion spray.
SMS1, SMS2, and SMSr overexpressed Hela cell preparation
Hela cells were purchased from the ATCC and maintained in growth medium (DMEM with 10% FBS) at 37°C in 5% CO2. Hela cells overexpressing SMS1, SMS2, or SMSr were established by transfection with pcDNA3.1 vector containing SMS1, SMS2, and SMSr cDNA, respectively, and maintained in medium with G418 (final concentration, 500 μg/ml) for every generation thereafter. RNAi was used to attenuate the activity of other two SMS isoforms when determining the Km and Vmax of one isoform. The RNAi target sequence for human SMS1 was 5′-AACTACACTCCCAGTACCTGG-3′ and SMSr 5′-CAAGAAGCTGGAATTTCTTGC-3′. The SMS2 siRNA were from Qiagen Cat#SI02758182.
Cells overexpressing one SMS isoform were subcultured in 10 cm dishes, and siRNAs for the other two isoform were transfected when cells were at 60% confluence. Three days after siRNA treatment, cells were washed with PBS three times and suspended in CPE synthase activity assay buffer [0.3 M sucrose, 15 mM KCl, 5 mM NaCl, 1 mM EDTA, 20 mM HEPES-KOH (pH 7.0), 100 μg/ml phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, and 1 μM leupeptin] after scraping. Cells were homogenated by glass bead homogenizer and centrifuged at 700 g for 10 min. The postnuclear supernatants were collected as the source of crude enzyme for determination of the Km and Vmax.
Km and Vmax determination
C6 NBD-Ceramide and C12 NBD-Ceramide (Avanti Polar Lipids, Alabaster, AL) were dissolved in DMSO as 1 mmol/l. PE (Cat#P7943) (Sigma-Aldrich, St. Louis, MO) was dissolved in DMSO as 10 mg/ml. For every CPE synthase assay, 5 μl PE was first added to a 0.7 ml reaction buffer containing 3.5 mg crude enzyme and mixed gently at room temperature for 10 min. The reaction was started by adding C6 NBD-Ceramide or C12 NBD-Ceramide to the system as a final concentration of 5, 7.5, 10, 15, and 20 μM, respectively. The reaction system was mixed thoroughly and kept at 37°C for 2 h. Chloroform/methanol (2/1; 0.7 ml) was added into the system, and the system was mixed vigorously to stop the reaction before lipid extraction. Organic-phase cells, after centrifugation at 9,000 g for 10 min, were collected and dried under N2 blow. The extracted lipids were separated by TLC with the solvent chloroform-methanol-25% ammonium (50/25/6). Every NBD-CPE band on silica gel was scraped off the plate and dissolved in 200 μl isopropanol by vortex. After brief centrifugation to quantify the NBD fluorescence intensity by a spectrometer BioTek Flx800, 150 μl isopropanol of NBD-CPE was removed. The pmol of NBD-CPE was calculated by an equation of NBD standard curve. The velocity of the reaction was expressed as pmol/mg/min. The [S]V curve the and 1/[S]1/V curve (Lineweaver Burk’s plot) were charted with Excel. Km and Vmax were calculated by Lineweaver Burk’s plot.
Lipid and lipoprotein measurements
Fasting plasma was collected for FPLC analysis and lipid measurements. Total cholesterol, phospholipids, and TGs and lipoproteins were assayed by enzymatic methods (Wako Pure Chemical Industries Ltd.). Plasma SM was measured as described (20).
Apolipoprotein measurement
Plasma apoE, apoB, and apoA-I levels were determined as described (19). Briefly, 0.2 µl of plasma was separated by SDS-PAGE (4–15% gradient polyacrylamide gels) and immunoblotted with polyclonal antibodies against apoE (Abcam), apoB (US Biological), and apoA-I (Abcam).
Lipid analysis
Sphingolipid analysis.
LC/ESI/MS/MS analysis of sphingolipids was performed using a TSQ Quantum Ultra-Triple quadrupole mass spectrometer (Thermo Fisher) equipped with an electrospray ionization probe and interfaced with the Agilent 1100 HPLC system. Lipid extracts were separated with an Xbridge C8 column (Waters). Mobile phase A was methanol-water-formic acid (80:20:0.4, v/v/v), and mobile phase B was methanol-acetonirtile (1:1, v/v). ESI/MS/MS was performed or MS analyses were performed online using ESI/MS/MS in the positive multiple reaction monitoring mode. Samples were extracted using a one-phase extraction method (methanol–chloroform) with internal standards. Ceramides, sphingosines, sphingosine-1-phosphate, and dihydrosphingosine-1-phosphate were quantified as the ratio of analyte to internal standard, and calibration curves were obtained by assaying serial dilutions of a mixture of sphingolipids.
CPE and SM analysis
For analyses of CPE and SM, 100 μl or 1 mg of the cell extracts were mixed with 1.5 ml of methanol. Then 100 μl of chloroform-methanol (9:1 v/v) containing 15:0-SM (1 nmol) and 17:0-CPE (0.25 nmol) internal standards and 0.5 ml of 0.3 M NaOH were added, and the samples were incubated at 37°C for 2 h. The solution was neutralized by the addition of 0.3 ml of 0.3 M HCl in water, and 2 ml of chloroform and 1 ml of water were added, followed by vigorous vortexing for 5 min. After centrifugation for 10 min at 3,000 rpm, the upper phase was removed, and the lower chloroform phase was washed twice with 2 ml of theoretical Folch upper phase. After drying under nitrogen gas, the lipids were dissolved in 0.2 ml LC-MS quality methanol, and 10 μl was injected into the LC/MS system.
