Abstract
Tissue cholesterol accumulation, macrophage infiltration, and inflammation are features of atherosclerosis and some forms of dermatitis. HDL and its main protein, apoAI, are acceptors of excess cholesterol from macrophages; this process inhibits tissue inflammation. Recent epidemiologic and clinical trial evidence questions the role of HDL and its manipulation in cardiovascular disease. We investigated the effect of ectopic macrophage apoAI expression on atherosclerosis and dermatitis induced by the combination of hypercholesterolemia and absence of HDL in mice. Hematopoietic progenitor cells were transduced to express human apoAI and transplanted into lethally irradiated LDL receptor−/−/apoAI−/− mice, which were then placed on a high-fat diet for 16 weeks. Macrophage apoAI expression reduced aortic CD4+ T-cell levels (−39.8%), lesion size (−25%), and necrotic core area (−31.6%), without affecting serum HDL or aortic macrophage levels. Macrophage apoAI reduced skin cholesterol by 39.8%, restored skin morphology, and reduced skin CD4+ T-cell levels. Macrophage apoAI also reduced CD4+ T-cell levels (−32.9%) in skin-draining lymph nodes but had no effect on other T cells, B cells, dendritic cells, or macrophages compared with control transplanted mice. Thus, macrophage apoAI expression protects against atherosclerosis and dermatitis by reducing cholesterol accumulation and regulating CD4+ T-cell levels, without affecting serum HDL or tissue macrophage levels.
Keywords: apolipoprotein AI, high density lipoprotein, gene therapy, hematopoietic progenitor cells, bone marrow transplant
Serum levels of HDL and its major protein apoAI are associated with decreased coronary heart disease (CHD) rates (1, 2). Antiatherogenic properties of HDL and apoAI are attributed to their reverse cholesterol transport capacity and anti-inflammatory effects (3, 4). However, there is evidence that some causes of low HDL are atheroprotective (5) and that very high HDL levels may not be associated with cardiovascular protection (6). These observations are supported by results of recent clinical trials reporting absence of cardiovascular benefits with pharmacologic maneuvers to increase HDL levels (7, 8). Thus, changes in serum HDL-cholesterol levels may not be a representation of the atheroprotective role of HDL (9). These data compel an investigation of the precise role of HDL and apoAI in tissue cholesterol homeostasis and in the regulation of the inflammatory response driven by cholesterol accumulation.
ApoAI is naturally expressed in hepatocytes and enterocytes but not in peripheral cells, including macrophages. Yet, apoAI is critical for the unloading of cholesterol from macrophages, with pathologic and clinical consequences ranging from atherosclerosis to xanthomatosis and skin inflammation (10). We and other groups have shown that ectopic expression of apoAI by macrophages (through transplantation of bone marrow cells transduced to express apoAI or derived from apoAI transgenic donors) reduces atherosclerosis by stimulating local cholesterol efflux (11–13). Removal of cholesterol from the aortic wall was mediated through increasing local apoAI concentration and upregulation of cellular cholesterol transporters (14). In addition, both endogenous and exogenous apoAI were shown to stimulate the secretion of macrophage apoE, another physiological driver of cholesterol efflux (15–17). Together, lipid-free apoAI, HDL-bound apoAI, and apoE act in synergy to extract cholesterol from macrophages, thus influencing both macrophage cholesterol accumulation, a hallmark of atherosclerosis, and macrophage phenotype adjustment to local inflammatory impulses (18, 19). Both macrophage and exogenous apoAI expression also reduce cytokine expression by macrophages and promote the switch to an anti-inflammatory macrophage phenotype (11, 20, 21).
In addition to atherosclerosis, dyslipidemia with disturbed reverse cholesterol transport can cause skin inflammation following local accumulation of cholesterol (22). Patients with atopic dermatitis, a chronic inflammatory disease, were shown to have higher levels of cholesterol accumulated in their skin compared with controls (23). It has been suggested that skin cholesterol levels provide insight into the presence and severity of atherosclerosis (24). One well-studied model of increased skin cholesterol accumulation due to disrupted cholesterol efflux capacity is the LDL receptor (LDLR)−/−/apoAI−/− mouse (25). When fed palm oil diet, a massive accumulation of cholesterol in the skin of these mice causes fatal dermatitis (26). LDLR−/−/apoAI−/− mice also show systemic inflammation with common autoimmune characteristics such as enlarged spleen and lymph nodes (LNs), and an increased CD4+ T-cell response (27). Adenoviral expression and systemic administration of apoAI to LDLR−/−/apoAI−/− mice rescued them from the fatal dyslipidemia-induced dermatitis by reducing skin cholesterol accumulation and inflammation (27, 28).
Because arterial and skin lesion formation and inflammation are driven by local accumulation of cholesterol-loaded macrophages, we set out to study whether expression of apoAI from macrophages is sufficient to rescue LDLR−/−/apoAI−/− mice from the phenotype of dyslipidemia-induced dermatitis and to determine its simultaneous effects on aortic, skin, and lymphatic inflammatory status.
We have generated chimeric mice expressing apoAI exclusively from macrophages via lentiviral transduction of hematopoietic progenitor cells (HPCs) followed by their transplantation into recipient mice lacking both LDLR and apoAI. Our results support a scenario in which the expression of macrophage apoAI protects against atherosclerosis and dermatitis both through removal of cholesterol from the aortic wall and skin, and reduction of CD4+ T-cell levels in the aortic wall, skin, and skin-draining LNs. These effects of macrophage apoAI were not associated with changes in serum apoAI and HDL levels or tissue macrophage numbers. Our results show that the correction of cholesterol overload in macrophages via ectopic apoAI expression reduces atherosclerosis burden and corrects the sterile dermatitis of severely hyperlipidemic mice, thus establishing a common platform for the development of a cell-based therapy of these conditions.
METHODS
Animals and diet
LDLR−/−/ApoAI−/− mice were obtained from Wake Forest University (WFU) and housed at Vanderbilt University Medical Center (VUMC). Mice were fed an atherogenic diet containing 0.1% cholesterol with 10% fat from palm oil, prepared in the diet kitchen of WFU, as described previously (26), and shipped to the animal facility of VUMC. Mice were maintained in a temperature-controlled room with a 12 h light/12 h dark cycle. All animal experiments were carried out in compliance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committees of the three institutions (VUMC, WFU, and Oregon Health and Science University).
