Abstract
The capacity of HDL to remove cholesterol from macrophages is inversely associated with the severity of angiographic coronary artery disease. The effect of human immunodeficiency virus (HIV) infection or its treatment on the ability of HDL particles to stimulate cholesterol efflux from human macrophages has never been studied. We evaluated the capacity of whole plasma and isolated HDL particles from HIV-infected subjects (n = 231) and uninfected controls (n = 200), as well as in a subset of 41 HIV subjects receiving highly active antiretroviral therapy (HAART) to mediate cholesterol efflux from human macrophages. Plasma cholesterol efflux capacity was reduced (−12%; P = 0.001) in HIV patients as compared with controls. HIV infection reduced by 27% (P < 0.05) the capacity of HDL subfractions to promote cholesterol efflux from macrophages. We observed a reduced ABCA1-dependent efflux capacity of plasma (−27%; P < 0.0001) from HIV-infected subjects as a result of a reduction in the efflux capacity of HDL3 particles. HAART administration restored the capacity of plasma from HIV patients to stimulate cholesterol efflux from human macrophages (9.4%; P = 0.04). During HIV infection, the capacity of whole plasma to remove cholesterol from macrophages is reduced, thus potentially contributing to the increased coronary heart disease in the HIV population. HAART administration restored the removal of cholesterol from macrophages by increasing HDL functionality.
Keywords: high density lipoprotein function, macrophage cholesterol efflux, reverse cholesterol transport, antiretroviral therapy, human immunodeficiency virus, highly active antiretroviral therapy
Coronary heart disease rates are expected to be higher as the human immunodeficiency virus (HIV) population ages. Controversy still exists as to whether HIV infection per se or its treatment increases the rate of coronary heart disease. Highly active antiretroviral therapy (HAART) has been associated with the occurrence of cardiovascular events in HIV-infected patients (1, 2). However, in the Strategies for Managment of Antiretroviral Therapy (SMART) trial, the episodic use of HAART was associated with an increased risk of CVD when compared with continuous HAART use (3). It is now well-established that atherosclerosis is a lipid-inflammatory-oxidative disease. Undoubtedly, endothelial dysfunction, immune activation, inflammation, and alterations in plasma lipids (4) are dynamic features of both untreated HIV infection and atherosclerosis (5, 6). Obviously HIV infection and its treatment exert differential effects on plasma lipids. The early epidemiological studies point up to the occurrence of dyslipidemia in early HIV infection far before HAART initiation (7). The most pronounced effect is attributed to HIV-induced hypocholesterolemia, characterized by a reduction in total cholesterol (TC), LDL cholesterol (LDL-C), and HDL cholesterol (HDL-C) levels (8, 9). Reverse pronounced changes are seen after HAART. While levels of LDL and TC are recovered or even increased, those of HDL-C are not. Low levels of HDL-C constitute a major risk factor for atherosclerosis (10). However, until now, therapeutic strategies aimed at increasing HDL-C levels have failed to significantly reduce cardiovascular mortality (11), indicating that circulating HDL-C levels constitute a weak marker to evaluate HDL particle functionality (12). Therefore, systemic availability of functional HDL is essential to maintain their anti-atherogenic actions, such as antioxidant and anti-inflammatory roles, as well as effective reverse cholesterol transport (RCT) pathway (13, 14). Importantly, the capacity of HDL to remove cholesterol from macrophages, thus representing a metric of HDL function, has been shown to be clinically relevant, as it is strongly and inversely associated with both carotid intima-media thickness and the severity of angiographic coronary artery disease; such observations being independent of HDL-C levels (15).
Earlier studies reported no significant alteration in the capacity of plasma from HIV-infected patients to mediate cholesterol efflux either from mouse or human macrophages (16, 17). However, the relative contribution of the ABCA1, scavenger receptor class B type I (SR-BI)/Cla1, and ABCG1 pathways to cholesterol export from macrophages is species specific (18). In human macrophages, ABCA1 is considered to be the major cholesterol transporter which interacts preferentially with lipid-poor ApoAI (19, 20). Of secondary importance, the SR-BI/Cla-1 receptor promotes bidirectional cholesterol fluxes and interacts mostly with large HDL particles in order to facilitate the exit of free cholesterol (FC) from macrophages (21). The ABCG1 transporter plays an important role in cholesterol efflux from mouse macrophages, however its contribution to promoting cholesterol efflux from human macrophages is mostly insignificant (18, 22).To date, no study has investigated the effect of HIV infection or its treatment on the ability of HDL particles to stimulate cholesterol efflux from human macrophages. We hypothesize that HIV infection might induce the appearance of dysfunctional HDL particles, thus reducing cholesterol efflux from human macrophages and favoring atherosclerosis progression. Chronic inflammation may directly lead to alterations in HDL composition, decreasing their ability to promote cholesterol from macrophages. Indeed, in patients with rheumatoid arthritis, an inverse correlation between markers of inflammation and the ability of HDL to promote cholesterol efflux was observed (23). Equally, an impairment of RCT from macrophage to feces has been observed in a rodent subacute endotoxemia model (24). The purpose of this study was to evaluate, in a large population, the effects of HIV infection, in the absence of antiretroviral therapy, on the capacity of plasma or isolated HDL particles to mediate FC efflux from human macrophages and from various cellular models, each representative of one specific efflux pathway. Then we assessed the impact of the HAART regimen on HDL functionality.
MATERIALS AND METHODS
Patients
The study was conducted in a total of 231 HIV-infected adult patients (177 men and 54 women) included in the metabolic substudy of the ANRS-Inserm COPANA Cohort (4), a longitudinal study of recently diagnosed HIV-infected patients naïve from HAART at entry. A total of 200 HIV-uninfected patients (147 men and 53 women) displaying fasting plasma lipid values within the normal range (TC <250 mg/dl, LDL-C <160 mg/dl, TGs <150 mg/dl) served as control subjects (Table 1). None of the control or HIV-infected patients were under current lipid-lowering therapy or obese (BMI <30 kg/m2). Approximately 38% of HIV-infected patients and 22% of HIV-uninfected subjects were current smokers. Clinical parameters of the HIV-infected population are presented in supplementary Tables 1 and 2. A subset of 41 subjects from the initial HIV-untreated population received antiretroviral therapy for at least 3 months and up to 5 years (31.5 ± 17.5 months) (Table 1). HAART regimens consisted of two reverse transcriptase inhibitors (tenofovir and emtricitabine) in association with either a protease inhibitor (atazanavir or lopinavir) boosted with ritonavir (n = 19 patients) or a non-nucleosidic transcriptase inhibitor (efavirenz) (n = 22 patients).
TABLE 1.
Clinical and biological characteristics of HIV-infected patients and HIV-uninfected controls
| HIV-Infected Patients | ||||
| HIV-Uninfected Controls (n = 200) | Before HAART (n = 231) | Before HAART (n = 41) | After HAART (n = 41) | |
| Clinical parameters | ||||
| Age, years | 43.3 ± 12.8 | 37.2 ± 9.4a | 38.3 ± 11.5 | |
| Gender, M/F | 147/53 | 177/54 | 36/5 | |
| BMI, kg/m2 | 27.1 ± 4.7 | 23.6 ± 3.4a | 23.8 ± 3.8 | 24.1 ± 4.3 |
| Plasma parameters (mg/dl) | ||||
| TC | 203.2 ± 43.9 | 173.0 ± 42.3a | 161.4 ± 25.3 | 198.6 ± 34.5c |
| TGs | 110.5 ± 49.5 | 104.1 ± 66.4 | 112.7 ± 39.2 | 116.3 ± 44.7 |
| LDL-C | 130.7 ± 41.2 | 108.2 ± 35.8a | 103.7 ± 28.6 | 126.2 ± 35.1c |
| HDL-C | 50.3 ± 16.4 | 44.2 ± 13.8a | 35.2 ± 8.1 | 49.1 ± 12.6c |
| apoB | 102.6 ± 27.3 | 81.8 ± 23.4a | 77.9 ± 12.7 | 85.9 ± 19.2c |
| apoAI | 144.4 ± 32.7 | 108.3 ± 23.4a | 97.2 ± 17.1 | 126.0 ± 21.9c |
| Endogenous plasma CETP activity (%) | 29.7 ± 7.2 | 27.6 ± 7.4b | 26.9 ± 6.5 | 29.0 ± 6.7d |
P < 0.0001 versus HIV-uninfected controls.