The CPE and SM species were quantified using LC/MS/MS. The chromatographic separation was carried out in the gradient mode using an Agilent 1100 HPLC system equipped with an Xbridge-C18 column (Waters). Analysis and quantification of CPE and SM were performed as reported previously with modification in concentration adjustment (21). To detect the CPE and SM, the column eluent was infused into the ESI source of the API 4000 Q-trap triple-quadrupole mass spectrometer operated in the positive ion mode using multiple reaction monitoring mode. The peak areas of the SM and CPE species and standards were obtained from the multiple reaction monitoring chromatograms with Analyst software (AB Sciex, Framingham, MA).
Tissue staining
Selected tissues were dissected from mice and put into 4% paraformaldehyde for fixation overnight. Tissues were paraffin embedded and sectioned (5 µm thick). Each section was deparaffinized and stained with hematoxylin and eosin.
Statistical analysis
Each experiment was conducted at least three times. Data are typically expressed as mean ± SD. Differences between two groups were analyzed by Student’s t-test. A P value of <0.05 was considered significant.
RESULTS
Mouse SMSr is also a CPE synthase
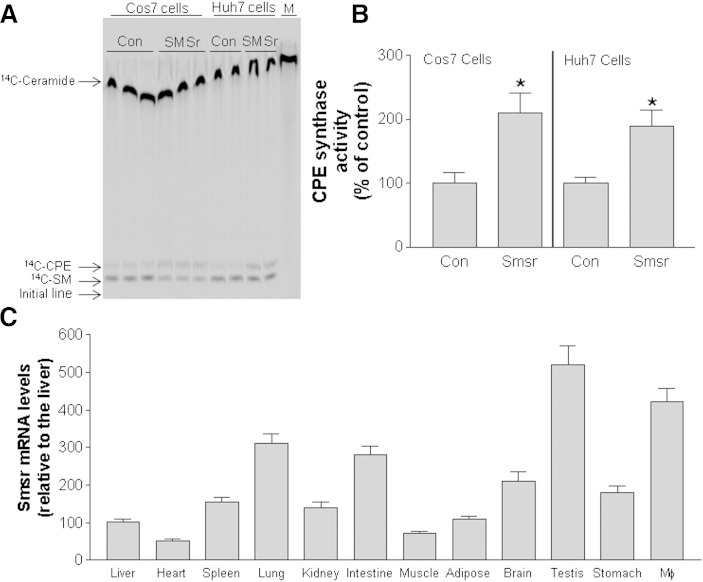
Previous studies indicated that human SMSr has CPE synthase activity (12). We transfected COS7 (a fibroblast-like cell line derived from monkey kidney tissue) and Huh7 (a human hepatoma cell line) cells with a mouse Smsr expression vector and measured CPE synthase activity in cell homogenates. Overexpression of mouse Smsr in both cell lines increased CPE synthase activity, indicating that mouse SMSr also has CPE synthase activity (Fig. 1A, B). We next measured mRNA levels using real-time PCR in 12 mouse tissues and found that mouse Smsr is ubiquitously expressed; the highest Smsr expression was seen in macrophages and testis (Fig. 1C).
Fig. 1.
Mouse SMSr has CPE synthase activity. We overexpressed Smsr in COS7 and Huh7 cells and measured CPE synthase activity in the cell homogenates. A: 14C-lipid separation on a TLC plate. Con, control; M, molecular markers. B: Quantitation of 14C-lipids in control (Con) and Smsr-overexpressing cells. Values are the mean ± SD of three experiments. *P < 0.01. C: Relative Smsr expression in different tissues determined by real-time PCR. Values are the mean ± SD of three mice. *P < 0.01.
Generation of Smsr KO mice
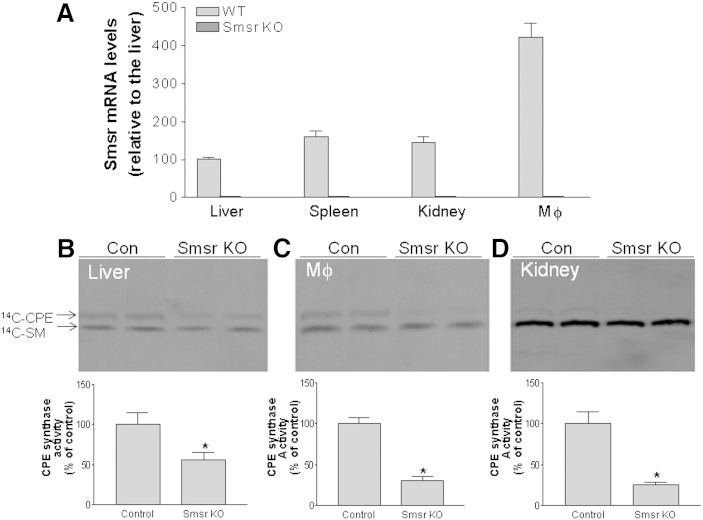
Mouse embryonic stem cells carrying the KO vector (Fig. 2A) were identified and injected into C57BL/6 blastocysts to generate chimeric mice. Male chimeras were bred with C57BL/6 female mice to transmit the Smsr-null allele through the germ line. Mice carrying the heterozygous Smsr-null allele were intercrossed to generate homozygous Smsr KO mice. The progeny were bred to reach a genetic background of at least 92.5% C57BL/6. Offspring from matings with heterozygous mice were born at the expected Mendelian ratio, indicating that SMSr is not essential during embryogenesis. Homozygous Smsr KO mice were viable and fertile. The different genotypes were confirmed by PCR using tail tip DNA as a template (Fig. 2B). We also used real-time PCR to confirm the lack of Smsr mRNA in several tissues, including liver, spleen, kidney, and macrophages (Fig. 3A).
