Abstract
Reverse cholesterol transport (RCT) contributes to the anti-atherogenic effects of HDL. Patients with the orphan disease, familial hypoalphalipoproteinemia (FHA), are characterized by decreased tissue cholesterol removal and an increased atherogenic burden. We performed an open-label uncontrolled proof-of-concept study to evaluate the effect of infusions with a human apoA-I-containing HDL-mimetic particle (CER-001) on RCT and the arterial vessel wall in FHA. Subjects received 20 infusions of CER-001 (8 mg/kg) during 6 months. Efficacy was assessed by measuring (apo)lipoproteins, plasma-mediated cellular cholesterol efflux, fecal sterol excretion (FSE), and carotid artery wall dimension by MRI and artery wall inflammation by 18F-fluorodeoxyglucose-positron emission tomography/computed tomography scans. We included seven FHA patients: HDL-cholesterol (HDL-c), 13.8 [1.8–29.1] mg/dl; apoA-I, 28.7 [7.9–59.1] mg/dl. Following nine infusions in 1 month, apoA-I and HDL-c increased directly after infusion by 27.0 and 16.1 mg/dl (P = 0.018). CER-001 induced a 44% relative increase (P = 0.018) in in vitro cellular cholesterol efflux with a trend toward increased FSE (P = 0.068). After nine infusions of CER-001, carotid mean vessel wall area decreased compared with baseline from 25.0 to 22.8 mm2 (P = 0.043) and target-to-background ratio from 2.04 to 1.81 (P = 0.046). In FHA-subjects, CER-001 stimulates cholesterol mobilization and reduces artery wall dimension and inflammation, supporting further evaluation of CER-001 in FHA patients.
Keywords: apolipoprotein A-I, reverse cholesterol transport, familial hypoalphalipoproteinemia
The large residual burden of CVD in patients receiving guideline-based medical treatment, underscores the need for additional therapeutic interventions (1). Strategies aimed at increasing HDL-cholesterol (HDL-c) have been pursued as a promising target in CVD prevention for more than two decades (2, 3), albeit without a clear CVD benefit. Both the cholesteryl ester transfer protein (CETP) inhibitors (4) and nicotinic acid derivatives (5), increasing HDL-c on top of standard-of-care by 25–40%, have failed to reduce CVD risk. The expectation of HDL-c as an anti-atherogenic target was further jeopardized by the Mendelian randomization studies, which revealed that common genetic variations affecting HDL-c levels were not associated with CVD risk (6). In retrospect, the strongest benefits for HDL-increasing strategies in experimental atherosclerosis were confined to interventions increasing apoA-I levels, the major protein constituent of the HDL particle. apoA-I has been shown to play a key role in the initial step of reverse cholesterol transport (RCT) (7, 8). Tissue cholesterol efflux was decreased in patients with the orphan disease of genetically-determined low HDL-c, familial hypoalphalipoproteinemia (FHA) (9), which coincided with accelerated atherogenesis (10, 11). Early results from infusion experiments with reconstituted HDL corroborated a therapeutic potential, showing increased fecal cholesterol excretion (12, 13), as well as a reduction in the cholesterol content in human atherosclerotic lesions (14). Intravenous ultrasonography trials in patients with acute coronary syndrome (ACS) (15, 16), however, failed to show a significant benefit of apoA-I infusion on coronary atheroma volume versus placebo, although apoA-I infusion was associated with a regression versus baseline. Accordingly, the CHI-SQUARE study, testing the effect of short-term infusion of CER-001 in patients after an ACS, also showed regression compared with baseline without a significant change compared with placebo infusion (17). A common factor in all these studies was, however, that all participants had normal HDL-c levels.
In the present proof-of-concept study, we hypothesized that infusions with CER-001, a discoidal HDL-mimetic particle containing recombinant human apoA-I in a complex with two natural phospholipids, would promote cholesterol mobilization from the artery wall in patients with FHA, characterized by clear reductions in apoA-I levels. To this end, we selected patients with molecularly diagnosed FHA (apoAI, ABCA1, or LCAT deficiency) and low apoA-I levels, who received a total of 20 infusions during 6 months. The efficacy was assessed by measuring plasma (apo)lipoprotein changes, plasma-mediated cellular cholesterol efflux, and fecal sterol excretion (FSE). Both at baseline and after 6 months, we also measured the effect of CER-001 on arterial wall dimensions and inflammation.
METHODS
This phase II study (International Clinical Trials Registry Platform, WHO, EUCTR2011-006188-23-NL) was conducted in accordance with the Declaration of Helsinki and in compliance with current Good Clinical Practices (ICH E6) and the requirements of the US Food and Drug Administration (21 CFR 312). The study (SAMBA) was designed jointly by the academic investigators and the sponsor (Cerenis™ Therapeutics, S.A., France), and was executed at the Academic Medical Center in Amsterdam, The Netherlands. The protocol was approved by the local institutional review board and all participants provided written informed consent.
Subjects
We recruited adult subjects (≥18 years of age) with an HDL-c deficiency based on hetero- or homozygosity for a mutation affecting at least one of three genes known to affect HDL-c concentration: apoA-I, ABCA1, and LCAT. If applicable, subjects were on stable lipid-lowering therapy at least 6 weeks before study initiation. Exclusion criteria included major renal (serum creatinine >2.0 mg/dl) and hepatic [alanine transaminase/ aspartate transaminase (AST) > twice the upper limit of normal] dysfunction.