P < 0.05 versus HIV-uninfected controls after adjustment for LDL-C, HDL-C, TG, age, and BMI.
P < 0.05 versus HIV-infected patients before HAART (n = 41).
P < 0.05 versus HIV-infected patients before HAART (n = 41) and after adjustment for LDL-C, HDL-C, TG, age, and BMI.
The study was performed in accordance with the ethical principles set forth in the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Biochemical analyses
Lipids and apolipoproteins of plasma and in isolated lipoprotein fractions were determined by using an autoanalyzer (Konelab 20) and commercial reagent kit from Roche Diagnostics for TC, from Thermo-Electron for TGs, HDL-C, apoAI, and apoB, from Diasys for phospholipids and FC, and from Pierce for total protein quantification (bicinchoninic acid assay reagent). Fasting plasma LDL-C was calculated using the Friedewald formula. Endogenous cholesteryl ester transfer from HDL to apoB-containing lipoproteins was assayed as previously described (25). Individual lipoprotein subfractions were isolated from plasma by isopycnic density gradient ultracentrifugation for 48 h at 40,000 rpm using a Beckman XL70 centrifuge and a SW41 rotor as previously described (26). All lipoprotein subfractions were analyzed for their lipid and protein contents. Total lipoprotein mass was calculated as the sum of the mass of individual lipid and protein components.
FC efflux assays
Efflux assays were performed using human THP-1 macrophages and several cellular models, Fu5AH, CHO-K1, CHO-hABCG1, and CHO-hABCA1, as previously described (27). 3H-cholesterol-labeled cells were incubated for 4 h at 37°C in the presence of 40-fold diluted total plasma or individual HDL subfractions [15 μg phospholipid/ml for cholesterol loaded-THP-1 macrophages; 10 μg phospholipid/ml for Fu5AH (SR-BI-dependent efflux); 5 μg phospholipid/ml for CHO-K1 and CHO-hABCG1; 10 μg apoAI/ml for CHO-hABCA1]. Fractional cholesterol efflux was calculated as the amount of the label recovered in the medium divided by the total label in each well. The background cholesterol efflux obtained in the absence of any acceptor was subtracted from the efflux obtained with samples. ABCG1-dependent efflux was calculated as the difference between efflux to CHO-hABCG1 and CHO-K1 cells. For CHO-hABCA1, the expression of ABCA1 was induced by tetracycline (1 μg/ml). The ABCA1-dependent efflux was calculated as the difference between efflux to activated CHO-hABCA1 and non-activated cells. Plasma efflux capacity was expressed as a relative efflux obtained by dividing the fractional efflux of sample values by those obtained with the standard plasma. The capacity of individual HDL subfractions to mediate FC efflux is expressed as the percentage of cholesterol efflux per mole of acceptor particle, as previously described (27). All efflux experiments were performed in triplicate for each sample.
Statistical analysis
Statistical analyses were performed with GraphPad Prism 4 and the R statistical software 2.12.2. Numerical variables are presented as mean ± SD. Mean differences between HIV-infected patients and HIV-uninfected controls were compared using an unpaired t-test. A paired t-test was used to assess the differences between baseline (before treatment) and following HAART (after treatment). In order to compare continuous variables between HIV-infected patients and HIV-uninfected controls, we used multivariate linear regression analysis adjusted for age and BMI. We considered the comparative parameter (cholesterol efflux measurements) as the predicted variable, whereas HIV infection, age, and BMI were considered as predictive variables. All P values are presented after adjustment for age and BMI differences and for plasma lipid levels or inflammatory makers when indicated. The results were considered to be statistically significant at P < 0.05.
RESULTS
Impact of HIV infection and HAART on plasma lipid and apolipoprotein levels and on lipoprotein subspecies distribution
HIV-infected patients displayed significant reductions in plasma levels of TC, LDL-C, and apoB compared with HIV-uninfected controls (Table 1). HIV-infected patients were equally characterized by significant reductions in HDL-C and apoAI levels as compared with uninfected controls. Similar fasting plasma TG levels were observed between HIV-infected patients and controls. Endogenous plasma cholesteryl ester transfer protein (CETP) activity was significantly reduced by 7.3% (P < 0.003) in HIV-infected patients in comparison with HIV-uninfected controls. However, it is important to note that HDL-C, LDL-C, TG, sex, and BMI represent independent modulators of endogenous plasma CETP activity (28). The difference in CETP activity between HIV-infected patients and HIV-uninfected controls remained significant after adjustment for those parameters (P < 0.05, Table 1).
As shown in supplementary Table 2, additional analyses were performed by dividing HIV-infected patients according to circulating levels of CD4+. Our analyses revealed that patients with the lowest CD4+ levels (<200 cells/mm3) displayed significantly reduced plasma levels of TC (−11%; P = 0.02), LDLC (−18%; P = 0.006), apoB (−10%; P = 0.005), and apoAI (−9%; P = 0.007) when compared with the subgroup with the highest levels of CD4+. Patients with the lowest CD4+ count (<200 cells/mm3) displayed significantly higher TG levels (47%) than those belonging to the highest quartile of CD4 cell count. Equally, levels of inflammatory markers such as IL-6, as well as those of viral charge, were significantly elevated in the subgroup of patients with the lowest CD4+ count. These observations suggest that the subgroup of patients with the lowest CD4+ count display a high risk to develop acquired immune deficiency syndrome (AIDS). Indeed, it has been previously reported that patients with AIDS displayed an increase in plasma TG levels and hepatic lipogenesis, as well as a decrease in TG clearance (29). By contrast, patients with HIV infection displayed plasma TG levels within the normal range, shedding light on the possibility that the decrease in TG clearance leading to an increase in plasma TG levels observed in patients with AIDS represents a consequence of AIDS development. HDL-C levels trend toward reduction (−13%) in patients with low CD4+ levels (<200 cells/mm3) as compared with those with higher CD4+ levels; however, such reduction did not reach statistical significance (P = 0.06). Interestingly, similar conclusions were reached when considering CD4+ count as a continuum (supplementary Table 3).