Fig. 3.
Effects of Smsr deficiency on tissue CPE synthase activity. A: Real-time PCR of Smsr mRNA in liver, spleen, kidney, and macrophages from Smsr KO and WT mice. B: CPE synthase activity of liver homogenates. Separation of 14C-CPE on a TLC plate (top) and quantitation of 14C-CPE (bottom). C: CPE synthase activity of macrophage (MΦ) homogenates. Separation of 14C-CPE on a TLC plate (top) and quantitation of 14C-CPE (bottom). D: CPE synthase activity of kidney homogenates. Separation of 14C-CPE on a TLC plate (top) and quantitation of 14C-CPE (bottom). Values are mean ± SD of five mice. *P < 0.001.
To evaluate whether SMSr is indeed a CPE synthase in vivo, we measured CPE synthase activity in liver, macrophage, and kidney from Smsr KO mice and from control mice. Smsr deficiency in mice significantly decreased CPE synthase activity in the liver (45%) (Fig. 3B), macrophage (69%) (Fig. 3C), and kidney (80%) (Fig. 3D), indicating, again, that SMSr is also a CPE synthase in mice.
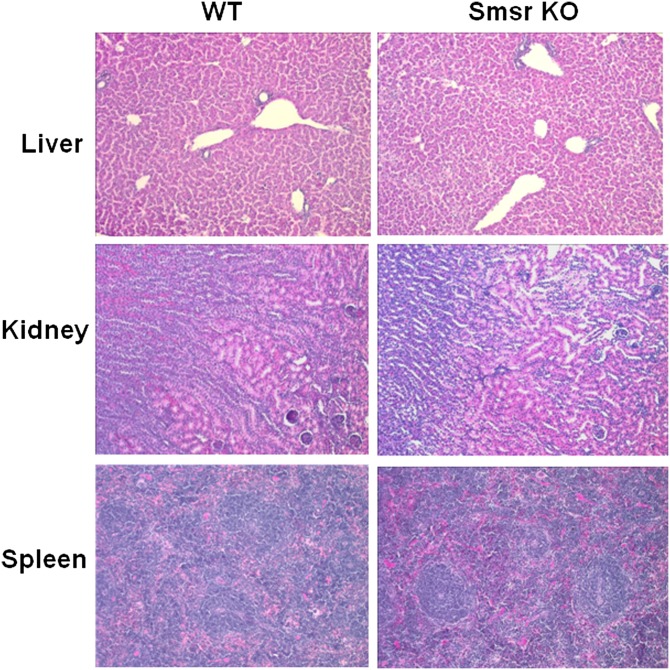
To investigate whether Smsr KO had any impact on tissue morphology, we stained sections from the liver, kidney, and spleen with hematoxylin and eosin. Smsr deficiency had no visible impact on the morphology of any of these tissues (Fig. 4).
Fig. 4.
Mouse tissue staining. Morphology of paraformaldehyde-fixed tissue (liver, kidney, and spleen) sections (5 μm thick) stained with hematoxylin and eosin examined by microscopy.
Effects of Smsr deficiency on CPE, ceramide, and other sphingolipid levels in plasma, liver, and macrophages
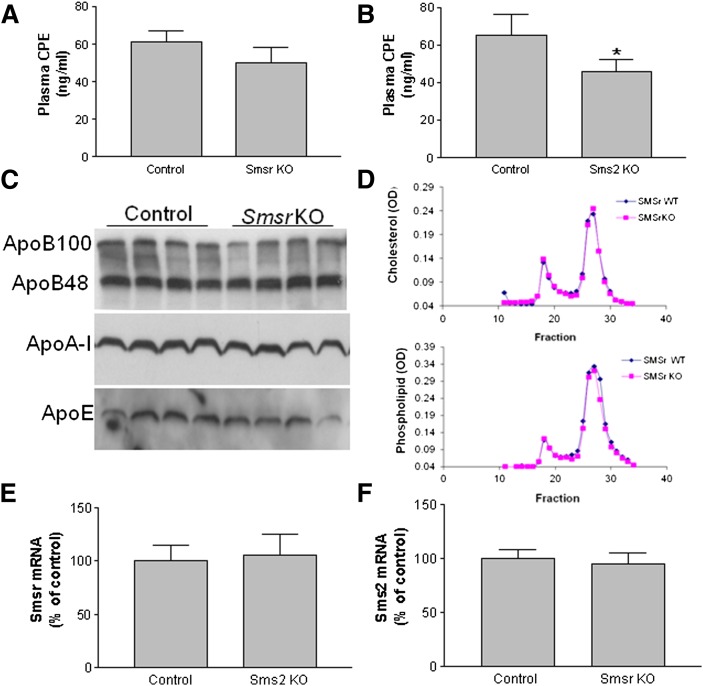
Because SMSr and SMS2 have CPE synthase activity (12, 13) that may contribute to blood CPE levels, we used LC/MS/MS to measure CPE levels in the blood of Smsr and Sms2 KO mice. We found that plasma CPE levels were extremely low (i.e., ∼20-fold lower than ceramide levels) (Table 1); moreover, plasma CPE levels were reduced in both KO mouse lines compared with their controls (Fig. 5A, B, supplementary Table 1), but the difference was statistically significant only for the Sms2 KO mice.