Design and intervention
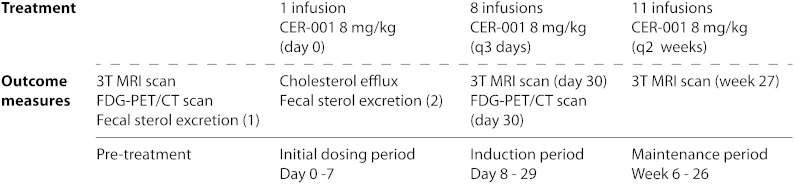
The study scheme is depicted in Fig. 1. At baseline, we subjected eligible FHA patients to 3.0 Tesla (3T) MRI and 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) scanning of the carotid arteries. Following the baseline examinations, the first of a total of 20 CER-001 infusions was administered (8 mg in apoA-I equivalents/kg body weight, infused over 60 minutes). CER-001 (Cerenis, France) is a negatively charged HDL-mimetic, consisting of recombinant human apoA-I and a combination of two naturally occurring phospholipids. The apoA-I component is expressed in mammalian Chinese hamster ovary cells and purified by a three-step column chromatography process. The phospholipid component consists of egg sphingomyelin and 1,2-dihexadecanoyl-sn-glycero-3-phospho-(1-rac-glycerol) (dipalmitoylphosphatidylglycerol) in a 97:3 weight ratio. The addition of dipalmitoylphosphatidylglycerol, a negatively charged phospholipid, in the CER-001 particle has been shown to minimize fusion of CER-001 with endogenous lipoproteins, thereby retaining the functional properties of the CER-001 particles after infusion (17a). The ratio of protein to total phospholipids in the CER-001 complex is 1:2.7 weight/weight. The drug product is a solution of the CER-001 complexes in phosphate-buffered sucrose/mannitol solution [10 mM phosphate buffer, 4.0% sucrose, 2.0% mannitol (pH 8.0)]. The concentration of CER-001 complexes in the formulation is expressed as the concentration of the apoA-I component. Intravenous infusions of CER-001 were administered over 1 h. One week after the first infusion, the second to ninth infusion of CER-001 were given every 3 days over a 3 week period after which another 11 infusions were given every 2 weeks. For the first, ninth (day 29), and last infusion (month 6), we withdrew blood prior to and at 1, 4, 8, and 24 h after the start of CER-001 infusion to evaluate lipid and lipoprotein parameters. Subjects remained fasting until the 4 h time point. Additionally, plasma-mediated cellular cholesterol efflux was measured in vitro in the blood samples obtained after the first infusion. After the ninth and twentieth infusion, the 3T MRI of the carotid arteries was repeated. The FDG-PET/CT scan was only repeated after the ninth infusion because of cumulative exposure to ionizing radiation (total exposure of 9.6 mSv per patient). FSE was determined at baseline and after the first CER-001 infusion in a subset of four subjects consenting to this additional analysis.
Fig. 1.
Study scheme. FSE studies were performed if the subject consented to these additional analyses.
Plasma lipids, lipoprotein profiles, and apoA-I
In all study participants, lipoprotein profiles were determined by HPLC using a Sepharose 6 column and enzymatically detected in-line for total cholesterol and unesterified cholesterol as performed by a contact research organization (Amatsi, Fontenille, France). The area under each of the peaks corresponding to lipoproteins with the sizes of VLDL, LDL, and HDL was integrated. Cholesterol ester levels were determined by subtracting the unesterified cholesterol from the total cholesterol in each fraction. The assays of total plasma and unesterified cholesterol were performed on the automated biochemical automator Pentra 400 (Horiba ABX Diagnostics, Montpellier, France) using cholesterol-oxidase enzymatic reaction. The assay of apoA-I was performed using a commercial ELISA kit (Assay Pro, Corona, CA). Intra-assay coefficient of variance was 4.8% with an inter-assay coefficient of variance of 7.21%.
Plasma-mediated cellular cholesterol efflux
Plasma-mediated cellular cholesterol efflux was quantified in vitro using whole plasma samples collected prior to and at the different time-points following initial administration of CER-001. Briefly, cultured J774 macrophages were labeled with [3H]cholesterol (2 μCi/ml) for 24 h. Following overnight equilibration, [3H]cholesterol release was measured after 4 h incubation with plasma. All assays were performed in triplicate. Plasma-mediated cellular cholesterol efflux was expressed as the percentage of the radioactivity released from cells into the medium relative to the total radioactivity in cells and medium (18).
FSE
Before and after CER-001 infusion, FSE was determined for a total of 8 days in a subpopulation of four subjects. At the beginning of the experiment, a blood and fecal sample was collected. Subsequently, participating FHA-patients were asked to adhere to a cholesterol-restricted diet (<250 mg cholesterol/day), to keep a dietary record, and to ingest 3 mg D4-sitostanol thrice daily until the end of the experiment. Sitostanol was used to correct for variations in fecal flow, as it is not absorbed by the intestines (19). For the assessment of FSE, consisting of fecal neutral sterols (FNSs) and bile acids, daily frozen fecal samples were thawed and homogenized with distilled water (1:1; w/w) from which 20 ml was dispensed into a 30 ml plastic tube. FNSs and bile acids were extracted as previously described (20, 21). Sterols were analyzed by capillary gas chromatography (Agilent 6890, Amstelveen, The Netherlands), equipped with a flame ionization detector and a CP Sil 19 capillary column (25 m × 0.25 mm × 0.2 μm; Chrompack BV, Middelburg, The Netherlands). Fecal D4-sitostanol was analyzed in its acetate derivatives by GC/MS (Agilent 7890A, Amstelveen, The Netherlands), using a ZB-5ms capillary column (30 m × 0.25 mm × 0.25 μm; Phenomex, Utrecht, The Netherlands). The daily excretion of neutral sterols and bile acids was calculated as relative amounts compared with the daily excretion of D4-sitostanol. Ultimately, daily sterol excretion was added together to determine total sterol excretion during the experiment.
3T MRI
MRI scans of both carotid arteries were obtained on a 3T MRI scanner (Philips Medical Systems, Best, The Netherlands) using a dedicated bilateral carotid coil (Shanghai Chenguang Medical Technologies, Shanghai, China). MRI scans were acquired according to a standardized protocol (22). In short, axial T1-weighted turbo-spin echo images were acquired in end-diastole from both common carotid arteries, using double inversion recovery preparation as black blood technique. In total, twelve 2 mm slices were acquired from each common carotid artery, with the most cranial slice being 8 mm below the carotid flow divider. Vessel wall dimensions were analyzed using dedicated measurement software (Vessel-Mass, Leiden University Medical Center, Leiden, The Netherlands). Before blinding, baseline and repeat scans were coregistered based on vessel wall morphology and distance from the carotid flow divider. One blinded reader performed all analyses. Lumen and outer wall contours were drawn manually in Vessel-Mass, after which mean vessel wall area (MVWA) was calculated.
FDG-PET/CT
Patients were subjected to FDG-PET/CT imaging at baseline and after the ninth CER-001 treatment. PET/CT imaging of the carotid arteries was performed as previously described (23, 24). In brief, PET/CT scans (Philips Gemini, Philips, Best, The Netherlands) of the neck region were obtained 90 minutes after FDG infusion (∼200 MBq, 5.5 mCi). FDG uptake in both the left and right carotid arterial wall was assessed by one blinded reader as follows; at least five regions of interest (ROIs) delineating the artery were drawn. Within each ROI, the maximal arterial standardized uptake value (SUVmax) was obtained as the maximal pixel activity within the ROI. For both the left and the right carotid artery, the mean SUVmax was derived by averaging the SUVmax of all ROIs. The maximal arterial target-to-background ratio (TBRmax) was calculated by correcting the mean SUVmax for the mean background blood activity in the venous blood pool, which was derived from the average of at least five ROIs within the jugular veins. The index vessel which was used as read-out parameter reflected the carotid artery with the highest FDG uptake at baseline (25).