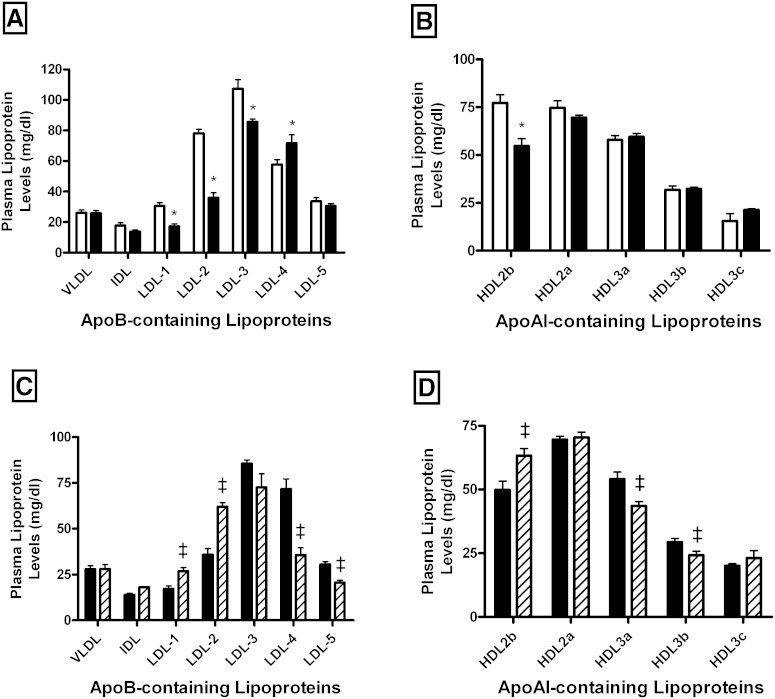
Figure 1 represents the distribution of apoB- and apoAI-containing lipoproteins isolated by isopycnic density gradient ultracentrifugation from HIV-infected patients and control subjects. As shown in Fig. 1A, similar plasma levels of TG-rich lipoprotein subfractions, i.e., VLDL and IDL, were observed in both groups of subjects. By contrast, HIV-infected patients displayed a significantly reduced LDL concentration (−21.6%; P < 0.0001) as compared with uninfected controls. Interestingly, such a reduction primarily reflected a marked and specific decrease in plasma levels of light LDL subspecies, LDL-1 (−43.7%; P < 0.0001) and LDL-2 (−54.1%; P < 0.0001), and to a lesser extent in those of LDL subfractions of intermediate density, LDL-3 (−20.3%; P < 0.0001), in comparison with controls. By contrast, plasma levels of the denser LDL subfractions appeared to be significantly increased (LDL-4, 24.2%; P < 0.05). Analysis of the distribution of apoAI-containing lipoprotein subspecies revealed that HIV infection was associated with a significant reduction (−18.2%; P < 0.005) in the mean plasma of HDL2 levels (124.3 ± 13.4 mg/dl and 151.9 ± 21.8 mg/dl in HIV-infected patients and controls, respectively), whereas those of the HDL3 subfraction were not significantly modified (113.2 ± 8.8 mg/dl and 105.1 ± 17.3 mg/dl in HIV-infected patients and controls, respectively). When individual HDL subfractions were considered, we observed a specific reduction in plasma levels of the largest HDL2b subfraction (−29.1%; P < 0.0001) in HIV-infected patients as compared with controls (Fig. 1B). HIV infection equally modified the qualitative features of circulating lipoprotein particles (supplementary Tables 4, 5).
Fig. 1.
Impact of HIV infection on plasma levels of lipoprotein subclasses isolated by gradient ultracentrifugation in HIV-infected subjects (n = 231; closed bars) and HIV-uninfected controls (n = 200; open bars) (A, B) and in a subset of HIV-infected patients before (n = 41; closed bars) and after HAART (n = 41; hatched bars) (C, D). A–C: Bar graphs showing levels of VLDL, IDL, and LDL subclasses. B–D: Bar graphs showing HDL subfraction levels. Values are mean ± SEM. *P < 0.05 versus controls; ‡P < 0.05 versus HIV-infected patients before treatment.
HAART induced a significant increase in plasma levels of TC, LDL-C, and HDL-C (Table 1). After an adjustment for HDL-C, LDL-C, and TG plasma levels, we observed a significant increase in CETP activity following HAART initiation (7.8%; P = 0.006). We presently observed that HAART corrects the apoB-containing lipoprotein profile toward an anti-atherogenic pattern. Indeed, HIV-infected subjects displayed a significant increase in light LDL-1 (1.5-fold; P < 0.005) and LDL-2 (1.7-fold; P < 0.0001) subfractions. In contrast, we observed a significant decrease in the small dense pro-atherogenic LDL-4 (−50.4%; P < 0.0003) and LDL-5 (−32.6%; P < 0.0005) in HAART-treated patients (Fig. 1C). In addition, plasma levels of large HDL2b subfractions significantly increased (26.9%; P = 0.009) following HAART administration (Fig. 1D), whereas those of small HDL were significantly reduced following HAART initiation (HDL3a, −19.6%; P < 0.005 and HDL3b, −17.5%; P < 0.05).
HIV infection reduces plasma and HDL efflux capacity via ABCA1 and SR-BI pathways independently of HDL subfraction levels: impact of HAART
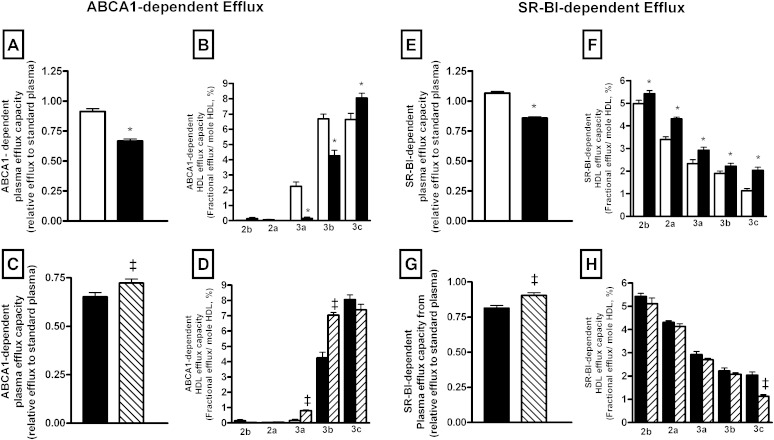
We explored the effect of HIV infection on the capacity of plasma to stimulate cholesterol removal from cells via each known specific efflux pathway. Interestingly, we observed that plasma from HIV-infected patients displayed a reduced capacity to stimulate cholesterol efflux via the ABCA1 pathway (−27%, P < 0.0001) in comparison with HIV-uninfected controls (Fig. 2A). In order to evaluate the contribution of various HDL subspecies in the reduced plasma efflux capacity associated with HIV infection, we evaluated the capacity of individual HDL subspecies to stimulate cholesterol efflux. It is well-established that ABCA1 interacts preferentially with small lipid-poor apoAI-containing lipoprotein. In good agreement with the known mechanism of action of ABCA1, we observed that large HDL2 particles isolated from HIV-infected or HIV-uninfected patients were quite inefficient in mediating cellular cholesterol efflux via the ABCA1 pathway (Fig. 2B, supplementary Fig. 1A). By contrast, HDL3 subspecies allowed removal of cholesterol from cells in an ABCA1-dependent manner, with the smallest HDL subspecies (HDL3b and HDL3c) displaying the most potent ability for cholesterol efflux. Interestingly, the ability of HDL3a and HDL3b subfractions to mediate cholesterol efflux via ABCA1 was markedly reduced in HIV-infected patients (HDL3a, −81%; P < 0.0001 and HDL3b, −34%; P = 0.01) as compared with controls.
Fig. 2.
A–D: Bar graphs showing ABCA1-dependent efflux capacity of plasma (A, C), or isolated HDL subspecies (B, D) determined in HIV-infected subjects (n = 231; closed bars) and HIV-uninfected controls (n = 200; open bars) (A, B) and in a subset of HIV-infected patients before (n = 41; closed bars) and after HAART (n = 41; hatched bars) (C, D). The ABCA1-dependent efflux was calculated as the difference between fractional cholesterol efflux to cells in the presence or absence of tetracycline after 4 h incubation in the presence of 40-fold-diluted plasma or isolated HDL particles. E–H: Bar graphs showing SR-BI-dependent efflux capacity of plasma (E, G) or isolated HDL subspecies (F, H) determined in HIV-infected subjects (n = 231; closed bars) and controls (n = 200; open bars) (E, F) and in a subset of HIV-infected patients before (n = 41; closed bars) and after HAART (n = 41; hatched bars) (G, H). The SR-BI-dependent cholesterol efflux was determined in cultured rat hepatoma Fu5AH cells expressing high levels of SR-BI. The capacity of HDL2 (2b and 2a) and HDL3 (3a, 3b, and 3c) subfractions to mediate FC efflux through the ABCA1 (B, C) and SR-BI (F, H) pathways and expressed per moles of HDL particles. Values are mean ± SEM. *P < 0.05 versus controls; ‡P < 0.05 versus HIV-infected patients before treatment.