TABLE 1.
Measurement of sphingolipids in tissues from Smsr KO and control mice by LC/MS/MS
| Mice | SM | CPE | Cer | DHCer | S1P | GlyCer | ||
| Plasma | μg/ml | ng/ml | ||||||
| WT | 93 ± 10 | 59 ± 6 | 1,115 ± 166 | 175 ± 23 | 451 ± 62 | 2,680 ± 391 | ||
| Smsr KO | 91 ± 5 | 51 ± 8 | 1,002 ± 118 | 193 ± 37 | 389 ± 41 | 2,559 ± 310 | ||
| Macrophages | μg/mg protein | ng/mg protein | ||||||
| WT | 24 ± 4 | 25 ± 4 | 858 ± 93 | 132 ± 29 | 168 ± 25 | |||
| Smsr KO | 26 ± 3 | 22 ± 5 | 804 ± 102 | 143 ± 20 | 154 ± 19 | |||
| Liver | μg/mg protein | ng/mg protein | ||||||
| WT | 9.5 ± 0.2 | 13 ± 2 | 293 ± 51 | 55 ± 6 | 137 ± 18 | |||
| Smsr KO | 9.3 ± 0.4 | 11 ± 3 | 274 ± 39 | 48 ± 9 | 122 ± 31 | |||
Values are the mean ± SD of five mice. Cer, ceramide; CPE, ceramide phosphoethanolamine; DHCer, dihydroceramide; Glycer, glucosylceramide; SM, sphingomyelin; S1P, sphingosine-1-phosphate.
Fig. 5.
Lipid and mRNA measurement. A: Measurement of plasma CPE by LC/MS/MS in Smsr KO and control mice. B: Measurement of plasma CPE by LC/MS/MS in Sms2 KO and control mice. Values are the mean ± SD of five mice. *P < 0.05. C: Plasma apoA-I, apoB, and apoE levels in four Smsr KO and four WT mice were determined by immunoblotting. D: Analysis of lipid distribution in mouse plasma by FPLC. A 200-μl aliquot of pooled plasma (from seven animals) was loaded onto a Sepharose 6B column and eluted with 50 mM Tris and 0.15 M NaCl (pH 7.5). An aliquot of each fraction was used for determination of total cholesterol (top) and total phospholipids (bottom). E: Real-time PCR with total RNA from the liver of Sms2 KO and control mice. F: Real-time PCR with total RNA from the liver of Smsr KO and control mice. Values are the mean ± SD of three mice.
It has been reported that CPE and/or SMSr can serve as sensors for ceramide homeostasis in cells (12, 14). We next examined levels of ceramide and other sphingolipids, including SM, dihydroceramide, glucosylceramide, and sphingosine-1-phosphate, in the circulation. We found that plasma ceramide and other sphingolipid levels did not differ significantly between Smsr KO mice and controls (Table 1; supplementary Tables 2–5).
We also measured plasma cholesterol, phospholipid, and triglyceride levels in Smsr KO and control animals because the levels of these lipids are closely associated with the development of atherosclerosis. We found no significant changes in these lipid levels in the Smsr KO mice (supplementary Table 6). This was also true for plasma apolipoprotein levels (Fig. 5C). In addition, the distribution of lipids was determined by FPLC of pooled plasma samples, and we did not observe any differences in cholesterol or phospholipid distribution between the Smsr KO and control mice (Fig. 5D).
Macrophage sphingolipids are involved in reverse cholesterol transport, a process associated with antiatherogenesis (22, 23). Because Smsr is highly expressed in macrophages, we next measured sphingolipids in these cells. Contrary to what we predicted, we did not find any significant changes in sphingolipids, including CPE and ceramide (Table 1, supplementary Tables 1–5) in the Smsr KO mice, indicating that neither CPE nor SMSr appears to be a sensor for ceramide homeostasis in macrophages.
The liver is one of the major organs for the production of SM-rich lipoproteins (24). Therefore, we measured sphingolipids in the liver and found that Smsr deficiency also had no significant effect on levels of CPE or other sphingolipids in the liver (Table 1, supplementary Tables 1–6).