Safety
For safety monitoring, blood withdrawals were performed directly prior to and 24 h after start of the first, second, fourth, seventh, ninth, thirteenth, sixteenth, and twentieth infusion (e.g., complete blood count, alkaline phosphatase, alanine transaminase, AST, bilirubin, lactate dehydrogenase, creatine phosphokinase). Anti-apoA-I antibody production was tested in samples taken prior to the first, fourth, ninth, thirteenth, sixteenth, and twentieth infusion. An electrochemiluminescent detection method was used for detection of anti-apoA-I antibodies with a sensitivity of 0.15 ng/ml. In case a subject tested positive for antibodies, the neutralizing potential of antibodies was determined by a cell-based assay, utilizing the ABCA1-mediated cholesterol efflux to CER-001 in J774 macrophages. Inhibitory potential of antibodies was quantified as a decrease in cholesterol efflux capacity. Additionally, the occurrence of adverse events (AEs) and medication changes were checked at every infusion and to test for hemodynamic changes, blood pressure was determined prior to and 2 h after the infusions.
Statistical analyses
Depending on the distribution, data are presented either as medians with interquartile ranges (IQRs) or means with SDs. For comparison of outcome data prior to versus after treatment, the Wilcoxon signed-rank test was used. In case of repeated measurements, we used the Friedman test to assess whether there was a significant difference in overall lipid parameters over time. If significance was found, post hoc analyses were performed using the Wilcoxon signed-rank test to do paired testing between all individual postinfusion lipid values and the preinfusion lipid values. Statistical analyses were performed using SPSS statistics version 20.0 (IBM, Chicago, IL). A P value of <0.05 was considered statistically significant.
Endpoints
The primary endpoint of the study comprised changes in apoA-I plasma concentration and plasma lipids. Secondary endpoints included changes in carotid MRI (MVWA) and FDG-PET/CT (TBRmax) and changes in FSE upon treatment. AEs and laboratory parameters (including antibody-development) were the key safety endpoints.
RESULTS
Baseline characteristics
Baseline characteristics are listed in Table 1. Seven FHA-patients (five male, two female) were included in whom the following mutations were identified: a homozygous mutation in two patients (for apoA-I and ABCA1 genes, respectively), double heterozygosity for apoA-I and ABCA1 genes in one patient, and four patients with a heterozygous mutation (for ABCA1 in two patients; for apoA-I and LCAT in one patient) (supplementary Table 1). Four subjects had coronary artery disease: two subjects had suffered from multiple myocardial infarctions, one subject underwent prophylactic coronary artery bypass grafting and one subject had received percutaneous coronary interventions for angina pectoris. Five patients were using lipid-lowering medication. The baseline lipid profile showed a median HDL-c of 13.8 mg/dl and a median apoA-I concentration of 28.7 mg/dl.
TABLE 1.
Baseline characteristics
| Subjects (n = 7) | |
| Female sex, n (%) | 2 (29) |
| Age, years | 51.1 [47.0–55.4] |
| BMI, kg/m2 | 28.4 [26.8–32.3] |
| Coronary artery disease, n (%) | 4 (57) |
| Lipid-lowering medication, n (%) | 5 (71) |
| Statin, n (%) | 5 (71) |
| Ezetimibe, n (%) | 3 (43) |
| Niacin, n (%) | 1 (14) |
| Systolic blood pressure, mmHg | 135 [107–143] |
| Diastolic blood pressure, mmHg | 82 [70–96] |
| Glucose, mmol/l | 4.9 [4.7–6.1] |
| Total cholesterol, mg/dl | 123.6 [93.8–163.3] |
| HDL-c, mg/dl | 13.8 [1.8–29.1] |
| LDL-c, mg/dl | 77.1 [53.5–101.0] |
| Triglycerides, mg/dl | 118.7 [87.7–298.5] |
| apoA-I, mg/dl | 28.7 [7.9–59.1] |
Data represent medians with IQRs unless specified otherwise.
Lipoprotein and apoA-I changes
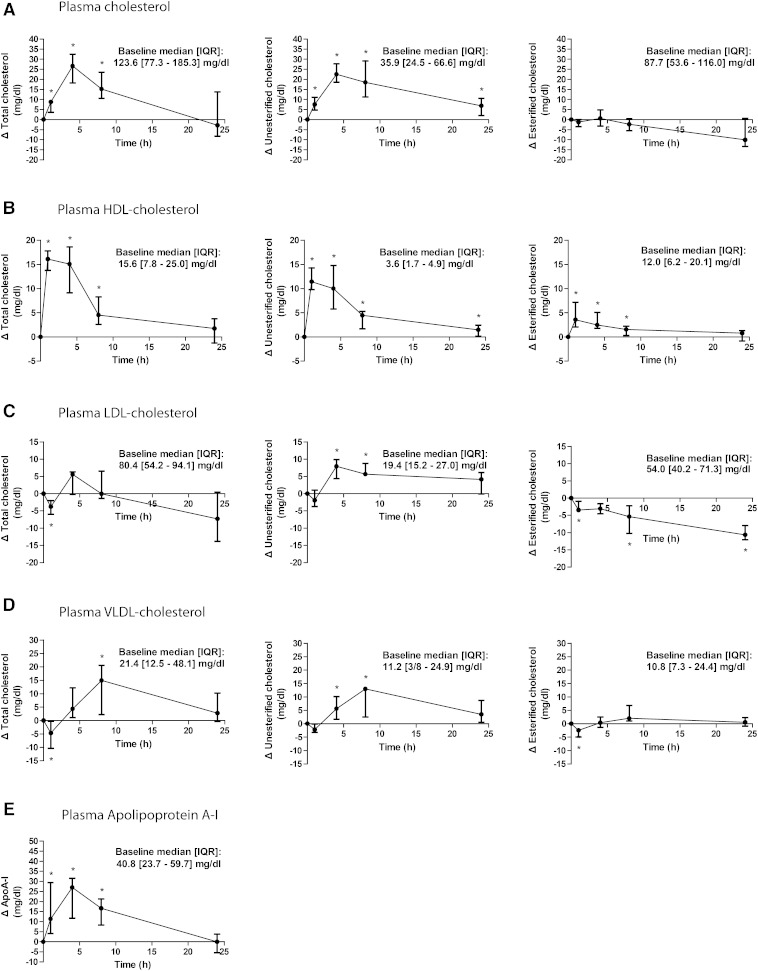
Figure 2 depicts the lipid profile changes after 1 month of CER-001 infusion. CER-001 increased apoA-I and HDL-c for at least 8 h after the start of the infusion with a peak in HDL-c directly after the completion of the infusion at t = 1 h (median 16.1 mg/dl versus pretreatment; P = 0.018) and for apoA-I levels 4 h after the start of the infusion (median 27.0 mg/dl; P = 0.018). Unesterified cholesterol in the HDL fraction increased markedly (11.5 mg/dl; P = 0.018), in combination with a modest increase of esterified cholesterol in the HDL fraction (median 3.6 mg/dl; P = 0.018). Analysis of VLDL-cholesterol (VLDL-c) and LDL-cholesterol (LDL-c) revealed small postdose increases predominantly in the unesterified cholesterol content, whereas esterification in these lipoproteins did not increase upon treatment. Six months of therapy resulted in similar postdose changes in lipoprotein and apoA-I levels (supplementary Fig. 1).