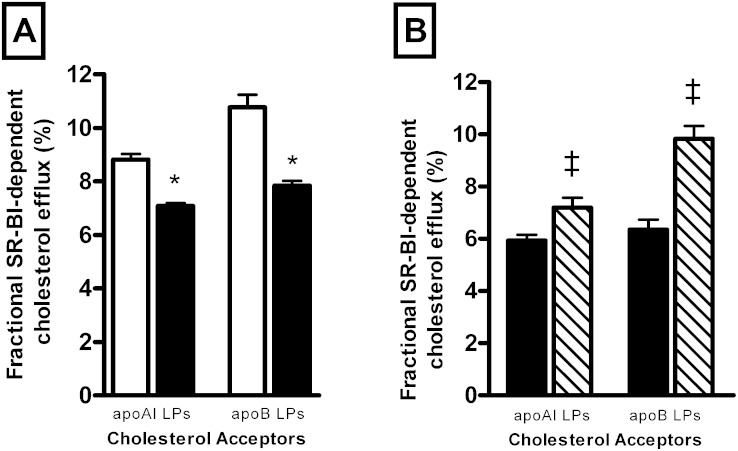
Equally, HIV infection induced a significant reduction in the capacity of the plasma to stimulate SR-BI dependent efflux (−21% P < 0.0001; Fig. 2E). As apoB-containing lipoproteins have been shown to equally contribute to cellular cholesterol efflux via SR-BI (30), we thus confirmed our observation by performing a cholesterol efflux assay using apoB-depleted plasma. As shown in Fig. 3A, apoB-depleted plasma from HIV-infected patients revealed a reduced ability (−19.6%; P < 0.0001) to mediate SR-BI-dependent efflux as compared with apoB-depleted plasma from controls. Evaluation of the capacity of isolated HDL particles to mediate SR-BI-dependent efflux revealed that all HDL subspecies isolated from HIV-infected patients displayed a significantly increased capacity to mediate cellular cholesterol efflux via the SR-BI pathway (Fig. 2F, supplementary Fig. 1C). By contrast, cellular cholesterol efflux via the ABCG1 pathway appeared not to be significantly affected by HIV infection (supplementary Fig. 2A).
Fig. 3.
A: Bar graphs showing efflux capacity of total plasma apoAI- or apoB-containing lipoproteins determined in HIV-infected subjects (n = 231; closed bars) and HIV-uninfected controls (n = 200; open bars). B: Bar graphs showing efflux capacity of total plasma apoAI- or apoB-containing lipoproteins in a subset of HIV-infected subjects before (n = 41; closed bars) and after HAART (n = 41; hatched bars). The SR-BI-dependent cholesterol efflux toward total plasma apoAI-containing lipoproteins was determined using 40-fold-diluted apoB-depleted plasma. The SR-BI-dependent cholesterol efflux toward total plasma apoB-containing lipoproteins was calculated as the difference between fractional cholesterol efflux measured in the presence of 40-fold-diluted plasma from that measured in the presence of 40-fold-diluted apoB-depleted plasma. Values are mean ± SEM. *P < 0.0001 versus controls; ‡P < 0.006 versus HIV-infected patients before treatment.
Taken together, our observations reveal that reduction in the capacity of whole plasma from HIV-infected patients to stimulate cholesterol efflux via the SR-BI pathway, as shown in Fig. 2E, results from a reduction in SR-BI-mediated efflux toward both apoB- and apoAI-containing lipoproteins (Fig. 3A). Reduction in circulating levels of cholesterol acceptors (Fig. 1A, B) primarily accounts for the reduction of plasma efflux through SR-BI, rather than from a defective ability of HDL particles to mediate cholesterol efflux from SR-BI. Multiple regression analysis indicated no significant difference in plasma efflux capacity between HIV-patient subgroups divided according to CD4+ levels, even after adjustment for plasma lipid levels or inflammatory markers (ABCA1: β = 0.04, P = 0.6; SR-BI: β = 0.06, P = 0.37; and ABCG1: β = 0.01, P = 0.8).
Interestingly, HAART significantly increased the capacity of plasma to stimulate cholesterol efflux via the ABCA1 pathway (11%; P = 0.001) (Fig. 2C). Mainly, we observed an increase in the intrinsic capacity of small HDL3a (5.2-fold; P < 0.0001) and HDL3b (66%; P < 0.0001) to stimulate cholesterol efflux via the ABCA1 pathway after HAART administration (Fig. 2D, supplementary Fig. 1B). Simultaneously, HAART significantly increased the capacity of plasma to stimulate cholesterol efflux via SR-BI (12%; P < 0.0001) (Fig. 2G). However, the intrinsic capacity of all HDL subfractions, with the exception of HDL3c, to stimulate cholesterol efflux via SR-BI was not affected by HAART (Fig. 2H, supplementary Fig. 1D). The capacity of apoB-depleted plasma to stimulate cholesterol efflux via SR-BI was significantly enhanced following HAART administration (10%; P < 0.0001) (Fig. 3B). HAART treatment increased the capacity of plasma to simulate cholesterol efflux via SR-BI (Fig. 2G) by increasing cholesterol efflux toward both apoAI- and apoB-containing lipoproteins (Fig. 3B) as a result of an increase in circulating levels of HDL and LDL particles (Fig. 1C, D), rather from an improvement of the intrinsic ability of HDL particles for SR-BI-dependent efflux (Fig. 2H). In addition, the HAART regimen induced a reduction in the capacity of plasma to stimulate cholesterol efflux via the ABCG1 pathway (−14%; P < 0.0001) (supplementary Fig. 1B).
Plasma and HDL-C efflux capacity from human macrophages is altered by HIV infection and restored following HAART
Inasmuch as we observed that HIV infection was associated with both major quantitative and qualitative alterations in circulating lipoprotein subspecies as well as a reduced plasma cholesterol efflux capacity via ABCA1 and SR-BI, we therefore analyzed the consequences of these alterations in human macrophages.
