Abstract
Little is known about whether cholesteryl ester transfer protein (CETP) genetic variation may modify the effect of weight-loss diets varying in fat content on changes in lipid levels. We analyzed the interaction between the CETP variant rs3764261 and dietary interventions on changes in lipid levels among 732 overweight/obese adults from a 2 year randomized weight-loss trial [Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST)], and replicated the findings in 171 overweight/obese adults from an independent 2 year weight-loss trial [Dietary Intervention Randomized Controlled Trial (DIRECT)]. In the POUNDS LOST, participants with the CETP rs3764261 CC genotype on the high-fat diet had larger increases in HDL cholesterol (P = 0.001) and decreases in triglycerides (P = 0.007) than those on the low-fat diet at 6 months, while no significant difference between these two diets was observed among participants carrying other genotypes. The gene-diet interactions on changes in HDL-cholesterol and triglycerides were replicated in the DIRECT (pooled P for interaction ≤ 0.01). Similar results on trajectory of changes in HDL cholesterol and triglycerides over the 2 year intervention were observed in both trials. Our study provides replicable evidence that individuals with the CETP rs3764261 CC genotype might derive greater effects on raising HDL cholesterol and lowering triglycerides by choosing a low-carbohydrate/high-fat weight-loss diet instead of a low-fat diet.
Keywords: cholesteryl ester transfer protein, Preventing Overweight Using Novel Dietary Strategies, Dietary Intervention Randomized Controlled Trial, gene-diet interaction
Unfavorable blood lipid levels, such as elevated levels of total cholesterol, LDL cholesterol, and triglycerides, and reduced levels of HDL cholesterol, have been associated with increased risk of CVDs (1–3). Lipid levels are determined by genetic and environmental factors, as well as their interactions (4, 5). There has been great interest in investigating cholesteryl ester transfer protein (CETP), a plasma glycoprotein that facilitates the transfer of cholesteryl ester and triglycerides between HDL and other lipoproteins (6), in relation to blood lipids. CETP deficiency due to CETP gene mutation causes increased HDL cholesterol levels (7, 8). In patients with low HDL cholesterol levels, CETP inhibition increased HDL cholesterol and decreased LDL cholesterol levels (9). Two previous meta-analyses have documented that CETP genetic variants related to moderate inhibition of CETP activity were associated with favorable blood lipid levels and decreased cardiovascular risk (10, 11). Several observational studies reported that CETP variants might interact with dietary factors, especially dietary fat intake, on HDL cholesterol levels (12–15), although the findings were not consistently replicated (11, 16–20). In addition, two small short-term diet intervention studies did not demonstrate the interaction between CETP genotype and dietary fat intake (21, 22). However, gene-diet interactions have rarely been investigated in long-term randomized intervention trials.
Recent genome-wide association studies (GWASs) have shown stronger associations between CETP genetic variants with HDL cholesterol levels than any other loci across the human genome (23–27). A common variant, rs3764261, located 2.5 kb upstream of CETP exhibited a strong association with HDL cholesterol levels (3.39 mg/dl per A-allele, P = 7.0 × 10−380), and the HDL-decreasing allele was also associated with elevated levels of LDL cholesterol and triglycerides (P ≤ 1.1 × 10−12) (27). To our knowledge, no study has investigated the interaction between this GWAS-identified CETP variant and dietary intake on lipid levels. Identification of gene-lifestyle interactions in relation to lipid levels may help to clarify the mechanisms underlying the development of dyslipidemia and CVD, and provide effective strategies for disease prevention. Therefore, in the current study, we examined whether the CETP rs3764261 genotype modulated changes in lipid levels in response to weight-loss diets varying in fat content in a 2 year randomized intervention study: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) (28). We also replicated our findings in an independent 2 year intervention study: the Dietary Intervention Randomized Controlled Trial (DIRECT) (29).