Generation of a lentiviral human apoAI cDNA expression construct
The lentiviral vector was prepared and expanded in our laboratory as previously described (29). In short, the self-inactivating (SIN) lentiviral vector (pWPT-WRPE), envelope plasmid pMD2.G, and packaging plasmid pCMV ΔR8.91 were kindly provided by Dr. Dider Trono (Lausanne, Switzerland). We have shown that transduction of HPCs with a green fluorescent protein (GFP)-expressing lentivirus under the human CD68 promoter direct transcriptional activity specifically in macrophages but not in B cells or T cells (17). The CD68 promoter (including the first intron) was amplified from human genomic DNA by PCR. The human apoAI cDNA with the growth hormone poly-A signal sequence was excised from an scavenger receptor type A (SRA)-apoAI clone previously generated in our laboratory (17). Schematic illustration of the complete structure of the GFP and apoAI constructs was adapted from one of our previous publications (17) and is shown in supplementary Fig. 1A, B, respectively.
Purification and enrichment of HPCs
Bone marrow cells from 8- to 10-week-old LDLR−/−/ApoAI−/− mice were harvested from femurs and tibias. HPCs were purified by depletion of the Lin+ cells with the Lineage Depletion SpinSep kit (Stem Cell Technologies). ScaI+/cKit+ cells were further selected from the Lin− cells using EasySep Scal+ and cKit+ selection kit (Stem Cell Technologies) to obtain the Scal+cKit+Lin− population.
Transplantation of HPCs into LDLR−/−/ApoAI−/− mice and quantitation of atherosclerotic lesions
HPCs were isolated from male or female LDLR−/−/ApoAI−/− mice at 8 to 10 weeks of age and then were left untreated, transduced with lentiviral-GFP, or transduced with lentiviral-apoAI at a multiplicity-of-infection of 30 using the Mammalian Transfection System (Promega) and cultured for 24 h in Stemspan media (Stem Cell technology). HPC transduction efficacy was 25.7% (supplementary Fig. 2, insert). Male or female LDLR−/−/ApoAI−/− mice at 12 to 14 weeks of age were lethally irradiated with 900 rads from a cesium γ source and transplanted with the HPCs (either apoAI transduced or nontransduced and GFP transduced as controls). After transplantation, mice were kept on an atherogenic diet containing 0.1% cholesterol with 10% fat from palm oil.
Analysis and quantitation of arterial lesions
At the end of the experiment, mice were euthanized and their hearts flushed with saline, embedded in OCT, and snap-frozen in dry ice. The inferior vena cava was cut to allow the perfusate to exit. Frozen sections of 10 μm thickness were taken in the region of the proximal aorta starting from the end of the aortic sinus and for 300 μm distally, according to the technique of Paigen et al. (30). Sections were stained with Oil Red O (ORO) and counterstained with hematoxylin. Quantitative analysis of lipid-stained lesions was performed on sections starting at the end of the aortic sinus. The lipid-stained lesions were measured by digitizing morphometry and reported as area (μm2 per lesion per mouse). For immunostaining, 5 µm thickness sections were taken in the region of the proximal aorta starting at the end of the aortic sinus.
Staining of CD3, CD4, MOMA-2, and hematoxylin and eosin in proximal aortic lesions
For CD3 and CD4 staining, 5 µm frozen sections were fixed in cold acetone, blocked with 4% BSA in PBS, then incubated with rat anti-mouse CD4-AlexaFluor 488 (Caltag Labs) and hamster anti-mouse CD3-AlexaFluor 647 (Invitrogen) antibody. Vectashield mounting medium with 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories Inc.) was used to stain nuclei. Monocyte/Macrophage Marker Antibody (MOMA)-2 staining was performed in 5 µm frozen sections as previously described (17). Necrosis was detected in 5 µm sections using Harris’s hematoxylin and eosin (H and E) staining and quantitated by measuring the acellular area in the intima versus total intimal area. Quantitative analyses of atherosclerotic lesion size, CD3, CD4, MOMA-2, and H and E staining were also performed using aortic sinus sections from the same mice.
LN cell isolation and cell-surface staining
LNs (axillary, brachial, superficial cervical, and inguinal) were collected and placed in 10% RPMI. Cells were isolated and stained as previously described (27). Acquisition of samples was done using a BD FACS-Calibur and analyzed using FlowJo software (TreeStar). Cell populations measured included effector/memory T cells (CD4+CD62Llow, CD8+CD62Llow), double-negative T cells (CD3+CD4−CD8−B220+), activated T cells (CD4+CD69high, CD8+CD69high), naïve B cells (IgM+B220+), dendritic cells (DCs) (CD3−CD11b+CD11c+), and macrophages (CD11c−CD11b+F4/80+). Antibodies used in surface staining including rat anti-mouse CD3, CD4, CD8, CD62L, CD69, IgM, B220, CD11c, CD11b, CD44, and F4/80 were obtained from BD Biosciences or eBioscience.
Skin cholesterol quantification and histology
Skin fragments were extracted using chloroform-methanol (2:1) as described previously (26). Cholesterol and other neutral lipids were isolated using the Kaluzny et al. (31) method and analyzed by mass spectrometry. For skin histology, freshly isolated skin fragments were collected and embedded into OCT, and 10 μm sections were stained with ORO or H and E. For immunostaining, sections were incubated with CD4 (BD biosciences), CD68 (Rockland), or CD11c (Pharmingen) antibodies as described previously (27).
Serum lipid and apoAI determination
Mice were fasted for 16 h prior to blood collection. Serum cholesterol or triglyceride measurements were performed using the Cholesterol or Triglyceride Reagent kit from RAICHEM, respectively. Mouse lipoproteins were separated from serum by size exclusion chromatography using a Superose 6 column on a fast-protein liquid chromatography system commonly used in our laboratory (32). ApoAI levels in mice serum were analyzed using the Apolipoprotein AI Human SimpleStep ELISA™ Kit from Abcam.