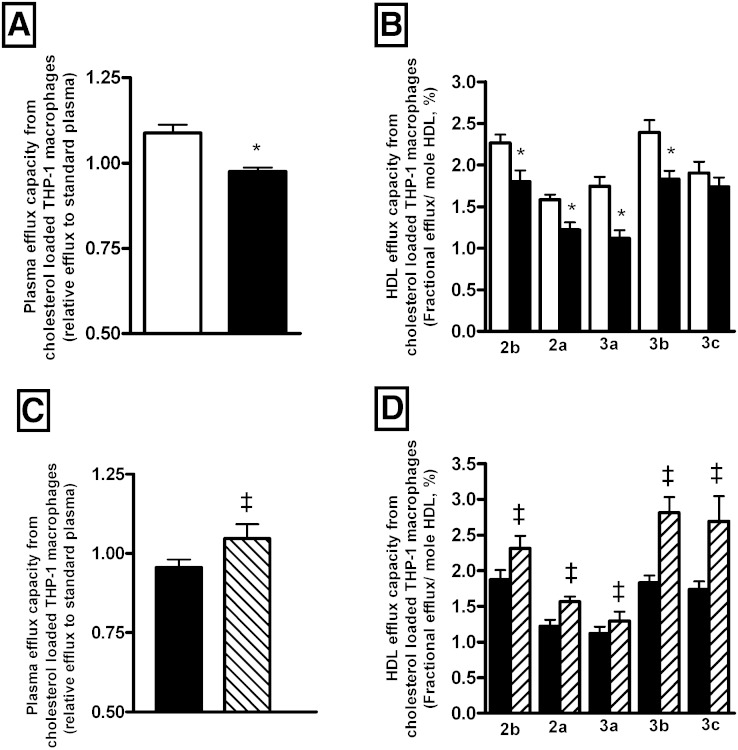
We observed that plasma from HIV-infected patients displayed a reduced capacity (−12%; P = 0.001) to remove cholesterol from human macrophages when compared with controls (Fig. 4A). Those observations remained significant after adjustment for HDL-C, LDL-C, or TG levels. Multiple regression analysis revealed no significant difference in plasma efflux capacity from THP-1 between HIV-patient subgroups divided according to CD4+ levels, even after adjustment for plasma lipid levels or inflammatory markers (β = 0.07; P = 0.354). As we could not exclude the possibility that either plasma may carry viral proteins that might affect cells and interfere with cholesterol efflux process (31), we evaluated plasma efflux capacity following pre-incubation of human THP-1 macrophages for 24 h in the presence of 10% plasma from either HIV-infected patients or HIV-uninfected controls. Data presented in supplementary Fig. 3 confirm that the capacity of plasma from HIV-infected patients to mediate cholesterol efflux capacity from human macrophages is reduced as compared with plasma from HIV-uninfected subjects. It is important to note that preincubation of cells with plasma from HIV patients appears to have an independent effect by reducing cholesterol efflux by approximately 30%. It is likely that such reductions primarily reflect a reduced capacity of HIV-infected plasma for cholesterol loading of THP-1 macrophages during the 24 h preincubation period prior to efflux measurement. Indeed, plasma from HIV-infected patients displays reduced circulating levels of LDL-C as compared with that of HIV-uninfected controls.
Fig. 4.
Bar graphs showing efflux capacity from cholesterol loaded human THP-1 macrophages of plasma (A, C) and isolated HDL subspecies (B, D) determined in HIV-infected subjects (n = 231; closed bars) (A, B), in HIV-uninfected controls (n = 200; open bars), and in a subset of HIV-infected patients before (n = 41; closed bars) and after HAART (n = 41; hatched bars) (C, D). Values are mean ± SEM. *P < 0.05 versus controls; ‡P < 0.05 versus HIV-infected patients before treatment.
Next, we evaluated the impact of HIV infection on the capacity of each HDL subfraction to remove cholesterol from human lipid-loaded macrophages. As shown in Fig. 4B and supplementary Fig. 1E, we observed that most HDL subspecies isolated from HIV-infected patients displayed a reduction in their capacity to stimulate macrophage cholesterol efflux when compared with HDL particles isolated from controls.
Most importantly, HAART was associated with a significant increase in plasma cholesterol efflux capacity from human THP-1 macrophages (10%; P = 0.04) (Fig. 4C). Interestingly, we observed that all individual HDL subfractions isolated from treated HIV patients displayed an increased intrinsic capacity to stimulate cholesterol efflux through human macrophages; HDL2b (28.3%; P < 0.03), HDL2a (28.4%; P < 0.02), HDL3a (15.8%; P < 0.05), HDL3b (53.7%; P < 0.003), and HDL3c (54.8%; P < 0.003) (Fig. 4D, supplementary Fig. 1F).
In the context of our study, we did not observe any significant difference in all measured parameters between HIV-infected patients receiving 2NRTI (tenofovir and emtricitabine) in association with a protease inhibitor (atazanavir or lopinavir) boosted with ritonavir (n = 19 patients) and those receiving 2NRTI (tenofivir and emtricitabine) in association with a NNRTI (efavirenz; n = 22 patients). Equally, we did not observe any significant impact of treatment duration on plasma cholesterol efflux capacity and on biological parameters (supplementary Table 6).
DISCUSSION
For the first time, we presently demonstrate that HIV infection reduces the capacity of the plasma to mediate cholesterol efflux from human macrophages. Such an alteration in the initial step of the RCT pathway might contribute to an accumulation of cholesterol into resident macrophages within the atherosclerotic plaque and thus might participate in the increased cardiovascular risk observed in HIV-infected subjects. Indeed, recent studies have correlated a decrease in cholesterol efflux from macrophages with the occurrence of coronary artery disease (15). First of all, our study demonstrates that HIV infection not only affects HDL-C levels but also, most importantly, HDL efflux capacities. Indeed HDL2b, HDL2a, and HDL3a from HIV-infected patients displayed a major alteration in their intrinsic capacity to mediate cholesterol efflux from human macrophages. Interestingly, and in contrast with our present observations, it has been previously reported that there is no difference in the capacity of plasma from HIV-infected patients to stimulate cholesterol efflux from macrophages. However, it is important to note that these latter studies have been conducted in a small number of subjects (16, 17) or using mouse macrophages to evaluate cholesterol efflux (16).
Few studies have evaluated the impact of HIV infection on HDL particle distribution. In particular, it has been previously reported that total HDL particle number is reduced during HIV infection (32–34). However, and in contrast with our present observations, it has been reported that the concentration of cholesterol in large HDL particles was unaltered in HIV-infected patients as compared with uninfected controls (32). Interruption of HAART was associated with a reduction in HDL particles of medium and small size (35), or only in HDL particles of medium size with no change in HDL particles of small size (36). We presently observed a specific reduction of the large HDL2b subfraction in HIV-infected patients when compared with controls. It is likely that such apparent conflicting observations might primarily result from differences in the method used to identify HDL subspecies.
It is well-established that cholesterol efflux from human macrophages primarily occurs through the ABCA1 pathway. Our present observations reveal that this major cholesterol efflux pathway is altered by HIV infection. ABCA1 facilitates FC efflux toward lipid-free/poor apoAI (37). However, there is a growing body of evidence indicating that ABCA1 not only promotes cholesterol efflux to lipid-free apoAI but also to HDL particles. Indeed, fibroblasts (38) and macrophages (18) isolated from patients carrying mutations for the ABCA1 gene exhibit an almost completely defective cholesterol efflux to apoAI, but equally a marked reduction of cholesterol efflux to HDL particles. Similarly, invalidation of Abca1 in mice confirmed that macrophage cholesterol efflux to HDL was markedly reduced in Abca1−/− mice, whereas cholesterol efflux to apoAI was totally absent (39). Consistent with these latter studies, we presently observed that HDL3 particles (HDL3c, HDL3b, and HDL3a) are able to promote cholesterol efflux via ABCA1, confirming that a subset of HDL subfraction, not just lipid-free apoAI, can act as cholesterol acceptors for ABCA1. Interestingly, HIV infection is associated with the appearance of circulating dysfunctional HDL3a and HDL3b subfractions displaying a reduced ability to remove macrophage cholesterol, likely via the ABCA1 pathway. A reduction in circulating levels of lipid-free/poor apoAI, such as prebeta1-HDL particles in HIV-infected patients, might equally participate in the alteration of plasma efflux capacity via ABCA1. Indeed, it has been previously reported that plasma levels of prebeta1-HDL particles were markedly reduced, approximately by 50%, in HIV-infected patients as compared with HIV-uninfected controls (16).