Effects of Smsr/Sms2 double deficiency on lipid levels in plasma, liver, and macrophages
We did not find that SMSr is a critical mediator of ceramide levels, as suggested in two previous cell culture studies (12, 14). Because SMS2 also has CPE synthase activity (13), we hypothesized that increased Sms2 expression might compensate for the loss of Smsr. To examine this possibility, we measured Sms2 mRNA levels in Smsr KO mice and Smsr mRNA levels in Sms2 KO mice and found no significant changes in expression of either gene (Fig. 5E, F). We then prepared Smsr/Sms2 double KO mice and used LC/MS/MS to measure sphingolipids in the plasma, liver, and macrophages of these animals. We found that CPE levels were significantly decreased in the liver and plasma but not in macrophages (Table 2) of the double KO mice compared with controls. As expected, SM levels in the plasma, liver, and macrophages were significantly reduced, whereas ceramide levels were significantly elevated (Table 2), likely due to loss of SMS2 activity (19).
TABLE 2.
Measurement of sphingolipids in tissues from Smsr/Sms2 KO and control mice by LC/MS/MS
| Mice | SM | CPE | Cer | DHCer | S1P | GlyCer |
| Plasma | μg/ml | ng/ml | ||||
| WT | 121 ± 10 | 68 ± 9 | 1,252 ± 220 | 231 ± 41 | 399 ± 51 | 2,291 ± 387 |
| Double KO | 93 ± 5a | 49 ± 5a | 1,702 ± 227a | 210 ± 34 | 361 ± 66 | 2,360 ± 209 |
| Macrophages | μg/mg protein | ng/mg protein | ||||
| WT | 31 ± 3 | 28 ± 8 | 774 ± 92 | 115 ± 29 | 182 ± 28 | |
| Double KO | 24 ± 4a | 23 ± 7 | 936 ± 25a | 137 ± 35 | 171 ± 38 | |
| Liver | μg/mg protein | ng/mg protein | ||||
| WT | 13.1 ± 0.3 | 19 ± 2 | 208 ± 29 | 45 ± 6 | 159 ± 23 | |
| Double KO | 9.5 ± 0.8a | 10 ± 3* | 302 ± 36a | 37 ± 3 | 167 ± 18 |
Values are mean ± SD of five mice. Cer, ceramide; CPE, ceramide phosphoethanolamine; DHCer, dihydroceramide; Double KO, Smsr/Sms2 KO mice; Glycer, glucosylceramide; SM, sphingomyelin; S1P, sphingosine-1-phosphate.
P < 0.01.
SMS1 has CPE synthase activity
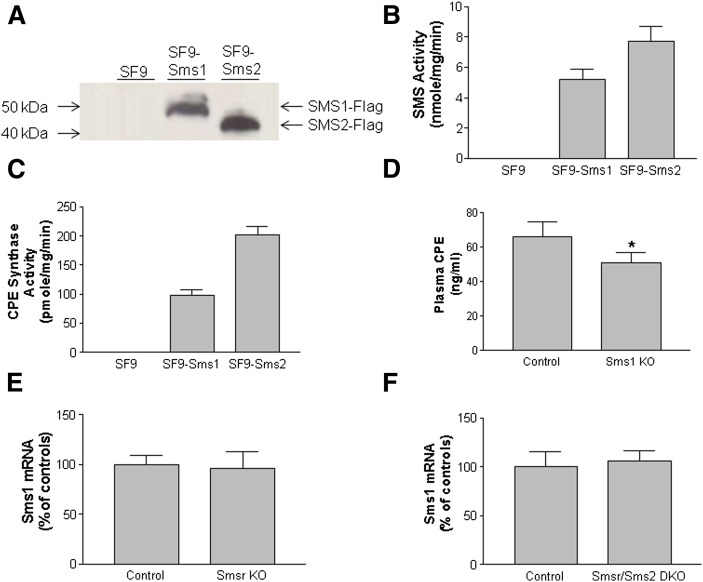
The above results suggested that CPE levels are not exclusively dependent on SMSr and SMS2, at least in macrophages. One possibility is that SMS1 may also have CPE synthase activity. Thus, we used the SF9 insect cell line, which has very low endogenous CPE synthase activity and is suitable for expression of recombinant proteins from a baculovirus vector (25). We found that expression of human Sms1-Flag or Sms2-Flag in SF9 cells (Fig. 6A) significantly increased SMS activity in the cell homogenates, reflected by C8-SM formation (Fig. 6B). Importantly, Sms1 or Sms2 expression stimulates C8-CPE formation (Fig. 6C), indicating that SMS1, like SMS2, has CPE synthase activity. Moreover, we measured CPE levels in Sms1 KO mice and found that Sms1 deficiency also reduced plasma CPE levels (Fig. 6D) (supplementary Table 7). We measured Sms1 mRNA levels in Smsr KO mice and Smsr/Sms2 double KO mice and found no significant changes compared with respective controls (Fig. 6E, F).
Fig. 6.
Mouse SMS1 and SMS2 have CPE synthase activity. A: SF9 insect cells were transfected with Sms1-Flag or Sms2-Flag, and Western blot was performed using anti-Flag antibody. B: SMS activity in SF9 insect cells transduced with Sms1-Flag and Sms2-Flag. C: CPE synthase activity in SF9 insect cells transduced with Sms1-Flag and Sms2-Flag. Values are mean ± SD of three experiments. D: Plasma CPE levels in Sms1 KO and control mice. E: SMS1 mRNA, measured by real-time PCR, in the liver of Smsr KO mice. F: SMS1 mRNA, measured by real-time PCR, in the liver of Smsr/ Sms2 bouble KO mice. Values are mean ± SD of five mice. *P < 0.05.