Statistical analyses
GraphPad Prism 6 software was used to carry out statistical analyses. The Mann-Whitney test was used to compare data between the two groups. Results are presented as mean ± standard deviation or percent ± coefficient of variance. Differences are reported as * P < 0.05, ** P < 0.01, and *** P < 0.001.
RESULTS
Macrophage apoAI expression does not affect serum lipid or HDL levels
After lethal irradiation, 12- to 14-week-old LDLR−/−/apoAI−/− mice were transplanted with 1 × 106 HPCs expressing apoAI (9 females and 9 males) and compared with control LDLR−/−/apoAI−/− mice transplanted with control HPC (10 females and 9 males). Sixteen weeks after transplantation of apoAI-expressing HPCs, circulating levels of apoAI were 438 ± 189 ng/dl, equivalent to 0.0003% of physiological levels (supplementary Fig. 2A). Feeding mice a palm oil diet for 16 weeks increased serum cholesterol and triglycerides similarly in control and apoAI recipients (cholesterol up 2.4-fold in controls and 2.1-fold in apoAI recipients, and triglycerides up 1.7-fold in controls and 2.1-fold in apoAI recipients; supplementary Fig. 2B and C, respectively). Consistent with low circulating apoAI, HPC expression of apoAI also did not affect serum HDL or other lipoprotein levels in LDLR−/−/apoAI−/− recipient mice (supplementary Fig. 2D).
Macrophage apoAI expression reduced size of aortic lesions
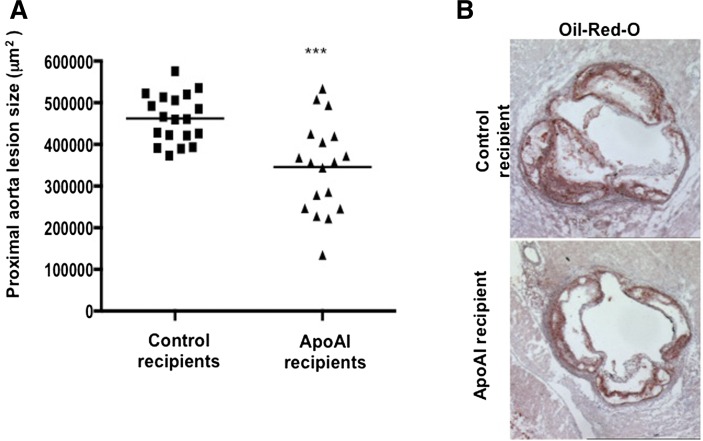
Wilhelm et al. (28) reported that LDLR−/−/apoAI−/− mice fed an atherogenic diet show a 2-fold increase in the severity of atherosclerosis compared with LDLR−/− mice. Sixteen weeks after transplantation, LDLR−/−/apoAI−/− recipient mice were euthanized, and a quantitative analysis of the extent of aortic atherosclerosis using ORO-stained sections of the proximal aorta was performed. The mean aortic lesion area of apoAI recipient mice was 25% less (345,861 ± 109,076 μm2) than that of control recipient mice (462,180 ± 57,671 μm2) (Fig. 1A). Representative ORO-stained aortic sections of the proximal aorta are shown in Fig. 1B. Interestingly, male apoAI recipient mice showed a greater reduction (35%) in aortic lesion area compared with females (15%).
Fig. 1.
Proximal aorta, lesion quantitation. A: Lesion size in the proximal aorta (ORO stained) of LDLR−/−/apoAI−/− recipient mice 16 weeks after HPC transplant and palm oil diet (control recipients: 11 females and 8 males; apoAI recipients: 8 females and 10 males). B: Representative ORO-stained proximal aorta of control recipient LDLR−/−/apoAI−/− (upper panel) and apoAI recipient LDLR−/−/apoAI−/− (lower panel) mice. *** P < 0.001.
Macrophage apoAI expression reduced levels of T cells in aortic lesions
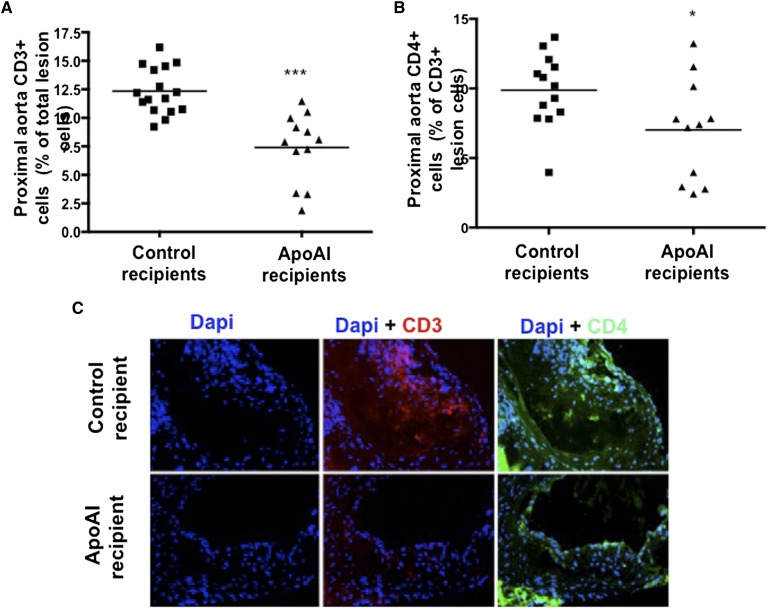
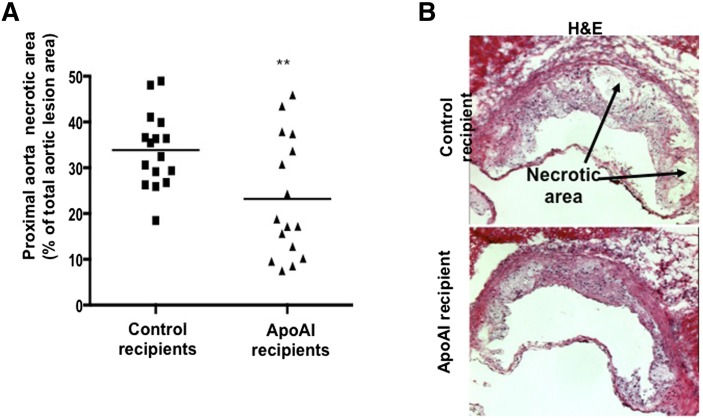
It was previously shown that macrophage apoAI reduces the levels of inflammatory monocyte/macrophages in the aortic lesion (11, 14). Figure 2A and B show lesion composition analyses, with apoAI recipient mice having significantly fewer CD3+ (7.0 ± 3.7% vs. 9.9 ± 2.6% in control recipients) and CD4+ (7.4 ± 3.0% vs. 12.3 ± 2.0% in control recipients) cells in the proximal aorta, respectively. Representative images of CD3 and CD4 staining in the proximal aorta are shown in Fig. 2C. ApoAI recipient mice also showed a smaller necrotic core area in the aortic lesion (23.2 ± 13.1% vs. 33.9 ± 8.2% in control recipients; Fig. 3A). Representative H-and-E-stained sections of the proximal aorta are presented in Fig. 3B. Interestingly, despite reduced CD4+ T cells and lesion size in apoAI recipients, the macrophage area in the aortic wall (MOMA-2 staining) did not differ compared with control recipient mice (supplementary Fig. 3A). In fact, MOMA-2 staining as a percentage of total area was significantly higher in apoAI recipients (44% vs. 31%, P = 0.022) compared with controls (supplementary Fig. 3B). Representative MOMA-2 stained sections of the proximal aorta are shown in supplementary Fig. 3C. No gender influences were seen in the effects of macrophage apoAI expression on atheroma T cells, macrophages, and necrotic core.