Fig. 2.
Lipoprotein profile changes and apoA-I kinetics after 1 month of treatment. Plasma was obtained at baseline and 1, 4, 8, and 24 h after the start of the ninth infusion. Changes in plasma cholesterol levels (A), HDL-c levels (B), LDL-c levels (C), VLDL-c levels (D), and apoA-I levels (E) following CER-001 infusion are depicted. Data represent baseline-corrected medians with IQRs. Values at every time point were compared with baseline. A P value <0.05 was considered statistically significant and is depicted with an asterisk.
Plasma-mediated cellular cholesterol efflux and FSE
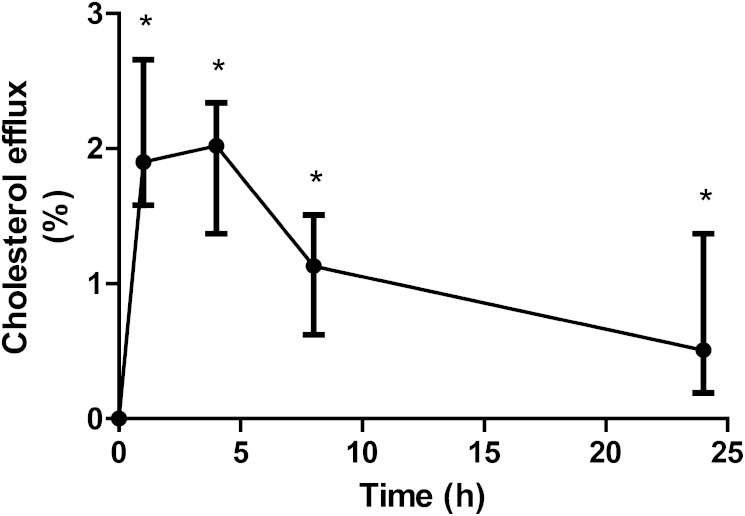
In vitro plasma-mediated cellular cholesterol efflux increased significantly upon CER-001 infusion. Plasma drawn 4 h after the start of the first CER-001 infusion showed an absolute increase of 1.9 ± 0.6% compared with baseline (Fig. 3), corresponding to a relative increase of 44 ± 26% (P = 0.018; Fig. 3). FSE was measured in a subset of four participants, who were all males with mutations either in the gene encoding for apoA-I and/or ABCA1 (supplementary Table 1). Characteristics such as weight, lipid profile, and cholesterol intake did not change between the baseline and post-CER-001 infusion measurements. After CER-001 treatment, there was a trend toward increased FSE (from 8.94 [6.43–11.76] g to 10.11 [6.81–12.91] g; P = 0.068), corresponding to an extra 0.68 [0.38–1.65] g of sterols being excreted during 8 days after treatment. This trend was caused by enhanced neutral sterol excretion (P = 0.068), whereas bile acid excretion was unaffected (P = 0.715). FSE increased in all four patients studied following CER-001 infusions, and we observed no apparent relation between genotype and effect of CER-001 infusion on FSE (supplementary Fig. 2).
Fig. 3.
Plasma-mediated cellular cholesterol efflux. Plasma-mediated cellular cholesterol efflux was analyzed in vitro using J774 macrophages. Cholesterol efflux capacity from plasma derived 1, 4, 8, and 24 h after infusion was compared with baseline efflux capacity. Data represent baseline-corrected medians with IQRs. A P value <0.05 was considered statistically significant and is depicted with an asterisk.
Carotid artery wall imaging
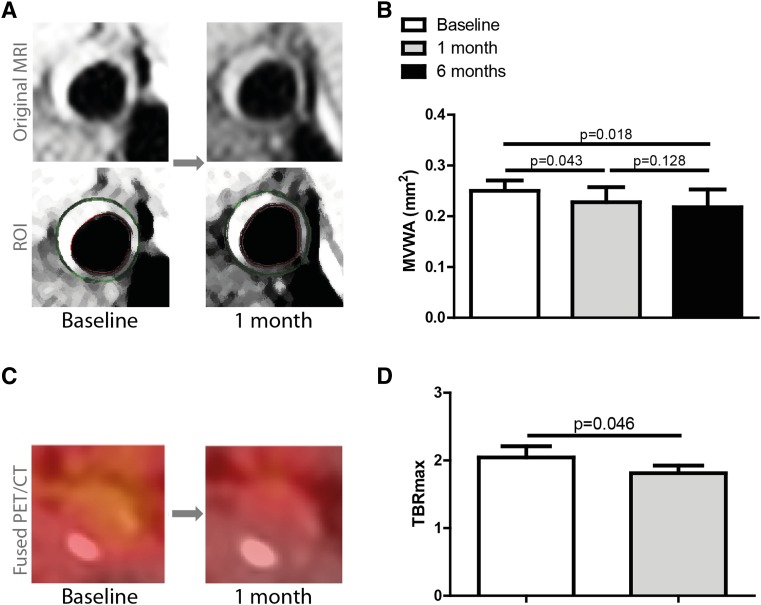
At baseline, median carotid MVWA was 25.0 mm2 [21.4–27.0 mm2] (Fig. 4A, B). None of the patients had overt atherosclerotic plaques at baseline, but MVWA was increased compared with that observed in healthy controls (22). Following nine infusions of CER-001 within a 1 month time frame, carotid MVWA decreased by a mean 4.6 ± 4.4% to 22.8 mm2 [20.8–25.7 mm2] (P = 0.043). After 6 months of infusions, the MVWA was 21.8 [20.7–25.3] (P = 0.018 vs. baseline; P = 0.128 versus month 1), corresponding to a total mean 6.7 ± 4.5% decrease versus baseline. With respect to the inflammatory activity, we found a baseline median TBRmax in the index vessel of 2.04 [1.51–2.21] (Fig. 4C, D). One month of CER-001 infusions reduced the TBRmax to 1.81 [1.49–1.92] (P = 0.046), corresponding to a mean percent reduction from baseline of 8.9 ± 12.7%.
Fig. 4.
Imaging results. MVWA and TBRmax of the carotid arteries, as assessed by MRI and FDG-PET/CT scan, respectively, were compared between baseline and after 1 month of nine CER-001 infusions. MVWA was also measured after 6 months with 11 additional CER-001 infusions. For TBRmax, the index vessel was chosen. Representative pre- and posttreatment 3T MRI and FDG-PET/CT scans are depicted in (A) and (C). In the case of the MRI, the original images and the ROI are shown. The results of both scans are shown in (B) and (D). Data represent medians with IQRs. A P value <0.05 was considered statistically significant.