Next, we sought to determine the Km and Vmax of SMS1, SMS2, and SMSr using NBD-C6-ceramide and NBD-C12-cermiden as substracts. Hela cells, which stably express SMS1 or SMS2 or SMSr, were established. The Km and Vmax of one isoform were determined when the other two isoforms were attenuated by siRNAs. Again, all members of SMS family have CPE synthase activity; however, they have different Km and Vmax (Table 3, supplementary Fig. 1).
TABLE 3.
CPE synthase Km and Vmax determination
| SMS1 | SMS2 | SMSr | |
| NBD-C6-Ceramide | |||
| Km (μM) | 48.79 | 12.50 | 10.16 |
| Vmax (pmol/mg/min) | 0.78 | 0.30 | 0.19 |
| NBD-C6-Ceramide | |||
| Km (μM) | 13.42 | 12.54 | 36.75 |
| Vmax (pmol/mg/min) | 0.12 | 0.11 | 0.28 |
Km and Vmas were measured as described in Materials and Methods. Also see supplementary Figures 1 and 2.
DISCUSSION
In this study, we demonstrated that mouse Smsr is expressed in all tissues examined and that it has CPE synthase activity. We also demonstrated, for the first time, that disruption of Smsr in mice results in 1) significant reduction of CPE synthase activity in the liver, kidney, and macrophages; 2) no significant reduction of CPE levels in the liver, plasma, and macrophages; and 3) marginal changes in the levels of other sphingolipids, including ceramide, SM, and sphingosine-1-phosphate, in the plasma, liver, and macrophages. Moreover, Smsr/Sms2 double deficiency did not show a complete depletion of CPE due to the activity of SMS1 as another CPE synthase. Overall, there is no CPE- or SMSr-mediated abnormal accumulation of ceramide in the tested tissues.
Insects like Drosophila melanogaster (26) do not synthesize SM but instead produce the SM analog CPE as a major membrane constituent (27). CPE is also produced in mammals by CPE synthase (28, 29). Both SMSr and SMS2 have CPE synthase activity (12, 13), and we demonstrated here that SMS1 also has this activity. Moreover, all three have their own Km and Vmax (Table 3, supplementary Figs. 1 and 2). The present study offers an opportunity to address the function of CPE and to examine whether it functions as a structural molecule or as a bioactive mediator of signaling in mammals.
We found that CPE synthase activity, and consequently CPE levels, were very low in the liver and macrophages (Table 1, Fig. 3B, C) and in the other tissues (data not shown), especially relative to ceramide and SM levels. Our finding that SMSr and SMS2, the two known CPE synthases, were only capable of producing trace amounts of CPE (Tables 1 and 2) is in line with previous reports (12, 14) and likely explains the generally low levels of CPE in plasma and tissues.
Unexpectedly, we detected a significant reduction in plasma CPE levels in Sms1 KO mice (Fig. 6B), suggesting that SMS1 also has CPE synthase activity. Indeed, we found that human SMS1 is capable of producing C8-CPE when expressed in SF9 insect cells (Fig. 6C). This result likely explains the presence of CPE in the plasma of Smsr/Sms2 double KO mice. SMS1 has CPE synthase activity, which was also observed by our collaborators (Dr. Willecke’s group, unpublished observations).
It has been reported that Smsr siRNA-mediated gene knockdown reduces CPE synthase activity and CPE levels in HeLa cells, leading to an accumulation of ceramide in the ER and a collapse of the early secretory pathway (12). It has been proposed that, under normal conditions, only small amounts of ceramide reach the SMSr active site (12) because ceramides synthesized on the cytosolic surface of the ER are continuously removed by CERT (2). SMSr-derived CPE might directly influence the activity of CERT in the ER. Thus, we predicted that Smsr or Smsr/Sms2 deficiency would result in reduced CPE production and ceramide accumulation in the tissues or circulation. However, although we did observe a reduction of CPE levels in the plasma of Smsr (not statistically significant), Sms2, and Smsr/Sms2 (Tables 1 and 2, Fig. 5B) KO mice, we did not observe a CPE reduction-mediated accumulation of ceramide. In fact, in the Sms1 KO mice, we observed a reduction in plasma ceramide levels (23). These results suggest that CPE is not a critical regulator of ceramide levels in vivo.
Recently, Tafesse et al. (14) reported that SMSr-catalyzed CPE production, although essential, is not sufficient to suppress ceramide-induced cell death and that SMSr-mediated ceramide homeostasis requires the N-terminal sterile α-motif, or the sterile α motif domain, of the enzyme. Similarly, the lack of ceramide accumulation in the Smsr KO mice further suggests that SMSr per se is also not a critical regulator of ceramide levels in vivo.