Fig. 2.
Proximal aorta, lesion T-cell analysis. A: Percent of CD3+ cells (out of total lesional cells) in proximal aortas of LDLR−/−/apoAI−/− recipient mice 16 weeks after HPC transplant and palm oil diet (control recipients: 8 females and 8 males; apoAI recipients: 6 females and 6 males). B: Percent of CD4+ cells (out of total lesional cells) in proximal aortas of LDLR−/−/apoAI−/− recipient mice 16 weeks after HPC transplant and palm oil diet (control recipients: 7 females and 6 males; apoAI recipients: 6 females and 5 males). C: Representative DAPI- (left), CD3- (middle), and CD4-stained (right) proximal aorta of control recipient LDLR−/−/apoAI−/− (upper panel) and apoAI recipient LDLR−/−/apoAI−/− (lower panel) mice. * P < 0.05, *** P < 0.001.
Fig. 3.
Proximal aorta, necrotic area quantitation. A: Percent of proximal aorta necrotic area (out of total aortic lesion area) of LDLR−/−/apoAI−/− recipient mice 16 weeks after HPC transplant and palm oil diet (control recipients: 8 females and 8 males; apoAI recipients: 8 females and 8 males). B: Representative H-and-E-stained proximal aorta of control recipient LDLR−/−/apoAI−/− (upper panel) and apoAI recipient LDLR−/−/apoAI−/− (lower panel) mice. ** P < 0.01.
Macrophage apoAI expression inhibits skin cholesterol accumulation and inflammation
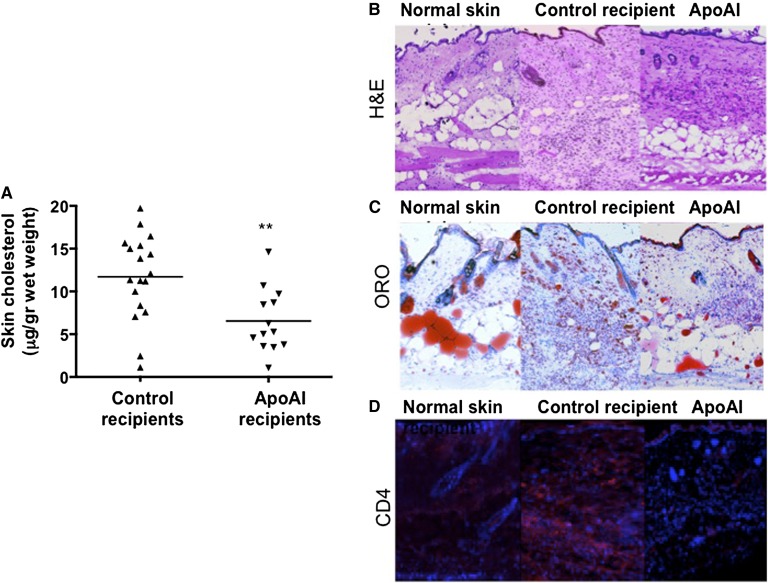
When fed palm oil diet, LDLR−/−/apoAI−/− mice accumulate cholesterol and inflammatory cells in the skin, causing a severe inflammatory phenotype eventually leading to death (26). Our results show that apoAI recipient mice accumulate lower levels of skin cholesterol compared with control recipient mice (6.5 ± 3.7 vs. 10.8 ± 5.3 μg/g wet weight, respectively; Fig. 4A). Representative H-and-E- and ORO-stained skin sections are presented in Fig. 4B and C, respectively. Interestingly, female apoAI recipient mice showed a greater reduction (38%) in skin cholesterol levels compared with males (28%).
Fig. 4.
Skin cholesterol accumulation and inflammation. A: Cholesterol level in skin tissue extracts of LDLR−/−/apoAI−/− recipient mice 16 weeks after HPC transplant and palm oil diet (control recipients: 10 females and 8 males; apoAI recipients: 7 females and 6 males). Representative proximal aorta of skin from healthy LDLR−/− (left panel), control recipient LDLR−/−/apoAI−/− (middle panel), and apoAI recipient LDLR−/−/apoAI−/− (right panel) mice of H-and-E-stained sections (B), ORO-stained sections (C), and CD4-immunostained (red) on top of DAPI-stained (blue) sections (D). ** P < 0.01.