MATERIALS AND METHODS
Subjects
The POUNDS LOST is a 2 year randomized clinical trial to compare the effects of energy-reduced diets with different compositions of macronutrients on weight loss. The study design, methods, and main results have been described in detail elsewhere (28, 30, 31). A total of 811 overweight and obese subjects (25 ≤ BMI ≤ 40 kg/m2) aged 30 to 70 years were randomly assigned to one of four diets; the target percentages of energy derived from fat, protein, and carbohydrate in the four diets were 20, 15, and 65%; 20, 25, and 55%; 40, 15, and 45%; and 40, 25, and 35%. After two years, 80% of the participants (n = 645) completed the trial. In the current study, 723 participants in whom CETP genotype and blood lipid data are available were included, and participants from the diet groups were combined for the comparison of low-fat diet (20%) and high-fat diet (40%). The POUNDS LOST study was approved by the human subjects committee at the Harvard School of Public Health and Brigham and Women’s Hospital, Boston, MA and the Pennington Biomedical Research Center of the Louisiana State University System, Baton Rouge, LA. All participants gave written informed consent.
The DIRECT, previously described in detail (29), is a 2 year randomized clinical trial to compare the effect of a low-fat diet, a Mediterranean diet, or a low-carbohydrate diet on long-term weight loss and various health parameters. A total of 322 overweight and obese subjects (BMI ≥ 27 kg/m2) aged 30 to 70 years were randomly assigned to one of the three diets. After 2 years, 85% of the participants (n = 272) completed the intervention. In the current study, we included 171 subjects from low-fat diet and low-carbohydrate (high-fat) diet groups for the replication analysis. The DIRECT study was approved and monitored by the human subjects committee of Soroka Medical Center and Ben-Gurion University. Each participant provided written informed consent.
Measurements
In the POUNDS LOST, body weight and waist circumference were measured in the morning before breakfast at baseline, 6 months, and 2 years. Height was measured at baseline. BMI was calculated as weight (kilograms)/height2 (meters squared). Fasting blood samples were obtained on 1 day at baseline, 6 months, and 2 years. Serum levels of total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides were measured by using the Beckman Synchron CX7 analyzer (Beckman Coulter) at the clinical laboratory at the Pennington Biomedical Research Center. To assess the dietary adherence across the intervention, dietary intake was assessed in a random sample of 50% of the participants, by a review of the 5 day diet record at baseline and by 24 h recall during a telephone interview on three nonconsecutive days at 6 months and 2 years. In addition, food frequency questionnaires were collected on all participants at baseline, 6 months, and 2 years.
In the DIRECT, body weight was measured every month and height was measured at baseline for BMI determination. A blood sample was drawn by venipuncture at 8:00 AM, after a 12 h fast, at baseline, 6 months, and 2 years. Serum levels of total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides were determined enzymatically with a Wako R-30 automatic analyzer in Leipzig University Laboratories, Leipzig, Germany. Adherence to the diets was evaluated by a validated food frequency questionnaire, and a subgroup of participants completed two repeated 24 h dietary recalls to verify dietary intakes. At baseline and at 6, 12, and 24 months of follow-up, the questionnaires were self-administered electronically through the workplace intranet.
Genotyping
In the POUNDS LOST, DNA was extracted from the buffy coat fraction of centrifuged blood using the QIAmp blood kit (Qiagen, Chatsworth, CA). The CETP SNP, rs3764261 (27), was genotyped using the OpenArray™ SNP genotyping system (BioTrove, Woburn, MA). In the DIRECT, DNA was extracted from peripheral leukocytes by phenol precipitation. Genotyping of SNP rs3764261 was done on a 7300 real-time PCR system with fluorescent TaqMan probes (Applied Biosystems, Foster City, CA). The genotype distribution was in Hardy-Weinberg equilibrium in both POUNDS LOST and DIRECT (P > 0.05).