Safety
No serious AEs or suspected unexpected serious adverse reactions were reported during this study. CER-001 infusions did not invoke blood pressure changes. Safety lab results showed no Hy’s law (26) cases and one three times the upper limit of normal elevation in AST in one patient while on treatment, who already had elevated AST at baseline. In four of the seven subjects, anti-apoA-I antibodies were absent throughout the study period. In one subject, with a homozygous apoA-I mutation, anti-apoA-I antibodies were present prior to CER-001 infusion. Another subject, with a heterozygous LCAT mutation, tested positive for antibodies in study week 18, whereas antibodies were absent at all measurements prior to and after this time point. The third subject testing positive for antibodies had a heterozygous apoA-I mutation, tested negative for antibodies at baseline and was positive for antibodies in study weeks 20 and 26. In none of these cases, the antibodies affected cholesterol efflux capacity to CER-001, as a marker of the neutralizing properties of anti-apoA-I antibodies.
DISCUSSION
In FHA patients, infusion of the HDL-mimetic CER-001 resulted in a significant increase in plasma apoA-I and HDL-c, with changes in both unesterified and esterified cholesterol content. These changes were accompanied by a significant increase in plasma-mediated cellular cholesterol efflux capacity in vitro, with a concomitant trend toward increased total sterol excretion in the feces. After nine infusions of CER-001 within 1 month, MVWA of the carotid artery decreased significantly compared with baseline, which persisted after an additional eleven infusions during the subsequent 5 months. The TBRmax of the carotid artery, reflecting arterial wall inflammation, was also reduced after nine infusions of CER-001. Collectively, the results of this proof-of-concept study imply that CER-001 stimulates RCT in orphan-disease FHA patients, leading to a reduction in both arterial wall thickness as well as arterial wall inflammation.
Plasma lipid changes following CER-001 infusion
Following infusion of CER-001, plasma apoA-I levels increased significantly with a return to baseline levels within 24 h, most likely reflecting the low dose administered. The increase in plasma HDL-c levels mirrored the increase in plasma apoA-I concentration, which is consistent with the stability of the CER-001 complex and different from previously reported apoA-I agents (12, 13). The increase in the unesterified cholesterol content and subsequent increase in esterified cholesterol content within the HDL fraction following CER-001 infusion indicates that the apoA-I within the CER-001 particle is recognized by endogenous LCAT, responsible for cholesterol esterification in plasma (27). However, the increased HDL-c following CER-001 infusion consisted predominantly of unesterified cholesterol, whereas normally the HDL fraction of cholesterol contains more esterified than unesterified cholesterol (28). These findings imply suboptimal esterification of the cholesterol mobilized by CER-001. Future studies are needed to determine whether the affinity of the recombinant apoA-I or the phospholipid composition of CER-001 contribute to a suboptimal cholesterol esterification.
Although the initial cholesterol mobilization induced by CER-001 is partitioned specifically to the HDL fraction, at later time points CER-001 infusion also induced a modest increase in unesterified cholesterol content in the VLDL and LDL fractions, whereas esterified cholesterol levels were not elevated. Similar effects have been reported upon apoA-I infusion in experimental models (29–31). The VLDL-c increase in response to apoA-I infusion has been attributed to both increased CETP-mediated cholesterol exchange between HDL-c and VLDL-c leading to higher VLDL-c, as well as to enhanced hepatic VLDL production reflecting an increased cholesterol flux toward the liver (29, 31). However, the observations that the increase in VLDL-c was confined to unesterified cholesterol in our participants, together with previous observations that CER-001 also increased VLDL-c in mice, which are naturally CETP-deficient (30, 32), argue against a CETP-driven mechanism, and hint toward an increased VLDL secretion. The mechanism by which CER-001 increases hepatic VLDL-c secretion remains to be elucidated.
Plasma-mediated cellular cholesterol efflux in vitro and FSE
We observed a mean baseline plasma-mediated cellular cholesterol efflux in vitro of 5.2% in FHA patients, which is lower compared with the previously reported 8–11% in healthy volunteers (33, 34). As expected, plasma-mediated cellular cholesterol efflux after CER-001 infusion showed a relative increase of 44%, indicating an excess capacity of the plasma to promote cholesterol efflux induced by CER-001 (30, 35). Recently, Rader and colleagues showed that the in vitro cholesterol efflux was inversely related to CVD events in two different, large cohorts (34, 36), lending support to a potentially beneficial effect of CER-001 in CVD. The ultimate step of RCT is elimination of cholesterol as bile acids and neutral sterols into the feces. We observed a trend toward an increased FSE after infusion of CER-001, which resulted in a mean additional 901 mg sterol excretion. An increase in sterol excretion following apoA-I infusion is supported by previous studies, in which infusion of higher dosages of apoA-I increased FNS excretion by 39–54% (12, 13). The additional 901 mg of sterol excretion observed over 8 days after infusion of a mean dose of 750 mg CER-001 is comparable (per gram of apoA-I infused) to that achieved after administration of a dose of 4 g apoA-I complexed with phosphatidylcholine, which induced an extra 5 g of sterol excretion over 9 days (12). Collectively, these data support the notion that CER-001 has a stimulatory effect on RCT. The preferred sources for the mobilization of cholesterol cannot, however, be determined in the present study. It is therefore difficult to separate the amount of cholesterol removed from the atherosclerotic vessel wall from that removed from other cholesterol compartments (37).
Carotid artery wall imaging
Following nine infusions of CER-001, a mean reduction of 4.6% in MVWA of the carotid arteries was observed compared with baseline. Combined with the plasma lipid changes and observed cholesterol fluxes, the reduction in MVWA presumably reflects the mobilization of cholesterol from the arterial wall. Previous data on experimental animal studies using apoA-I infusions support this mechanism (30, 38). It has proven more challenging, however, to demonstrate an effect of infusing apoA-I-containing particles on atherosclerosis in humans. A single reconstituted HDL-infusion significantly reduced the lipid content in atherosclerotic plaques (14). In line with these data, multiple infusions of apoA-IMilano complexes, reconstituted apoA-I complexes, CER-001, or reinfusion of autologous HDL after extracorporeal HDL-c delipidation, also resulted in regression of coronary atheroma volume over baseline in post-ACS patients (15–17, 39). None of these studies, however, reached a significant change compared with placebo.