It was previously shown that systemic apoAI could regulate migration of T cells, macrophages, and DCs in LDLR−/−/apoAI−/− mice (28). We further found that apoAI recipient mice had lower levels of skin CD4+ T cells (−69%) compared with controls (Fig. 4D). In contrast, macrophage (CD68+) and DC (CD11c+) staining of skin sections did not show differences between apoAI and control recipient mice, both showing larger staining areas compared with skin sections from normal LDLR−/− mice on palm oil diet (supplementary Fig. 4A, B).
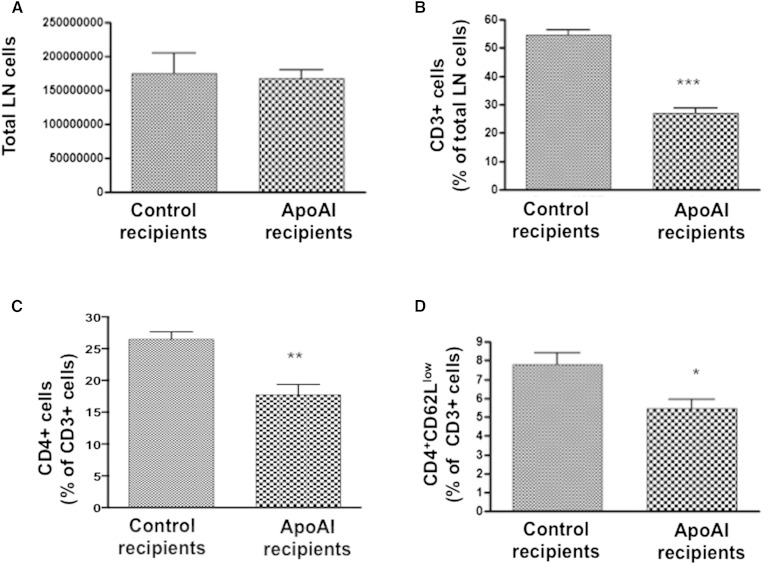
Macrophage apoAI expression reduced CD4+ T cells in skin-draining LNs
In skin-draining LNs (axillary, brachial, superficial cervical, and inguinal) there was no difference in the total number of cells between apoAI recipient or control LDLR−/−/apoAI−/− mice (Fig. 5A). However, skin-draining LNs of apoAI recipient mice showed a significant reduction in CD3+ cells (−49.9%), CD4+ cells (−32.9%), and effector/memory CD4 (CD4+CD62Llow) cells (−30.1%) (Fig. 5A–D, respectively). No differences were found in other T-cell populations, such as activated CD4 (CD4+CD69high), CD8+, activated CD8 (CD8+CD69high), effector/memory CD8 (CD8+CD62Llow), or double-negative T cells (CD3+B220+CD4−CD8−) (supplementary Fig. 5A–E, respectively). In addition, no differences were found in B cell (IgM+B220+), DC (CD3−CD11b+CD11c+), or macrophage (CD11c−CD11b+F4/80+) levels in LN cells (supplementary Fig. 5F–H, respectively).
Fig. 5.
Skin-draining LN inflammation. A: Total number of cells in axillary, brachial, superficial cervical, and inguinal LNs of LDLR−/−/apoAI−/− recipient mice 16 weeks after HPC transplant and palm oil diet. B: Percent of CD3+ cells out of total LN cells in LDLR−/−/apoAI−/− recipient mice 16 weeks after HPC transplant and palm oil diet. C: Percent of CD4+ cells out of total CD3+ cells in LDLR−/−/apoAI−/− recipient mice 16 weeks after HPC transplant and palm oil diet. D: Percent of effector/memory CD4 cells (CD4+CD62Llow) out of total CD3+ cells in LDLR−/−/apoAI−/− recipient mice 16 weeks after HPC transplant and palm oil diet. * P < 0.05, ** P < 0.01, *** P < 0.001.
DISCUSSION
Of all the major risk factors for CHD, HDL is the only one without a clear therapeutic mandate (7, 33). One of the main roles of HDL and apoAI is to drive cholesterol efflux from peripheral cells in general and macrophages in particular, thus preventing foam cell formation (34) and reducing inflammation (35). Reduced capacity to efflux cholesterol from macrophages by HDL in serum is a predictor for atherosclerosis in mice and humans (36–38). Perturbation of macrophage cholesterol efflux from macrophages via systemic gene deletion of sterol O-acyltransferase 1 (also known as ACAT1) increases atherosclerosis and causes accumulation of cholesterol and macrophage infiltration in the skin leading to dermatitis in mice (39). The phenotype of dyslipidemia-induced dermatitis is also caused by the loss of ACAT1 exclusively in macrophages (39). Human studies have also shown a link between skin cholesterol accumulation and atherosclerosis (24, 40). Here, we investigated whether expression of apoAI from macrophages affects both atherosclerosis and dermatitis in mice under conditions of dyslipidemia and complete absence of reverse cholesterol transport. Our results support a scenario in which macrophage expression of apoAI regulates cholesterol loading and CD4+ T-cell levels to reduce atherosclerosis and rescue the severe dermatitis phenotype without affecting serum lipids and HDL levels or tissue macrophage numbers.
The goal of our study was to investigate the effects of macrophage apoAI expression in the aortic wall, skin, and skin-draining LNs. Because cholesterol-induced dermatitis and atherosclerosis are both characterized by cellular cholesterol accumulation and cholesterol-induced inflammation, macrophage-based apoAI expression is an attractive therapeutic target for the control of both conditions. HPC transplantation is a reliable approach to study the effect of manipulations of macrophage gene expression on atherosclerosis in the mouse (41), with translational applications for a cell-based therapy in humans (42).