There are two possible explanations for the discrepancy between this study and the previous studies. First, the previous two studies were conducted in Hela cells (12, 14), a well-known immortal cell line derived from human cervical cancer cells (30). The CPE or SMSr might mediate ceramide metabolism in Hela cells but might not in normal cells. Further, it has been noted that Smsr knockdown in multiple cell lines did not alter sphingolipid biosynthesis (15). Second, there are differences between ex vivo and in vivo studies, and the former might not fully reflect the situation in the latter. It is well known that inhibition of microsomal TG transfer protein results in hepatic TG accumulation in humans (31). However, the microsomal TG transfer protein inhibitor has a very marginal effect on TG levels in HepG2 cells (32), a human hepatoma cell line.
Smsr is the most conserved member in the Sms family (3) and was expressed to varying degrees in all 12 tissues examined in this study (Fig. 1C), but it has no SMS activity (12). Given the fact that tissue CPE levels are extremely low and that SMS1 and SMS2 have CPE synthase activity, it is not clear why SMSr is needed and so highly conserved in the animal kingdom. An Sms1/Sms2/Smsr triple KO mouse line, in which SM synthase and CPE synthase activity are eliminated in vivo, may help to uncover the central function for SMSr. We are in the process of preparing these mice.
In conclusion, mouse Smsr deficiency reduces CPE levels through reduction of CPE synthase activity. However, the deficiency has no impact on tissue ceramide levels. All members of sphingomyelin synthase gene family (SMSr, SMS1, and SMS2) have CPE synthase activity.
Supplementary Material
Acknowledgments
The authors thank Drs. Yan Q. Chen, David Yurek, and Ming-Shang Kuo from Eli Lilly for assistance in performing the Sms1 and Sms2 expression in SF9 insect cells and LC/MS analysis.
Footnotes
Abbreviations:
- CERT
- ceramide transport protein
- CPE
- ceramide phosphoethanolamine
- ER
- endoplasmic reticulum
- SMS
- sphingomyelin synthase
- SMSr
- sphingomyelin synthase-related protein
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures and seven tables.
REFERENCES
- 1.Merrill A. H., Jr 1983. Characterization of serine palmitoyltransferase activity in Chinese hamster ovary cells. Biochim. Biophys. Acta. 754: 284–291. [DOI] [PubMed] [Google Scholar]
- 2.Hanada K., Kumagai K., Yasuda S., Miura Y., Kawano M., Fukasawa M., Nishijima M. 2003. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 426: 803–809. [DOI] [PubMed] [Google Scholar]
- 3.Huitema K., van den Dikkenberg J., Brouwers J. F., Holthuis J. C. 2004. Identification of a family of animal sphingomyelin synthases. EMBO J. 23: 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaoka S., Miyaji M., Kitano T., Umehara H., Okazaki T. 2004. Expression cloning of a human cDNA restoring sphingomyelin synthesis and cell growth in sphingomyelin synthase-defective lymphoid cells. J. Biol. Chem. 279: 18688–18693. [DOI] [PubMed] [Google Scholar]
- 5.Ichikawa S., Sakiyama H., Suzuki G., Hidari K. I., Hirabayashi Y. 1996. Expression cloning of a cDNA for human ceramide glucosyltransferase that catalyzes the first glycosylation step of glycosphingolipid synthesis. Proc. Natl. Acad. Sci. USA. 93: 4638–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simons K., Ikonen E. 1997. Functional rafts in cell membranes. Nature. 387: 569–572. [DOI] [PubMed] [Google Scholar]
- 7.Li Z., Hailemariam T. K., Zhou H., Li Y., Duckworth D. C., Peake D. A., Zhang Y., Kuo M. S., Cao G., Jiang X. C. 2007. Inhibition of sphingomyelin synthase (SMS) affects intracellular sphingomyelin accumulation and plasma membrane lipid organization. Biochim. Biophys. Acta. 1771: 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyaji M., Jin Z. X., Yamaoka S., Amakawa R., Fukuhara S., Sato S. B., Kobayashi T., Domae N., Mimori T., Bloom E. T., et al. 2005. Role of membrane sphingomyelin and ceramide in platform formation for Fas-mediated apoptosis. J. Exp. Med. 202: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Luit A. H., Budde M., Zerp S., Caan W., Klarenbeek J. B., Verheij M., Van Blitterswijk W. J. 2007. Resistance to alkyl-lysophospholipid-induced apoptosis due to downregulated sphingomyelin synthase 1 expression with consequent sphingomyelin- and cholesterol-deficiency in lipid rafts. Biochem. J. 401: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tafesse F. G., Ternes P., Holthuis J. C. 2006. The multigenic sphingomyelin synthase family. J. Biol. Chem. 281: 29421–29425. [DOI] [PubMed] [Google Scholar]