As observed for ABCA1, our present study reveals that SR-BI-dependent plasma efflux capacity is significantly attenuated by HIV infection. SR-BI contributes to cholesterol efflux in human macrophages by interacting with mature HDL particles (21). However, HIV infection appears to significantly influence the qualitative features of HDL particles, as it improves their intrinsic capacity to stimulate cholesterol efflux via the SR-BI pathway. This observation indicates that the qualitative features of HDL particles induced by HIV infection are unlikely responsible alone for the observed reduction in SR-BI-dependent plasma efflux capacity. In good agreement with our observation, it has been previously reported that the capacity of serum from patients displaying a chronic inflammatory status displayed a reduced capacity to remove cholesterol through the SR-BI pathway (40). In addition, it has been previously demonstrated that apoB-containing lipoproteins participate in the flux of cholesterol to SR-BI along with HDL lipoproteins (30). As a matter of fact, in vitro studies have also demonstrated that SR-BI can interact with LDL particles and mediate cellular cholesterol export to large buoyant LDL particles (41). Our present study demonstrates that HIV infection specifically reduces plasma levels of the largest LDL subfractions; therefore, it is likely that such modifications might partially contribute to the reduced ability of plasma to mediate macrophage cholesterol efflux via the SR-BI pathway. In the present study, we used the release of labeled cell cholesterol to quantify efflux. As cholesterol influx may occur during incubation of cells in the presence of HDL- or apoB-containing lipoproteins, we cannot exclude the possibility that under our experimental conditions, isotopic release of cellular cholesterol was associated with an increase in cellular cholesterol content due to a significant level of cholesterol influx into the cells, leading in some ways to an overestimation of cholesterol efflux (42). Nonetheless, cholesterol influx does not appear to be quantitatively significant at short periods of cell incubation (4 h), as performed in the present study, as compared with cholesterol efflux (42–45). Interestingly, no significant impact of HIV infection on the capacity of plasma to stimulate cholesterol efflux through the ABCG1 pathway has been observed. ABCG1 has an important role in the removal of cholesterol from mouse macrophages (46), however its contribution in cholesterol efflux from human macrophages has been shown to be less important (18). We thus conclude that HIV infection is associated with an altered capacity of plasma to mediate removal of cholesterol from macrophages as a result of defective ABCA1- and SR-BI-dependent cholesterol efflux.
Approximately 15% of the HIV-infected patients presently studied displayed plasma levels of TGs above 1.5 g/ml. Most of these patients belonged to the subgroup of HIV-infected patients with the lowest CD4+ count (<200 cells/mm3). Hypertriglyceridemia is frequently associated with a low HDL-C phenotype resulting from a reduction in large HDL particles. Hydrolysis of TGs by hepatic lipase reduces HDL particle size and favors dissociation of apoAI from HDL. Such a reduction in the circulating level of large HDL particles has been shown to be associated with reduced SR-BI-dependent cellular cholesterol efflux, whereas the increased fraction of lipid-poor/free apoAI could enhance efflux via the ABCA1 pathway (47). However, in the context of the present study, we did not observe a significant impact of hypertriglyceridemia on plasma or HDL efflux capacity among HIV-infected patients.
Importantly, our present study highlights the fact that HAART is able to restore the capacity of plasma to stimulate cholesterol efflux from human macrophages as a result of an increase in both ABCA1- and SR-BI-dependent efflux pathways. Interestingly HAART was able to restore the intrinsic capacity of small HDL3b to stimulate cholesterol efflux via ABCA1, which was markedly reduced in HIV-infected patients.
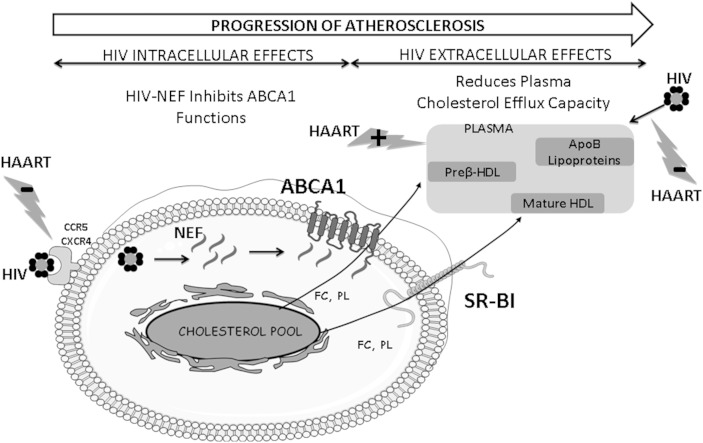
Data reported here, together with those of the literature, allow us to propose an integrated mechanism of the consequences of HIV infection and its treatment on cholesterol homeostasis in human macrophages (Fig. 5). Both intracellular and extracellular mechanisms of HIV interact together and favor the progression of atherosclerosis. HAART restores HDL-mediated cholesterol efflux from macrophages either directly by acting on its metabolism or indirectly by suppressing viral charge and thus eliminating HIV adverse effects on HDL functionality. We thus propose that the overall increase in cardiovascular risk in HIV-infected patients receiving HAART appears not to be associated with an alteration of the initial step of the RCT pathway, i.e., the capacity of HDL particles to stimulate macrophage cholesterol efflux.
Fig. 5.
Integrated mechanism of the intracellular and extracellular consequences of HIV infection that control cholesterol homeostasis in human macrophages. HIV penetrates into macrophages through a direct interaction with CCR5 and CXCR4 receptors. It has been formerly demonstrated that HIV-specific protein Nef binds to ABCA1 and inhibits its efflux functions in human macrophages (31). Thus, by reducing cholesterol efflux through ABCA1 toward circulating lipid-poor/lipid-free apoAI and to small HDL3 subspecies, HIV infection potentially alters plasma cholesterol efflux capacity from human macrophages. In parallel, following HIV infection, major structural and functional modifications in plasma lipoproteins occur resulting in a reduction of the capacity of HDL particles to stimulate cholesterol efflux through the ABCA1 pathway. Taken together those effects of HIV infection on human macrophage homeostasis might significantly increase the risk of cardiovascular disease and favor the progression of atherosclerosis in HIV-infected patients. Increasing the capacity of plasma to stimulate cholesterol efflux via ABCA1, the major macrophage cholesterol transporter, is an interesting approach to reduce cardiovascular risk in this population. HAART restores HDL-mediated cholesterol efflux from macrophages either directly by acting on its metabolism or indirectly by suppressing viral charge and thus eliminating HIV adverse effects on HDL functionality.
In conclusion, we demonstrate for the first time that HDL particles generated as a consequence of HIV infection are dysfunctional regarding their major anti-atherogenic property, i.e., cellular cholesterol efflux, known as the initial step of the RCT pathway. Plasma and HDL from HIV-infected subjects displayed an alteration in their capacity to stimulate cholesterol efflux through ABCA1, the main efflux pathway in human macrophages. We reveal a beneficial role of antiretroviral therapy in restoring HDL-C efflux capacity from human macrophages. In this context, current therapeutic strategies aim to increase the number of functional HDL particles by either increasing the hepatic production of apoAI or by intravascular infusion of reconstituted HDL or apoAI mimetic. Those therapeutic approaches have been demonstrated to reduce atheroma volume (48–50). Such therapies might be considered in HIV-infected subjects not only to stimulate the first step of RCT and to normalize lipoprotein distribution toward an atherogenic profile, but also to improve the damages on atherosclerotic lesions caused by the chronic inflammatory activation induced by HIV infection.
Supplementary Material
Acknowledgments
The authors are grateful to all members of the ANRS COPANA cohort study for patients’ recruitment and follow up. They warmly thank Dr. Wendy Jessup for the kind gift of ABCG1-CHOK1 cells and ABCA1-CHOK1 established by Ingrid Gelissen and by Kim Mi-Jurng, respectively.