ApoAI has functional characteristics similar to that of apoE (43); however, macrophages do not naturally make apoAI and instead tremendously upregulate apoE to serve as the physiological driver of cholesterol efflux under conditions leading to foam cell transformation (44, 45). ApoE affects atherosclerosis both by reducing serum lipid levels (46, 47) and by exerting local effects on the forming atheroma (48). In contrast, transgenic expression of apoAI from macrophages has been shown to reduce atherogenesis in mice exclusively via local effects and without altering serum lipid levels (12, 13, 17). In this study, LDLR−/−/apoAI−/− recipient mice transplanted with apoAI-expressing HPC showed significantly less aortic lesion size compared with recipient mice transplanted with control HPC. ApoAI expressed by HPCs accumulated in serum in extremely low levels (i.e., ∼300,000 times less than physiological levels), too low to affect serum cholesterol, triglyceride, or HDL levels. It must be noted that the clear antiatherogenic power of macrophage-produced apoAI we observed in the current study, with extremely advanced, complex, and large aortic lesions, may suggest much larger effects under conditions of initial atherosclerosis development and, most importantly, under conditions conducive to atherosclerosis regression.
Aortic lesions contain multiple hematopoietic cell populations, including macrophages, T cells, and DCs (49). Modulation of the local macrophage inflammatory response is able to affect lesion composition (50, 51), and apoAI modulates macrophage cytokine production and phenotypic stance (20, 21). Our data show that macrophage apoAI exerts local anti-inflammatory effects in the atheroma, with reduced lesional accumulation of CD3+ and CD4+ T cells. In addition, macrophage apoAI reduces the formation of necrotic core in the vessel wall, a key determinant of lesion stability. Both T-cell recruitment and necrotic core formation are indicators of advanced stage aortic lesions; interestingly, macrophage apoAI expression did not affect macrophage numbers per unit area in the atherosclerotic lesion.
Local lipid trafficking is of primary importance for the structural and functional integrity of the skin barrier (52). In patients with dermatitis, a disease caused by altered skin barrier and loss of immune regulation, skin cholesterol levels are significantly higher compared with healthy controls (23). In addition, skin cholesterol levels correlate with the extent of atherosclerosis measured by coronary artery angiography, coronary calcium score, or carotid intima media thickness (24). Feingold et al. (53) have previously shown that microscopic skin lesions enriched in cholesterol and with features of inflammation are present in mice with altered lipoprotein metabolism (e.g., apoE−/− mice). A more severe phenotype of skin cholesterol accumulation is found when serum cholesterol is elevated and cholesterol efflux pathways are inhibited (e.g., apoE−/−/ACAT1−/− mice) (39). Another model of dysfunctional apoB-containing lipoprotein metabolism and cholesterol efflux is the LDLR−/−/apoAI−/− mouse, which develops severe dermatitis on a high-fat diet containing palm oil (26). Here we show that macrophage apoAI expression reduced skin cholesterol accumulation, restored skin morphology, and rescued LDLR−/−/apoAI−/− mice from their severe skin phenotype, often lethal. Control recipient mice started to develop skin lesions around 12–13 weeks, as was previously described by Zabalawi et al. (26), while apoAI recipients developed smaller skin lesions at ∼14–15 weeks mainly limited to areas around the ears. No behavioral differences were observed between mice of the two groups. Our experimental model shows that apoAI expressed locally by macrophages reduces CD3+ and CD4+ T cell levels in the skin, and total CD4+ and effector/memory CD4+ cells in skin-draining LNs. In contrast, macrophage apoAI expression did not affect macrophages or other cells of the hematopoietic lineage in the skin or skin-draining LNs.
We conclude that macrophage apoAI inhibits dyslipidemia-induced dermatitis and atherosclerosis directly through reduced cholesterol accumulation and regulation of CD4+ T cells in the aortic wall, skin, and skin-draining LNs, all without affecting tissue macrophage content or serum HDL and apoAI levels. Our results also suggest that production of apoAI by macrophages does not affect macrophage recruitment into the aortic wall or skin caused by the initial lipid insult. However, the reduced lipid loading of macrophages within the tissue inhibits further aggravation of inflammation and subsequent T-cell response. These results also suggest that transplantation of apoAI-transduced HPCs may be developed as a cell-based therapy for treatment of cholesterol-related dermatitis, such as eczema, with the additional benefit of protecting against atherosclerosis.
Supplementary Material
Footnotes
Abbreviations:
- DAPI
- 4,6-diamidino-2-phenylindole
- DC
- dendritic cell
- GFP
- green fluorescent protein
- H and E
- hematoxylin and eosin
- HPC
- hematopoietic progenitor cell
- LDLR
- LDL receptor
- LN
- lymph node
- ORO
- Oil Red O
This work was supported by National Institutes of Health Grants R01 HL057986 and HL106845 (S.F.), PO1 HL116263 (M.F.L.), DK59637 (Lipid, Lipoprotein and Atherosclerosis Core of the Vanderbilt Mouse Metabolic Phenotype Centers), and R01 HL112270 and HL112276 (M.G.S-T.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures.