- 11.Yeang C., Varshney S., Wang R., Zhang Y., Ye D., Jiang X. C. 2008. The domain responsible for sphingomyelin synthase (SMS) activity. Biochim. Biophys. Acta. 1781: 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vacaru A. M., Tafesse F. G., Ternes P., Kondylis V., Hermansson M., Brouwers J. F., Somerharju P., Rabouille C., Holthuis J. C. 2009. Sphingomyelin synthase-related protein SMSr controls ceramide homeostasis in the ER. J. Cell Biol. 185: 1013–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ternes P., Brouwers J. F., van den Dikkenberg J., Holthuis J. C. 2009. Sphingomyelin synthase SMS2 displays dual activity as ceramide phosphoethanolamine synthase. J. Lipid Res. 50: 2270–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tafesse F. G., Vacaru A. M., Bosma E. F., Hermansson M., Jain A., Hilderink A., Somerharju P., Holthuis J. C. 2014. Sphingomyelin synthase-related protein SMSr is a suppressor of ceramide-induced mitochondrial apoptosis. J. Cell Sci. 127: 445–454. [DOI] [PubMed] [Google Scholar]
- 15.Siow D. L., Wattenberg B. W. 2012. Mammalian ORMDL proteins mediate the feedback response in ceramide biosynthesis. J. Biol. Chem. 287: 40198–40204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang X. C., Paultre F., Pearson T. A., Reed R. G., Francis C. K., Lin M., Berglund L., Tall A. R. 2000. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 20: 2614–2618. [DOI] [PubMed] [Google Scholar]
- 17.Park T. S., Panek R. L., Mueller S. B., Hanselman J. C., Rosebury W. S., Robertson A. W., Kindt E. K., Homan R., Karathanasis S. K., Rekhter M. D. 2004. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 110: 3465–3471. [DOI] [PubMed] [Google Scholar]
- 18.Hojjati M. R., Li Z., Jiang X. C. 2005. Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim. Biophys. Acta. 1737: 44–51. [DOI] [PubMed] [Google Scholar]
- 19.Liu J., Zhang H., Li Z., Hailemariam T. K., Chakraborty M., Jiang K., Qiu D., Bui H. H., Peake D. A., Kuo M. S., et al. 2009. Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arterioscler. Thromb. Vasc. Biol. 29: 850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hojjati M. R., Jiang X. C. 2006. Rapid, specific, and sensitive measurements of plasma sphingomyelin and phosphatidylcholine. J. Lipid Res. 47: 673–676. [DOI] [PubMed] [Google Scholar]
- 21.Lee S. Y., Kim J. R., Hu Y., Khan R., Kim S. J., Bharadwaj K. G., Davidson M. M., Choi C. S., Shin K. O., Lee Y. M., et al. 2012. Cardiomyocyte specific deficiency of serine palmitoyltransferase subunit 2 reduces ceramide but leads to cardiac dysfunction. J. Biol. Chem. 287: 18429–18439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J., Huan C., Chakraborty M., Zhang H., Lu D., Kuo M. S., Cao G., Jiang X. C. 2009. Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circ. Res. 105: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z., Fan Y., Liu J., Li Y., Huan C., Bui H. H., Kuo M. S., Park T. S., Cao G., Jiang X. C. 2012. Impact of sphingomyelin synthase 1 deficiency on sphingolipid metabolism and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 32: 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z., Li Y., Chakraborty M., Fan Y., Bui H. H., Peake D. A., Kuo M. S., Xiao X., Cao G., Jiang X. C. 2009. Liver-specific deficiency of serine palmitoyltransferase subunit 2 decreases plasma sphingomyelin and increases apolipoprotein E levels. J. Biol. Chem. 284: 27010–27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wickham T. J., Davis T., Granados R. R., Shuler M. L., Wood H. A. 1992. Screening of insect cell lines for the production of recombinant proteins and infectious virus in the baculovirus expression system. Biotechnol. Prog. 8: 391–396. [DOI] [PubMed] [Google Scholar]
- 26.Vacaru A. M., van den Dikkenberg J., Ternes P., Holthuis J. C. 2013. Ceramide phosphoethanolamine biosynthesis in Drosophila is mediated by a unique ethanolamine phosphotransferase in the Golgi lumen. J. Biol. Chem. 288: 11520–11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao R. P., Yuan C., Allegood J. C., Rawat S. S., Edwards M. B., Wang X., Merrill A. H., Jr, Acharya U., Acharya J. K. 2007. Ceramide transfer protein function is essential for normal oxidative stress response and lifespan. Proc. Natl. Acad. Sci. USA. 104: 11364–11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malgat M., Maurice A., Baraud J. 1986. Sphingomyelin and ceramide-phosphoethanolamine synthesis by microsomes and plasma membranes from rat liver and brain. J. Lipid Res. 27: 251–260. [PubMed] [Google Scholar]
- 29.Malgat M., Maurice A., Baraud J. 1987. Sidedness of ceramide-phosphoethanolamine synthesis on rat liver and brain microsomal membranes. J. Lipid Res. 28: 138–143. [PubMed] [Google Scholar]
- 30.Rahbari R., Sheahan T., Modes V., Collier P., Macfarlane C., Badge R. M. 2009. A novel L1 retrotransposon marker for HeLa cell line identification. Biotechniques. 46: 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuchel M., Bloedon L. T., Szapary P. O., Kolansky D. M., Wolfe M. L., Sarkis A., Millar J. S., Ikewaki K., Siegelman E. S., Gregg R. E., et al. 2007. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N. Engl. J. Med. 356: 148–156. [DOI] [PubMed] [Google Scholar]
- 32.Borradaile N. M., de Dreu L. E., Barrett P. H., Behrsin C. D., Huff M. W. 2003. Hepatocyte apoB-containing lipoprotein secretion is decreased by the grapefruit flavonoid, naringenin, via inhibition of MTP-mediated microsomal triglyceride accumulation. Biochemistry. 42: 1283–1291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.