Footnotes
Abbreviations:
- AIDS
- acquired immune deficiency syndrome
- CETP
- cholesteryl ester transfer protein
- FC
- free cholesterol
- HAART
- highly active antiretroviral therapy
- HDL-C
- HDL cholesterol
- HIV
- human immunodeficiency virus
- LDL-C
- LDL cholesterol
- RCT
- reverse cholesterol transport
- SR-BI
- scavenger receptor class B type I
- TC
- total cholesterol
The ANRS COPANA cohort study is funded by the Inserm-ANRS Paris, France. This work was supported by INSERM, UPMC and by a research grant from Sidaction foundation. P.E.K. was the recipient of a Research Fellowship from the New French Atherosclerosis Society. E.F.V. was a recipient of a Research Fellowship from the French Ministry of Research and Technology. The authors have no conflicts of interest to disclose.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures and six tables.
REFERENCES
- 1.Delaney J. A., Scherzer R., Biggs M. L., Shliplak M. G., Polak J. F., Currier J. S., Kronmal R. A., Wanke C., Bacchetti P., O’Leary D., et al. 2010. Associations of antiretroviral drug use and HIV-specific risk factors with carotid intima-media thickness. AIDS. 24: 2201–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friis-Møller N., Sabin C. A., Weber R., d’Arminio Monforte A., El-Sadr W. M., Reiss P., Thiebaut R., Morfeldt L., De Wit S., Pradier C., et al. 2003. Combination antiretroviral therapy and the risk of myocardial infarction. N. Engl. J. Med. 349: 1993–2003. [DOI] [PubMed] [Google Scholar]
- 3.El-Sadr W. M., Lundgren J., Neaton J. D., Gordin F., Abrams D., Arduino R. C., Babiker A., Burman W., Clumeck N., Cohen C. J., et al. 2006. CD4+ count-guided interruption of antiretroviral treatment. N. Engl. J. Med. 355: 2283–2296. [DOI] [PubMed] [Google Scholar]
- 4.Boufassa F., Goujard C., Viard J. P., Carlier R., Lefebvre B., Yeni P., Bouchaud O., Capeau J., Meyer L., Vigouroux C. 2012. Immune deficiency could be an early risk factor for altered insulin sensitivity in antiretroviral-naive HIV-1-infected patients: the ANRS COPANA cohort. Antivir. Ther. 17: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Lorenzo F., Collot-Teixeira S., Boffito M., Feher M., Gazzard B., McGregor J. L. 2008. Metabolic-inflammatory changes, and accelerated atherosclerosis in HIV patients: rationale for preventative measures. Curr. Med. Chem. 15: 2991–2999. [DOI] [PubMed] [Google Scholar]
- 6.Kedzierska K., Crowe S. M. 2001. Cytokines and HIV-1: interactions and clinical implications. Antivir. Chem. Chemother. 12: 133–150. [DOI] [PubMed] [Google Scholar]
- 7.Riddler S. A., Smit E., Cole S. R., Li R., Chmiel J. S., Dobs A., Palella F., Visscher B., Evans R., Kingsley L. A. 2003. Impact of HIV infection and HAART on serum lipids in men. JAMA. 289: 2978–2982. [DOI] [PubMed] [Google Scholar]
- 8.Rose H., Woolley I., Hoy J., Dart A., Bryant B., Mijch A., Sviridov D. 2006. HIV infection and high-density lipoprotein: the effect of the disease vs the effect of treatment. Metabolism. 55: 90–95. [DOI] [PubMed] [Google Scholar]
- 9.Shor-Posner G., Basit A., Lu Y., Cabrejos C., Chang J., Fletcher M., Mantero-Atienza E., Baum M. K. 1993. Hypocholesterolemia is associated with immune dysfunction in early human immunodeficiency virus-1 infection. Am. J. Med. 94: 515–519. [DOI] [PubMed] [Google Scholar]
- 10.Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. 1977. High density lipoprotein as a protective factor against coronary heart disease. The Framingham study. Am. J. Med. 62: 707–714. [DOI] [PubMed] [Google Scholar]
- 11.Michos E. D., Sibley C. T., Baer J. T., Blaha M. J., Blumenthal R. S. 2012. Niacin and statin combination therapy for atherosclerosis regression and prevention of cardiovascular disease events: reconciling the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes) trial with previous surrogate endpoint trials. J. Am. Coll. Cardiol. 59: 2058–2064. [DOI] [PubMed] [Google Scholar]
- 12.Hewing B., Moore K. J., Fisher E. A. 2012. HDL and cardiovascular risk: time to call the plumber? Circ. Res. 111: 1117–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camont L., Chapman M. J., Kontush A. 2011. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol. Med. 17: 594–603. [DOI] [PubMed] [Google Scholar]
- 14.Florentin M., Liberopoulos E. N., Wierzbicki A. S., Mikhailidis D. P. 2008. Multiple actions of high-density lipoprotein. Curr. Opin. Cardiol. 23: 370–378. [DOI] [PubMed] [Google Scholar]
- 15.Khera A. V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rose H., Hoy J., Woolley I., Tchoua U., Bukrinsky M., Dart A., Sviridov D. 2008. HIV infection and high density lipoprotein metabolism. Atherosclerosis. 199: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose H., Low H., Dewar E., Bukrinsky M., Hoy J., Dart A., Sviridov D. 2013. The effect of HIV infection on atherosclerosis and lipoprotein metabolism: a one year prospective study. Atherosclerosis. 229: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larrede S., Quinn C. M., Jessup W., Frisdal E., Olivier M., Hsieh V., Kim M. J., Van. Eck M., Couvert P., Carrie A., et al. 2009. Stimulation of cholesterol efflux by LXR agonists in cholesterol loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler. Thromb. Vasc. Biol. 29: 1930–1936. [DOI] [PubMed] [Google Scholar]
- 19.Adorni M. P., Zimetti F., Billheimer J. T., Wang N., Rader D. J., Phillips M. C., Rothblat G. H. 2007. The roles of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 48: 2453–2462. [DOI] [PubMed] [Google Scholar]
- 20.Jessup W., Gelissen I. C., Gaus K., Kritharides L. 2006. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr. Opin. Lipidol. 17: 247–257. [DOI] [PubMed] [Google Scholar]
- 21.Vergeer M., Korporaal S. J., Franssen R., Meurs I., Out R., Hovingh G. K., Hoekstra M., Sierts J. A., Dallinga-Thie G. M., Motazacker M. M., et al. 2011. Genetic variant of the scavenger receptor BI in humans. N. Engl. J. Med. 364: 136–145. [DOI] [PubMed] [Google Scholar]
- 22.Wang N., Lan D., Chen W., Matsuura F., Tall A. R. 2004. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA. 101: 9774–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charles-Schoeman C., Lee Y. Y., Grijalva V., Amjadi S., FitzGerald J., Ranganath V. K., Taylor M., McMahon M., Paulus H. E., Reddy S. T. 2012. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann. Rheum. Dis. 71: 1157–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGillicuddy F. C., de la Llera Moya M., Hinkle C. C., Joshi M. R., Chiquoine E. H., Billheimer J. T., Rothblat G. H., Reilly M. P. 2009. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 119: 1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guérin M., Dolphin P. J., Chapman M. J. 1994. A new in vitro method for the simultaneous evaluation of cholesteryl ester exchange and mass transfer between HDL and apoB-containing lipoprotein subspecies. Identification of preferential cholesteryl ester acceptors in human plasma. Arterioscler. Thromb. 14: 199–206. [DOI] [PubMed] [Google Scholar]