REFERENCES
- 1.Castelli W. P., Garrison R. J., Wilson P. W., Abbott R. D., Kalousdian S., Kannel W. B. 1986. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. J. Am. Med. Assoc. 256: 2835–2838. [PubMed] [Google Scholar]
- 2.Assmann G., Cullen P., Schulte H. 1998. The Munster Heart Study (PROCAM). Results of follow-up at 8 years. Eur. Heart J. 19 (Suppl. A): A2–11. [PubMed] [Google Scholar]
- 3.Rosenson R. S., Brewer H. B., Jr, Davidson W. S., Fayad Z. A., Fuster V., Goldstein J., Hellerstein M., Jiang X. C., Phillips M. C., Rader D. J., et al. 2012. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 125: 1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maxfield F. R., Tabas I. 2005. Role of cholesterol and lipid organization in disease. Nature. 438: 612–621. [DOI] [PubMed] [Google Scholar]
- 5.Sirtori C. R., Calabresi L., Franceschini G., Baldassarre D., Amato M., Johansson J., Salvetti M., Monteduro C., Zulli R., Muiesan M. L., et al. 2001. Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: the Limone sul Garda study. Circulation. 103: 1949–1954. [DOI] [PubMed] [Google Scholar]
- 6.Hirano K., Yamashita S., Kuga Y., Sakai N., Nozaki S., Kihara S., Arai T., Yanagi K., Takami S., Menju M., et al. 1995. Atherosclerotic disease in marked hyperalphalipoproteinemia. Combined reduction of cholesteryl ester transfer protein and hepatic triglyceride lipase. Arterioscler. Thromb. Vasc. Biol. 15: 1849–1856. [DOI] [PubMed] [Google Scholar]
- 7.Kingwell B. A., Chapman M. J., Kontush A., Miller N. E. 2014. HDL-targeted therapies: progress, failures and future. Nat. Rev. Drug Discov. 13: 445–464. [DOI] [PubMed] [Google Scholar]
- 8.Mohammadpour A. H., Akhlaghi F. 2013. Future of cholesteryl ester transfer protein (CETP) inhibitors: a pharmacological perspective. Clin. Pharmacokinet. 52: 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rader D. J., Hovingh G. K. 2014. HDL and cardiovascular disease. Lancet. 384: 618–625. [DOI] [PubMed] [Google Scholar]
- 10.Rader D. J., Alexander E. T., Weibel G. L., Billheimer J., Rothblat G. H. 2009. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 50 (Suppl.): S189–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L., Sharifi B. G., Pan T., Song L., Yukht A., Shah P. K. 2006. Bone marrow transplantation shows superior atheroprotective effects of gene therapy with apolipoprotein A-I Milano compared with wild-type apolipoprotein A-I in hyperlipidemic mice. J. Am. Coll. Cardiol. 48: 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Major A. S., Dove D. E., Ishiguro H., Su Y. R., Brown A. M., Liu L., Carter K. J., Linton M. F., Fazio S. 2001. Increased cholesterol efflux in apolipoprotein AI (ApoAI)-producing macrophages as a mechanism for reduced atherosclerosis in ApoAI((−/−)) mice. Arterioscler. Thromb. Vasc. Biol. 21: 1790–1795. [DOI] [PubMed] [Google Scholar]
- 13.Ishiguro H., Yoshida H., Major A. S., Zhu T., Babaev V. R., Linton M. F., Fazio S. 2001. Retrovirus-mediated expression of apolipoprotein A-I in the macrophage protects against atherosclerosis in vivo. J. Biol. Chem. 276: 36742–36748. [DOI] [PubMed] [Google Scholar]
- 14.Su Y. R., Ishiguro H., Major A. S., Dove D. E., Zhang W., Hasty A. H., Babaev V. R., Linton M. F., Fazio S. 2003. Macrophage apolipoprotein A-I expression protects against atherosclerosis in ApoE-deficient mice and up-regulates ABC transporters. Mol. Ther. 8: 576–583. [DOI] [PubMed] [Google Scholar]
- 15.Bielicki J. K., McCall M. R., Forte T. M. 1999. Apolipoprotein A-I promotes cholesterol release and apolipoprotein E recruitment from THP-1 macrophage-like foam cells. J. Lipid Res. 40: 85–92. [PubMed] [Google Scholar]
- 16.Kockx M., Rye K. A., Gaus K., Quinn C. M., Wright J., Sloane T., Sviridov D., Fu Y., Sullivan D., Burnett J. R., et al. 2004. Apolipoprotein A-I-stimulated apolipoprotein E secretion from human macrophages is independent of cholesterol efflux. J. Biol. Chem. 279: 25966–25977. [DOI] [PubMed] [Google Scholar]
- 17.Su Y. R., Blakemore J. L., Zhang Y., Linton M. F., Fazio S. 2008. Lentiviral transduction of apoAI into hematopoietic progenitor cells and macrophages: applications to cell therapy of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 28: 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linton M. F., Fazio S. 2003. Macrophages, inflammation, and atherosclerosis. Int. J. Obes. Relat. Metab. Disord. 27 (Suppl. 3): S35–S40. [DOI] [PubMed] [Google Scholar]
- 19.Kontush A., Chapman M. J. 2006. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 58: 342–374. [DOI] [PubMed] [Google Scholar]
- 20.Li Q., Zhai Z., Ma W., Qian Z. 2014. Anti-inflammatory effects of apoprotein AI are mediated via modulating macrophage polarity. Zhonghua. Xin Xue Guan Bing Za Zhi. 42: 132–135. [PubMed] [Google Scholar]
- 21.Yin K., Tang S. L., Yu X. H., Tu G. H., He R. F., Li J. F., Xie D., Gui Q. J., Fu Y. C., Jiang Z. S., et al. 2013. Apolipoprotein A-I inhibits LPS-induced atherosclerosis in ApoE(−/−) mice possibly via activated STAT3-mediated upregulation of tristetraprolin. Acta Pharmacol. Sin. 34: 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feingold K. R. 2013. Innate immunity stimulates permeability barrier homeostasis. J. Invest. Dermatol. 133: 1925–1927. [DOI] [PubMed] [Google Scholar]
- 23.Di Nardo A., Wertz P., Giannetti A., Seidenari S. 1998. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm. Venereol. 78: 27–30. [DOI] [PubMed] [Google Scholar]
- 24.Mancini G. B., Chan S., Frohlich J., Kuramoto L., Schulzer M., Abbott D. 2002. Association of skin cholesterol content, measured by a noninvasive method, with markers of inflammation and Framingham risk prediction. Am. J. Cardiol. 89: 1313–1316. [DOI] [PubMed] [Google Scholar]
- 25.Zabalawi M., Bharadwaj M., Horton H., Cline M., Willingham M., Thomas M. J., Sorci-Thomas M. G. 2007. Inflammation and skin cholesterol in LDLr−/−, apoA-I−/− mice: link between cholesterol homeostasis and self-tolerance? J. Lipid Res. 48: 52–65. [DOI] [PubMed] [Google Scholar]