Several distinct differences between these studies and our study deserve closer attention. First, we treated patients with severely lowered HDL-c levels (mean 15.5 mg/dl), whereas previous intravenous ultrasonography trials included postACS patients with mean HDL-c baseline levels of approximately 40 mg/dl (15–17). The potential impact of the absolute HDL-c level on CVD risk is supported by epidemiological studies showing the steepest association between CVD risk and HDL-c levels in patients with markedly lowered HDL-c levels (2, 3). More recently, we substantiated the impact of low HDL-c on tissue cholesterol efflux, particularly in patients with very low HDL-c levels (9), which is compatible with the concept that the best therapeutic result of apoA-I infusion can be obtained in those patients with the lowest apoA-I levels. As the limited number of subjects included in the present study does not allow for subgroup analysis, further studies are needed to determine to what extent the response to apoA-I treatment depends upon the baseline apoA-I levels and/or baseline genotype. Second, we used a high dosing frequency of nine infusions within a 1 month period, compared with only 4–6 weekly infusions in previous apoA-I infusion trials in ACS patients (15–17). The return to baseline of plasma apoA-I within 24 h after the infusion of 8 mg/kg CER-001 could imply that a higher dosing frequency might have a stronger impact. The lack of further improvement in carotid MVWA following 5 months of treatment in a lower dosing frequency (bi-weekly infusions) lends further support to this concept.
In addition, repetitive infusion of CER-001 resulted in a significant 8.9% mean decrease in arterial wall FDG uptake (TBRmax) in the index carotid artery. In the context of atherosclerosis, FDG uptake is used as an estimate of arterial wall inflammation (23). Potent anti-inflammatory effects of apoA-I/HDL have been reported previously. In experimental models, preβ-HDL administration markedly attenuated the inflammatory responses in a collar-induced atherosclerosis model in rabbits (38) and LDL-receptor knockout mice (30). In line with these observations, Shaw et al. (14) observed a marked decrease in the number of macrophages and inflammatory markers in plaques from patients with peripheral artery disease treated with reconstituted HDL. The mechanisms contributing to this anti-inflammatory effect have recently been extensively reviewed (40, 41). The anti-inflammatory effect of CER-001 infusions may also relate to the removal of cholesterol from the arterial wall, as it was shown that acute LDL-c lowering had the capacity to reduce inflammatory activity even in advanced atherosclerotic lesions within a 2 week timeframe (42). In support of this concept, we corroborated a direct anti-inflammatory effect of LDL-c lowering in patients with familial hypercholesterolemia, in whom LDL-apheresis was associated with a 14% reduction of arterial target-to-background ratio (43). A third explanation for the anti-inflammatory effect of CER-001 treatment could relate to the scavenger effect of the HDL particle for pro-inflammatory intermediates in which the HDL particle extracts pro-inflammatory oxidized sterols from the arterial wall (44).
Safety
CER-001 infusions were generally well-tolerated and study-drug-related AEs were mild. No hemodynamic changes were observed. Laboratory safety parameters did not reveal any clinically relevant signal. Treatment-emergent antibodies to apoA-I were observed in two subjects. The antibodies in one of these two subjects spontaneously reverted to negative prior to the last dose of CER-001. The other subject, with a heterozygous mutation for apoA-I, tested positive for antibodies intermittently. Additional testing however showed none of the antibodies to have neutralizing properties.
Study limitations
The major limitation of the present study relates to the limited sample size, combined with the heterogeneity of genetic mutations of the included subjects. The limited number of participants is, however, a direct consequence of the rare prevalence of genetic mutations resulting in severe apoA-I/HDL-deficiency (45–47), precluding the inclusion of larger numbers of patients with the orphan disease FHA. Second, the present study did not include a separate placebo infusion. In this respect, it should be noted that in previous trials evaluating the effect of apoA-I mimetics on atherosclerotic burden (15–17), a beneficial effect of the compound was observed compared with baseline, but not to placebo. In the present study, however, placebo infusion could not be implemented in view of the rarity of the disease combined with the intensity of the study protocol. Third, the data on the FSE should be interpreted with caution. Only four patients consented to participate in this intensive sub-study, whereas one patient also continued the use of ezetimibe, which is known to affect FSE (48). In addition, the patients were studied in an outpatient setting, resulting in imprecise control of dietary cholesterol intake, which may also impact FSE.
Notwithstanding these limitations, the results from our proof-of-concept study do support further clinical evaluation of the HDL-mimetic CER-001 in patients with FHA using a randomized placebo-controlled design. Awaiting such trial(s), the present data have provided a basis for the European Medicines Agency to grant two orphan designations for the use of CER-001 in the treatment of patients with apoA-I deficiency or ABCA1 deficiency (August 2014).
CONCLUSION
The present proof-of-concept study shows that CER-001, a recombinant human apoA-I-containing HDL-mimetic particle, stimulates RCT in FHA patients. Given the observed reduction in carotid MVWA and arterial wall inflammation, our data imply that the mobilized cholesterol may originate at least partly from the atherosclerotic vessel wall. Collectively, these findings support further clinical evaluation of the effect of CER-001 in larger trials in patients with FHA.
Supplementary Material
Acknowledgments
The authors would like to thank A. W. M. Schimmel for her work on plasma lipid analysis and R. Boverhof for his work on the FSE experiments. The authors thank R. Snoeks, A. M. van der Berg-Faaij, and W. M. de Jong for their assistance with performing the MRI and FDG-PET/CT scans.
Footnotes
Abbreviations:
- ACS
- acute coronary syndrome
- AE
- adverse event
- AST
- aspartate transaminase
- CETP
- cholesteryl ester transfer protein
- FDG
- 18F-fluorodeoxyglucose
- FHA
- familial hypoalphalipoproteinemia
- FNS
- fecal neutral sterol
- FSE
- fecal sterol excretion
- HDL-c
- HDL-cholesterol
- IQR
- interquartile range
- LDL-c
- LDL-cholesterol
- MVWA
- mean vessel wall area
- PET/CT
- positron emission tomography/computed tomography
- RCT
- reverse cholesterol transport
- ROI
- region of interest
- SUVmax
- maximal standardized uptake value
- 3T
- 3.0 Tesla
- TBRmax
- maximal target-to-background ratio
- VLDL-c
- VLDL-cholesterol
Part of the research for this work was supported by a grant from The Netherlands Heart Foundation [2011-B019: generating the best evidence-based pharmaceutical targets for atherosclerosis (GENIUS)]. J-L.D., C.H.K., R.B., and J.F.P. are employed by Cerenis. R.D.S. has received consultant and lecturing fees from Astra Zeneca, Amgen, Aegerion, Biolab, Boehringer Ingelheim, Bristol Myers Squibb, Genzyme, Pfizer, Novartis, Eli Lilly, Sanofi, Regeneron, and Unilever. G.K.H. has received lecturing fees from Amgen, Sanofi, MSD, Eli Lilly, and Cerenis. G.K.H. also participates in the MODE study (involving CER-001). E.S.S. has received lecturing fees from Amgen, Sanofi, Eli Lily and Torrent. E.S.S. also served as principal investigator for this study on CER-001 infusion in patients with hypoalphalipoproteinemia. J.J.P.K. is a consultant to and receives honoraria from Cerenis, Medicines Company, CSL-Behring, as well as from Dezima Pharmaceuticals, Eli Lilly, MSD, Isis Pharmaceuticals, and Boehringer Ingelheim. J.J.P.K. was also member of the steering committee of the CHI-SQUARE study (involving CER-001).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures and one table.