- 26.Guerin M., Lassel T. S., Le Goff W., Farnier M., Chapman M. J. 2000. Action of atorvastatin in combined hyperlipidemia: preferential reduction of cholesteryl ester transfer from HDL to VLDL1 particles. Arterioscler. Thromb. Vasc. Biol. 20: 189–197. [DOI] [PubMed] [Google Scholar]
- 27.Villard E. F., El Khoury P., Duchene E., Bonnefont-Rousselot D., Clement K., Bruckert E., Bittar R., Le Goff W., Guerin M. 2012. Elevated CETP activity improves plasma cholesterol efflux capacity from human macrophages in women. Arterioscler. Thromb. Vasc. Biol. 32: 2341–2349. [DOI] [PubMed] [Google Scholar]
- 28.Villard E. F., Federspiel M. C., Cherfils C., Fesel-Fouquier V., Bruckert E., Clement K., Bonnefont-Rousselot D., Le Goff W., Bittar R., Couvert P., et al. 2013. Endogenous CETP activity as a predictor of cardiovascular risk: determination of the optimal range. Atherosclerosis. 227: 165–171. [DOI] [PubMed] [Google Scholar]
- 29.Grunfeld C., Pang M., Doerrler W., Shigenaga J. K., Jensen P., Feingold K. R. 1992. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J. Clin. Endocrinol. Metab. 74: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 30.Hoang A., Drew B. G., Low H., Remaley A. T., Nestel P., Kingwell B. A., Sviridov D. 2012. Mechanism of cholesterol efflux in humans after infusion of reconstituted high-density lipoprotein. Eur. Heart J. 33: 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mujawar Z., Rose H., Morrow M. P., Pushkarsky T., Dubrovsky L., Mukhamedova N., Fu Y., Dart A., Orenstein J. M., Bobryshev Y. V., et al. 2006. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 4: e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aragonés G., Beltrán-Debón R., Rull A., Rodríguez-Sanabria F., Fernández-Sender L., Camps J., Joven J., Alonso-Villaverde C. 2010. Human immunodeficiency virus-infection induces major changes in high-density lipoprotein particle size distribution and composition: the effect of antiretroviral treatment and disease severity. Clin. Chem. Lab. Med. 48: 1147–1152. [DOI] [PubMed] [Google Scholar]
- 33.Riddler S. A., Li X., Otvos J., Post W., Palella F., Kingsley L., Visscher B., Jacobson L. P., Sharrett A. R. 2008. Antiretroviral therapy is associated with an atherogenic lipoprotein phenotype among HIV-1-infected men in the Multicenter AIDS Cohort Study. J. Acquir. Immune Defic. Syndr. 48: 281–288. [DOI] [PubMed] [Google Scholar]
- 34.Tien P. C., Schneider M. F., Cox C., Cohen M., Karim R., Lazar J., Young M., Glesby M. J. 2010. HIV, HAART, and lipoprotein particle concentrations in the Women’s Interagency HIV Study. AIDS. 24: 2809–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duprez D. A., Kuller L. H., Tracy R., Otvos J., Cooper D. A., Hoy J., Neuhaus J., Paton N. I., Friis-Moller N., Lampe F., et al. 2009. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis. 207: 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lampe F. C., Duprez D. A., Kuller L. H., Tracy R., Otvos J., Stroes E., Cooper D. A., Hoy J., Paton N. I., Friis-Moller N., et al. 2010. Changes in lipids and lipoprotein particle concentrations after interruption of antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 54: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oram J. F., Vaughan A. M. 2000. ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins. Curr. Opin. Lipidol. 11: 253–260. [DOI] [PubMed] [Google Scholar]
- 38.Remaley A. T., Schumacher U. K., Stonik J. A., Farsi B. D., Nazih H., Brewer H. B., Jr 1997. Decreased reverse cholesterol transport from Tangier disease fibroblasts. Acceptor specificity and effect of brefeldin on lipid efflux. Arterioscler. Thromb. Vasc. Biol. 17: 1813–1821. [DOI] [PubMed] [Google Scholar]
- 39.Out R., Jessup W., Le Goff W., Hoekstra M., Gelissen I. C., Zhao Y., Kritharides L., Chimini G., Kuiper J., Chapman M. J., et al. 2008. Coexistence of foam cells and hypocholesterolemia in mice lacking the ABC transporters A1 and G1. Circ. Res. 102: 113–120. [DOI] [PubMed] [Google Scholar]
- 40.Ripollés Piquer B., Nazih H., Bourreille A., Segain J. P., Huvelin J. M., Galmiche J. P., Bard J. M. 2006. Altered lipid, apolipoprotein, and lipoprotein profiles in inflammatory bowel disease: consequences on the cholesterol efflux capacity of serum using Fu5AH cell system. Metabolism. 55: 980–988. [DOI] [PubMed] [Google Scholar]
- 41.Tréguier M., Moreau M., Sposito A., Chapman M. J., Huby T. 2007. LDL particle subspecies are distinct in their capacity to mediate free cholesterol efflux via the SR-BI/Cla-1 receptor. Biochim. Biophys. Acta. 1771: 129–138. [DOI] [PubMed] [Google Scholar]
- 42.Weibel G. L., Drazul-Schrader D., Shivers D. K., Wade A. N., Rothblat G. H., Reilly M. P., de la Llera-Moya M. 2014. Importance of evaluating cell cholesterol influx with efflux in determining the impact of human serum on cholesterol metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 34: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Catalano G., Julia Z., Frisdal E., Vedie B., Fournier N., Le Goff W., Chapman M. J., Guerin M. 2009. Torcetrapib differentially modulates the biological activities of HDL2 and HDL3 particles in the reverse cholesterol transport pathway. Arterioscler. Thromb. Vasc. Biol. 29: 268–275. [DOI] [PubMed] [Google Scholar]
- 44.Gelissen I. C., Harris M., Rye K. A., Quinn C., Brown A. J., Kockx M., Cartland S., Packianathan M., Kritharides L., Jessup W. 2006. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler. Thromb. Vasc. Biol. 26: 534–540. [DOI] [PubMed] [Google Scholar]
- 45.Rothblat G. H., Phillips M. C. 1982. Mechanism of cholesterol efflux from cells. Effects of acceptor structure and concentration. J. Biol. Chem. 257: 4775–4782. [PubMed] [Google Scholar]
- 46.Wang N., Ranalletta M., Matsuura F., Peng F., Tall A. R. 2006. LXR-induced redistribution of ABCG1 to plasma membrane in macrophages enhances cholesterol mass efflux to HDL. Arterioscler. Thromb. Vasc. Biol. 26: 1310–1316. [DOI] [PubMed] [Google Scholar]
- 47.Fournier N., Francone O., Rothblat G., Goudouneche D., Cambillau M., Kellner-Weibel G., Robinet P., Royer L., Moatti N., Simon A., et al. 2003. Enhanced efflux of cholesterol from ABCA1-expressing macrophages to serum from type IV hypertriglyceridemic subjects. Atherosclerosis. 171: 287–293. [DOI] [PubMed] [Google Scholar]
- 48.Diditchenko S., Gille A., Pragst I., Stadler D., Waelchli M., Hamilton R., Leis A., Wright S. D. 2013. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 33: 2202–2211. [DOI] [PubMed] [Google Scholar]
- 49.Haase C. L., Tybjaerg-Hansen A., Grande P., Frikke-Schmidt R. 2010. Genetically elevated apolipoprotein A-I, high-density lipoprotein cholesterol levels, and risk of ischemic heart disease. J. Clin. Endocrinol. Metab. 95: E500–E510. [DOI] [PubMed] [Google Scholar]
- 50.Waksman R., Torguson R., Kent K. M., Pichard A. D., Suddath W. O., Satler L. F., Martin B. D., Perlman T. J., Maltais J. A., Weissman N. J., et al. 2010. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J. Am. Coll. Cardiol. 55: 2727–2735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.