- 26.Zabalawi M., Bhat S., Loughlin T., Thomas M. J., Alexander E., Cline M., Bullock B., Willingham M., Sorci-Thomas M. G. 2003. Induction of fatal inflammation in LDL receptor and ApoA-I double-knockout mice fed dietary fat and cholesterol. Am. J. Pathol. 163: 1201–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilhelm A. J., Zabalawi M., Owen J. S., Shah D., Grayson J. M., Major A. S., Bhat S., Gibbs D. P., Jr, Thomas M. J., Sorci-Thomas M. G. 2010. Apolipoprotein A-I modulates regulatory T cells in autoimmune LDLr−/−, ApoA-I−/− mice. J. Biol. Chem. 285: 36158–36169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilhelm A. J., Zabalawi M., Grayson J. M., Weant A. E., Major A. S., Owen J., Bharadwaj M., Walzem R., Chan L., Oka K., et al. 2009. Apolipoprotein A-I and its role in lymphocyte cholesterol homeostasis and autoimmunity. Arterioscler. Thromb. Vasc. Biol. 29: 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavori H., Fan D., Giunzioni I., Zhu L., Linton M. F., Fogo A. B., Fazio S. 2014. Macrophage-derived apoESendai suppresses atherosclerosis while causing lipoprotein glomerulopathy in hyperlipidemic mice. J. Lipid Res. 55: 2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paigen B., Mitchell D., Reue K., Morrow A., Lusis A. J., LeBoeuf R. C. 1987. Ath-1, a gene determining atherosclerosis susceptibility and high density lipoprotein levels in mice. Proc. Natl. Acad. Sci. USA. 84: 3763–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaluzny M. A., Duncan L. A., Merritt M. V., Epps D. E. 1985. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J. Lipid Res. 26: 135–140. [PubMed] [Google Scholar]
- 32.Tavori H., Fan D., Blakemore J. L., Yancey P. G., Ding L., Linton M. F., Fazio S. 2013. Serum proprotein convertase subtilisin/kexin type 9 and cell surface low-density lipoprotein receptor: evidence for a reciprocal regulation. Circulation. 127: 2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. 2001. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). J. Am. Med. Assoc. 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 34.Vaya J., Szuchman A., Tavori H., Aluf Y. 2011. Oxysterols formation as a reflection of biochemical pathways: summary of in vitro and in vivo studies. Chem. Phys. Lipids. 164: 438–442. [DOI] [PubMed] [Google Scholar]
- 35.Moore K. J., Sheedy F. J., Fisher E. A. 2013. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13: 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singaraja R. R., Fievet C., Castro G., James E. R., Hennuyer N., Clee S. M., Bissada N., Choy J. C., Fruchart J. C., McManus B. M., et al. 2002. Increased ABCA1 activity protects against atherosclerosis. J. Clin. Invest. 110: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khera A. V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X. M., Tang W. H., Mosior M. K., Huang Y., Wu Y., Matter W., Gao V., Schmitt D., Didonato J. A., Fisher E. A., et al. 2013. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler. Thromb. Vasc. Biol. 33: 1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Accad M., Smith S. J., Newland D. L., Sanan D. A., King L. E., Jr, Linton M. F., Fazio S., Farese R. V., Jr 2000. Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA:cholesterol acyltransferase 1. J. Clin. Invest. 105: 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zawydiwski R., Sprecher D. L., Evelegh M. J., Horsewood P., Carte C., Patterson M. 2001. A novel test for the measurement of skin cholesterol. Clin. Chem. 47: 1302–1304. [PubMed] [Google Scholar]
- 41.Aparicio-Vergara M., Shiri-Sverdlov R., de Haan G., Hofker M. H. 2010. Bone marrow transplantation in mice as a tool for studying the role of hematopoietic cells in metabolic and cardiovascular diseases. Atherosclerosis. 213: 335–344. [DOI] [PubMed] [Google Scholar]
- 42.Felfly H., Haddad G. G. 2014. Hematopoietic stem cells: potential new applications for translational medicine. J. Stem Cells. 9: 163–197. [PubMed] [Google Scholar]
- 43.Vedhachalam C., Narayanaswami V., Neto N., Forte T. M., Phillips M. C., Lund-Katz S., Bielicki J. K. 2007. The C-terminal lipid-binding domain of apolipoprotein E is a highly efficient mediator of ABCA1-dependent cholesterol efflux that promotes the assembly of high-density lipoproteins. Biochemistry. 46: 2583–2593. [DOI] [PubMed] [Google Scholar]
- 44.Dove D. E., Linton M. F., Fazio S. 2005. ApoE-mediated cholesterol efflux from macrophages: separation of autocrine and paracrine effects. Am. J. Physiol. Cell Physiol. 288: C586–C592. [DOI] [PubMed] [Google Scholar]
- 45.Tall A. R., Costet P., Wang N. 2002. Regulation and mechanisms of macrophage cholesterol efflux. J. Clin. Invest. 110: 899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linton M. F., Atkinson J. B., Fazio S. 1995. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 267: 1034–1037. [DOI] [PubMed] [Google Scholar]
- 47.Linton M. F., Fazio S. 1999. Macrophages, lipoprotein metabolism, and atherosclerosis: insights from murine bone marrow transplantation studies. Curr. Opin. Lipidol. 10: 97–105. [DOI] [PubMed] [Google Scholar]
- 48.Fazio S., Babaev V. R., Murray A. B., Hasty A. H., Carter K. J., Gleaves L. A., Atkinson J. B., Linton M. F. 1997. Increased atherosclerosis in mice reconstituted with apolipoprotein E null macrophages. Proc. Natl. Acad. Sci. USA. 94: 4647–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galkina E., Ley K. 2009. Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 27: 165–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yancey P. G., Ding Y., Fan D., Blakemore J. L., Zhang Y., Ding L., Zhang J., Linton M. F., Fazio S. 2011. Low-density lipoprotein receptor-related protein 1 prevents early atherosclerosis by limiting lesional apoptosis and inflammatory Ly-6Chigh monocytosis: evidence that the effects are not apolipoprotein E dependent. Circulation. 124: 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy A. J., Akhtari M., Tolani S., Pagler T., Bijl N., Kuo C. L., Wang M., Sanson M., Abramowicz S., Welch C., et al. 2011. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 121: 4138–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feingold K. R. 2009. The outer frontier: the importance of lipid metabolism in the skin. J. Lipid Res. 50 (Suppl.): S417–S422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feingold K. R., Elias P. M., Mao-Qiang M., Fartasch M., Zhang S. H., Maeda N. 1995. Apolipoprotein E deficiency leads to cutaneous foam cell formation in mice. J. Invest. Dermatol. 104: 246–250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.