REFERENCES
- 1.Fruchart J-C., Davignon J., Hermans M. P., Al-Rubeaan K., Amarenco P., Assmann G., Barter P., Betteridge J., Bruckert E., Cuevas A., et al. 2014. Residual macrovascular risk in 2013: what have we learned? Cardiovasc. Diabetol. 13: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emerging Risk Factors Collaboration, Di Angelantonio E., Sarwar N., Perry P., Kaptoge S., Ray K. K., Thompson A., Wood A. M., Lewington S., Sattar N., et al. 2009. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 302: 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel W. B., Castelli W. P., Gordon T. 1979. Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Ann. Intern. Med. 90: 85–91. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz G. G., Olsson A. G., Abt M., Ballantyne C. M., Barter P. J., Brumm J., Chaitman B. R., Holme I. M., Kallend D., Leiter L. A., et al. 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367: 2089–2099. [DOI] [PubMed] [Google Scholar]
- 5.Boden W. E., Probstfield J. L., Anderson T., Chaitman B. R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., Weintraub W. 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267. [DOI] [PubMed] [Google Scholar]
- 6.Voight B. F., Peloso G. M., Orho-Melander M., Frikke-Schmidt R., Barbalic M., Jensen M. K., Hindy G., Hólm H., Ding E. L., Johnson T., et al. 2012. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet. 380: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Zanotti I., Reilly M. P., Glick J. M., Rothblat G. H., Rader D. J. 2003. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 108: 661–663. [DOI] [PubMed] [Google Scholar]
- 8.Duverger N., Kruth H., Emmanuel F., Caillaud J. M., Viglietta C., Castro G., Tailleux A., Fievet C., Fruchart J. C., Houdebine L. M., et al. 1996. Inhibition of atherosclerosis development in cholesterol-fed human apolipoprotein A-I-transgenic rabbits. Circulation. 94: 713–717. [DOI] [PubMed] [Google Scholar]
- 9.Holleboom A. G., Jakulj L., Franssen R., Decaris J., Vergeer M., Koetsveld J., Luchoomun J., Glass A., Hellerstein M. K., Kastelein J. J., et al. 2013. In vivo tissue cholesterol efflux is reduced in carriers of a mutation in APOA1. J. Lipid Res. 54: 1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bochem A. E., van Wijk D. F., Holleboom A. G., Duivenvoorden R., Motazacker M. M., Dallinga-Thie G. M., de Groot E., Kastelein J. J., Nederveen A. J., Hovingh G. K., et al. 2013. ABCA1 mutation carriers with low high-density lipoprotein cholesterol are characterized by a larger atherosclerotic burden. Eur. Heart J. 34: 286–291. [DOI] [PubMed] [Google Scholar]
- 11.Duivenvoorden R., Holleboom A. G., van den Bogaard B., Nederveen A. J., de Groot E., Hutten B. A., Schimmel A. W., Hovingh G. K., Kastelein J. J., Kuivenhoven J. A., et al. 2011. Carriers of lecithin cholesterol acyltransferase gene mutations have accelerated atherogenesis as assessed by carotid 3.0-T magnetic resonance imaging. J. Am. Coll. Cardiol. 58: 2481–2487. [Erratum. 2012. J. Am. Coll. Cardiol. 59: 196.] [DOI] [PubMed] [Google Scholar]
- 12.Eriksson M., Carlson L. A., Miettinen T. A., Angelin B. 1999. Stimulation of fecal steroid excretion after infusion of recombinant proapolipoprotein A-I: potential reverse cholesterol transport in humans. Circulation. 100: 594–598. [DOI] [PubMed] [Google Scholar]
- 13.Nanjee M. N., Cooke C. J., Garvin R., Semeria F., Lewis G., Olszewski W. L., Miller N. E. 2001. Intravenous apoA-I/lecithin discs increase pre-beta-HDL concentration in tissue fluid and stimulate reverse cholesterol transport in humans. J. Lipid Res. 42: 1586–1593. [PubMed] [Google Scholar]
- 14.Shaw J. A., Bobik A., Murphy A., Kanellakis P., Blombery P., Mukhamedova N., Woollard K., Lyon S., Sviridov D., Dart A. M. 2008. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ. Res. 103: 1084–1091. [DOI] [PubMed] [Google Scholar]
- 15.Nissen S. E., Tsunoda T., Tuzcu E. M., Schoenhagen P., Cooper C. J., Yasin M., Eaton G. M., Lauer M. A., Sheldon W. S., Grines C. L., et al. 2003. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 290: 2292–2300. [DOI] [PubMed] [Google Scholar]
- 16.Tardif J-C., Grégoire J., L’Allier P. L., Ibrahim R., Lespérance J., Heinonen T. M., Kouz S., Berry C., Basser R., Lavoie M. A., et al. 2007. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 297: 1675–1682. [DOI] [PubMed] [Google Scholar]
- 17.Tardif J-C., Ballantyne C. M., Barter P., Dasseux J-L., Fayad Z. A., Guertin M-C., Kastelein J. J., Keyserling C., Klepp H., Koenig W., et al. 2014. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur. Heart J. 35: 3277–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Goffinet M., Baron R., Tardy C., Sy G., Schwendeman A., Keyserling C. H., Wetterau J., Barbaras R., Lalwani N., Dasseux J-L. 2011. CER-001, a new generation of HDL mimetic: characterization and determination of in vitro and in vivo activities; ATVB Scientific Sessions. P364. [Google Scholar]
- 18.de la Llera-Moya M., Drazul-Schrader D., Asztalos B. F., Cuchel M., Rader D. J., Rothblat G. H. 2010. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler. Thromb. Vasc. Biol. 30: 796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinemann T., Kullak-Ublick G-a., Pietruck B., Bergmann K. 1991. Mechanisms of action of plant sterols on inhibition of cholesterol absorption. Eur. J. Clin. Pharmacol. 40: S59–S63. [PubMed] [Google Scholar]
- 20.Arca M., Montali A., Ciocca S., Angelico F., Cantafora A. 1983. An improved gas-liquid chromatographic method for the determination of fecal neutral sterols. J. Lipid Res. 24: 332–335. [PubMed] [Google Scholar]
- 21.Setchell K. D., Lawson A. M., Tanida N., Sjövall J. 1983. General methods for the analysis of metabolic profiles of bile acids and related compounds in feces. J. Lipid Res. 24: 1085–1100. [PubMed] [Google Scholar]
- 22.Duivenvoorden R., de Groot E., Elsen B. M., Laméris J. S., van der Geest R. J., Stroes E. S., Kastelein J. J., Nederveen A. J. 2009. In vivo quantification of carotid artery wall dimensions: 3.0-Tesla MRI versus B-mode ultrasound imaging. Circ. Cardiovasc. Imaging. 2: 235–242. [DOI] [PubMed] [Google Scholar]