Statistical analysis
The primary outcome of the study was the percent change in lipid levels from baseline to 6 months of intervention. Percent changes in lipid levels at 2 years were the secondary outcomes because the adherence to diets declined and blood lipid levels changed slowly or rebounded between 6 months and 2 years in the POUNDS LOST. General linear models for continuous variables and χ2 tests for categorical variables were applied for the comparison according to the CETP genotype groups at baseline. Differences in total energy and nutrient intakes between low-fat and high-fat diet groups by the CETP genotype groups at baseline, 6 months, and 2 years were examined using general linear models. We compared changes in blood lipids between low-fat and high-fat diet groups by CETP genotype groups at 6 months and 2 years using general linear models adjusted for age, sex, ethnicity, baseline BMI, and lipid-lowering medication use. Gene-diet intervention interactions were tested by including the genotype-by-diet interaction terms in the models. In addition, we used generalized estimating equations to evaluate the trajectory of changes in blood lipids by CETP genotype groups over the two-year intervention, with age, sex, ethnicity, time point, and diet group as explanatory variables in the models. Similar analyses were repeated in the DIRECT to replicate the results in the POUNDS LOST. Results from the two trials were pooled by inverse-variance-weighted fixed-effects meta-analyses. Because of the small number of minor homozygotes of the CETP SNP rs3764261 (n = 65 in the POUNDS LOST and 25 in the DIRECT,), heterozygotes and minor homozygotes (CA and AA genotypes) were combined in the analyses. All reported P values are nominal and two-sided and a P value of 0.05 was considered statistically significant. Statistical analyses were performed with SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Baseline characteristics
Baseline characteristics of participants from the two trials, according to the CETP genotype, are shown in Table 1. The genotype frequencies were similar between men and women, among ethnic groups, and across the diet groups (all P ≥ 0.32). Participants with CC genotype had lower HDL cholesterol levels at baseline compared with those without this genotype in both the POUNDS LOST (P < 0.001) and the DIRECT (P = 0.05), consistent with previous reports (27).We did not observe significant differences in LDL cholesterol and triglyceride levels between the CETP genotype groups (all P > 0.08).
TABLE 1.
Baseline characteristics of the study participants in the POUNDS LOST and DIRECT trials
| Characteristic | POUNDS LOST | DIRECT | ||||
| CC (n = 332) | CA/AA (n = 391) | a | CC (n = 80) | CA/AA (n = 91) | a | |
| Age, years | 51.4 ± 9.2 | 50.7 ± 9.3 | 0.32 | 50.9 ± 6.0 | 50.8 ± 6.3 | 0.95 |
| Sex, n (%) | 0.59 | 0.75 | ||||
| Male | 133 (40.1) | 149 (38.1) | 69 (86.2) | 80 (87.9) | ||
| Female | 199 (59.9) | 242 (61.9) | 11 (13.8) | 11 (12.1) | ||
| Ethnic group, n (%) | 0.90 | — | ||||
| White | 262 (78.9) | 314 (80.3) | 80 (100) | 91 (100) | ||
| Black | 53 (16.0) | 58 (14.9) | — | — | ||
| Hispanic, Asian, or other | 17 (5.1) | 19 (4.9) | — | — | ||
| Diet groups, n (%) | 0.59 | 0.68 | ||||
| Low-fat diets | 164 (49.4) | 201 (51.4) | 43 (53.7) | 46 (50.5) | ||
| High-fat diets | 168 (50.6) | 190 (48.6) | 37 (46.3) | 45 (49.5) | ||
| Weight, kg | 92.7 ± 15.6 | 93.8 ± 15.6 | 0.13 | 92.0 ± 14.5 | 90.2 ± 12.4 | 0.29 |
| BMI, kg/m22 | 32.4 ± 3.9 | 32.9 ± 3.9 | 0.06 | 31.0 ± 3.4 | 30.4 ± 3.3 | 0.25 |
| Total cholesterol, mg/dl | 199.1 ± 35.4 | 204.5 ± 38.2 | 0.05 | 191.2 ± 35.8 | 200.9 ± 39.0 | 0.09 |
| LDL cholesterol, mg/dl | 124.0 ± 30.6 | 126.4 ± 33.3 | 0.31 | 114.3 ± 34.1 | 123.5 ± 34.0 | 0.08 |
| HDL cholesterol, mg/dl | 47.0 ± 12.5 | 50.3 ± 15.3 | <0.001 | 36.7 ± 9.3 | 39.2 ± 9.1 | 0.05 |
| Triglycerides, mg/dl | 144.9 ± 92.4 | 142.7 ± 80.7 | 0.59 | 164.4 ± 66.7 | 161.5 ± 78.1 | 0.84 |
Data are means ± SD unless otherwise indicated.