- 23.Tawakol A., Migrino R. Q., Bashian G. G., Bedri S., Vermylen D., Cury R. C., Yates D., LaMuraglia G. M., Furie K., Houser S., et al. 2006. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J. Am. Coll. Cardiol. 48: 1818–1824. [DOI] [PubMed] [Google Scholar]
- 24.Rudd J. H. F., Myers K. S., Bansilal S., Machac J., Rafique A., Farkouh M., Fuster V., Fayad Z. A. 2007. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J. Am. Coll. Cardiol. 50: 892–896. [DOI] [PubMed] [Google Scholar]
- 25.Fayad Z. A., Mani V., Woodward M., Kallend D., Abt M., Burgess T., Fuster V., Ballantyne C. M., Stein E. A., Tardif J. C., et al. 2011. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 378: 1547–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Björnsson E. 2006. Drug-induced liver injury: Hy’s rule revisited. Clin. Pharmacol. Ther. 79: 521–528. [DOI] [PubMed] [Google Scholar]
- 27.Jonas A. 2000. Lecithin cholesterol acyltransferase. Biochim. Biophys. Acta. 1529: 245–256. [DOI] [PubMed] [Google Scholar]
- 28.Kontush A., de Faria E. C., Chantepie S., Chapman M. J. 2004. Antioxidative activity of HDL particle subspecies is impaired in hyperalphalipoproteinemia: relevance of enzymatic and physicochemical properties. Arterioscler. Thromb. Vasc. Biol. 24: 526–533. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Berbée J. F. P., Stroes E. S., Smit J. W., Havekes L. M., Romijn J. A., Rensen P. C. 2011. CETP expression reverses the reconstituted HDL-induced increase in VLDL. J. Lipid Res. 52: 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tardy C., Goffinet M., Boubekeur N., Ackermann R., Sy G., Bluteau A., Cholez G., Keyserling C., Lalwani N., Paolini J. F., et al. CER-001, an HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis. 232: 110-118. [DOI] [PubMed] [Google Scholar]
- 31.Shah P. K., Yano J., Reyes O., Chyu K-Y., Kaul S., Bisgaier C. L., Drake S., Cercek B. 2001. High-dose recombinant apolipoprotein A-I(Milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 103: 3047–3050. [DOI] [PubMed] [Google Scholar]
- 32.Jiao S., Cole T. G., Kitchens R. T., Pfleger B., Schonfeld G. 1990. Genetic heterogeneity of lipoproteins in inbred strains of mice: analysis by gel-permeation chromatography. Metabolism. 39: 155–160. [DOI] [PubMed] [Google Scholar]
- 33.Diditchenko S., Gille A., Pragst I., Stadler D., Waelchli M., Hamilton R., Leis A, Wright S. D. 2013. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 33: 2202–2211. [DOI] [PubMed] [Google Scholar]
- 34.Khera A. V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X., Collins H. L., Ranalletta M., Fuki I. V., Billheimer J. T., Rothblat G. H., Tall A. R., Rader D. J. 2007. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 117: 2216–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohatgi A., Khera A., Berry J. D., Givens E. G., Ayers C. R., Wedin K. E., Neeland I. J., Yuhanna I. S., Rader D. R., de Lemos J. A., et al. 2014. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 371: 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenson R. S., Brewer H. B., Jr., Davidson W. S., Fayad Z. A., Fuster V., Goldstein J., Hellerstein M., Jiang X. C., Phillips M. C., Rader D. J., et al. 2012. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 125: 1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholls S. J., Cutri B., Worthley S. G., Kee P., Rye K-A., Bao S., Barter P. J. 2005. Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler. Thromb. Vasc. Biol. 25: 2416–2421. [DOI] [PubMed] [Google Scholar]
- 39.Waksman R., Torguson R., Kent K. M., Pichard A. D., Suddath W. O., Satler L. F., Martin B. D., Perlman T. J., Maltais J. A., Weissman N. J., et al. 2010. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J. Am. Coll. Cardiol. 55: 2727–2735. [DOI] [PubMed] [Google Scholar]
- 40.Rye K-A., Barter P. J. 2014. Cardioprotective functions of HDLs. J. Lipid Res. 55: 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westerterp M., Bochem A. E., Yvan-Charvet L., Murphy A. J., Wang N., Tall A. R. 2014. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ. Res. 114: 157–170. [DOI] [PubMed] [Google Scholar]
- 42.Feig J. E., Parathath S., Rong J. X., Mick S. L., Vengrenyuk Y., Grauer L., Young S. G., Fisher E. A. 2011. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 123: 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Wijk D. F., Sjouke B., Figueroa A., Emami H., van der Valk F. M., MacNabb M. H., Hemphill L. C., Schulte D. M., Koopman M. G., Lobatto M. E., et al. 2014. Nonpharmacological lipoprotein apheresis reduces arterial inflammation in familial hypercholesterolemia. J. Am. Coll. Cardiol. 64: 1418–1426. [DOI] [PubMed] [Google Scholar]
- 44.Navab M., Reddy S. T., Van Lenten B. J., Buga G. M., Hough G., Wagner A. C., Fogelman A. M. 2012. High-density lipoprotein and 4F peptide reduce systemic inflammation by modulating intestinal oxidized lipid metabolism: novel hypotheses and review of literature. Arterioscler. Thromb. Vasc. Biol. 32: 2553–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haase C. L., Frikke-Schmidt R., Nordestgaard B. G., Tybjærg-Hansen A. 2012. Population-based resequencing of APOA1 in 10,330 individuals: spectrum of genetic variation, phenotype, and comparison with extreme phenotype approach. PLoS Genet. 8: e1003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haase C. L., Tybjærg-Hansen A., Qayyum A. A., Schou J., Nordestgaard B. G., Frikke-Schmidt R. 2012. LCAT, HDL cholesterol and ischemic cardiovascular disease: a Mendelian randomization study of HDL cholesterol in 54,500 individuals. J. Clin. Endocrinol. Metab. 97: E248–E256. [DOI] [PubMed] [Google Scholar]
- 47.Frikke-Schmidt R., Nordestgaard B. G., Stene M. C. A., Sethi A. A., Remaley A. T., Schnohr P., Grande P., Tybjaerg-Hansen A. 2008. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 299: 2524–2532. [DOI] [PubMed] [Google Scholar]
- 48.Davidson M. H., Voogt J., Luchoomun J., Decaris J., Killion S., Boban D., Glass A, Mohammad H., Lu Y., Villegas D., et al. 2013. Inhibition of intestinal cholesterol absorption with ezetimibe increases components of reverse cholesterol transport in humans. Atherosclerosis. 230: 322–329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.