P values were calculated by χ2 test for categorical variables, and general linear models for continuous variables.
Diet adherence and nutrient intake
Consistent with the overall POUNDS LOST (28) and DIRECT (29) trial results, the reported dietary intakes (total energy, fat, protein, and carbohydrate) confirmed that participants modified their intake of macronutrients in the direction of the intervention (Table 2). Participants in the high-fat diet groups reported higher dietary fat intake and lower carbohydrate intake than those in the low-fat diet groups at 6 months and 2 years similarly across genotypes (all P < 0.05).
TABLE 2.
Energy and nutrient intakes according to diet and genotype groups
| Dietary intake | POUNDS LOST | DIRECT | ||||||
| CC | CA/AA | CC | CA/AA | |||||
| Low-fat Diets | High-fat Diets | Low-fat Diets | High-fat Diets | Low-fat Diets | High-fat Diets | Low-fat Diets | High-fat Diets | |
| Total energy (kcal/day) | ||||||||
| Baseline | 1,927 ± 578 | 2,019 ± 590 | 1,975 ± 516 | 1,946 ± 564 | 2,397 ± 856 | 2,434 ± 954 | 2,573 ± 2530 | 2,601 ± 1759 |
| 6 month | 1,672 ± 599 | 1,645 ± 510 | 1,551 ± 478 | 1,626 ± 509 | 1,959 ± 500 | 1,982 ± 610 | 2,086 ± 942 | 1,887 ± 608 |
| 2 years | 1,562 ± 475 | 1,618 ± 517 | 1,581 ± 479 | 1,378 ± 458a | 1,957 ± 704 | 1,945 ± 791 | 1,874 ± 688 | 1,965 ± 764 |
| Fat (% of energy) | ||||||||
| Baseline | 36.5 ± 6.6 | 36.9 ± 5.7 | 36.9 ± 5.7 | 37.5 ± 5.9 | 30.3 ± 5.5 | 32.0 ± 5.5 | 32.0 ± 4.1 | 31.2 ± 5.7 |
| 6 month | 25.1 ± 6.9 | 33.7 ± 7.2a | 26.9 ± 7.8 | 34.5 ± 7.0a | 31.1 ± 3.2 | 40.7 ± 7.3a | 32.3 ± 4.6 | 36.8 ± 5.4a |
| 2 years | 28.3 ± 8.8 | 36.7 ± 5.9a | 26.7 ± 6.7 | 32.4 ± 8.6a | 31.4 ± 3.5 | 40.2 ± 5.5a | 32.1 ± 5.1 | 37.9 ± 5.3a |
| Carbohydrate (% of energy) | ||||||||
| Baseline | 45.5 ± 8.7 | 44.1 ± 7.7 | 45.2 ± 7.0 | 43.9 ± 7.4 | 53.5 ± 9.2 | 51.3 ± 7.7 | 50.8 ± 6.6 | 51.8 ± 9.3 |
| 6 month | 56.5 ± 9.6 | 47.3 ± 8.7a | 54.2 ± 10.7 | 45.0 ± 7.5a | 51.4 ± 6.0 | 38.5 ± 9.1a | 48.9 ± 7.3 | 44.9 ± 7.0a |
| 2 years | 51.3 ± 10.5 | 43.7 ± 7.1a | 53.0 ± 9.5 | 47.4 ± 11.2a | 51.0 ± 6.1 | 39.0 ± 6.3a | 50.0 ± 6.0 | 42.2 ± 7.3a |
| Protein (% of energy) | ||||||||
| Baseline | 17.9 ± 3.6 | 18.4 ± 3.3 | 18.1 ± 3.3 | 18.1 ± 3.2 | 18.0 ± 3.2 | 18.5 ± 2.2 | 18.4 ± 3.1 | 18.6 ± 4.6 |
| 6 month | 19.4 ± 4.0 | 19.8 ± 4.8 | 20.1 ± 4.2 | 20.8 ± 4.8 | 19.4 ± 3.0 | 22.7 ± 3.4a | 20.3 ± 3.8 | 20.4 ± 3.0 |
| 2 years | 19.6 ± 4.2 | 20.0 ± 4.9 | 20.8 ± 3.5 | 20.7 ± 5.6 | 19.3 ± 3.6 | 22.3 ± 2.6a | 18.9 ± 2.7 | 21.4 ± 4.7a |
Data are means ± SD.
P < 0.05 for the comparison between the low- and high-fat diet groups.
Interaction between CETP genotype and diet intervention on changes in lipids
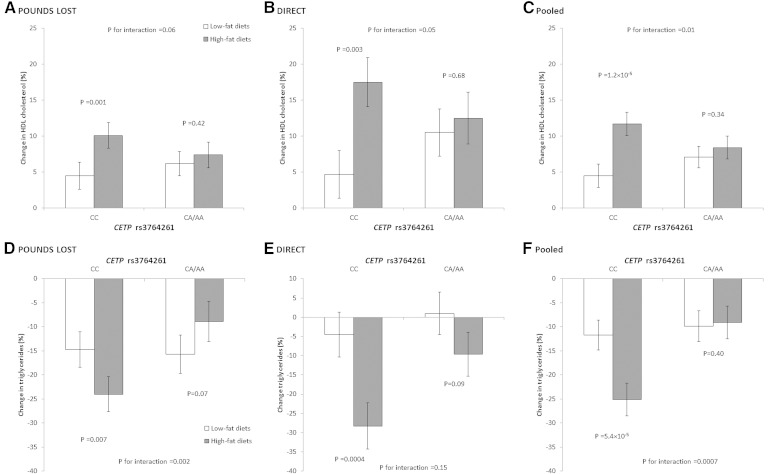
We observed a borderline significant interaction between the CETP genotype and dietary fat intervention on changes in HDL cholesterol levels at 6 months in the POUNDS LOST (P for interaction = 0.06) (Fig. 1A), adjusted for age, sex, ethnicity, baseline BMI, and lipid-lowering medication use. Among the CETP CC genotype, participants in the high-fat diet group showed a greater increase in HDL cholesterol levels compared with those in the low-fat diet group (10.1 vs. 4.5%, P = 0.001). There was no significant difference in changes of HDL cholesterol levels by diet fat interventions among participants with CA/AA genotypes (7.4 vs. 6.2%, P = 0.43). A similar gene-diet intervention interaction was replicated in the DIRECT (P for interaction = 0.05) (Fig. 1B). In the combined data from these two trials, we found a significant gene-diet intervention interaction (P for interaction = 0.01), and there was a significant difference in increases of HDL cholesterol levels between high-fat and low-fat diet interventions among participants with the CETP CC genotype (11.7 vs. 4.5%, P < 0.001) (Fig. 1C). There was no significant interaction between CETP genotype and dietary interaction on weight loss (data not shown). After further adjustment for changes in body weight, the results were attenuated but remained significant in the combined data (P for interaction = 0.02).
Fig. 1.
Percent changes in HDL cholesterol and triglyceride levels according to diet and genotype groups at 6 months. Data are means ± SE, adjusted for age, sex, ethnicity (POUNDS LOST only), baseline BMI, and lipid-lowering medication use. Data from two trials were pooled by inverse-variance-weighted fixed-effects meta-analyses.
In addition, we found a significant gene-diet intervention interaction on changes in triglycerides at 6 months in the POUNDS LOST (P for interaction = 0.002) (Fig. 1D). Among the CETP CC genotype, participants in the high-fat diet group had a greater decrease in triglyceride levels compared with those in the low-fat diet group (−24.0 vs. −14.7%, P = 0.007). There was no significant difference in changes in triglyceride levels between the high-fat and low-fat diet interventions among participants without CC genotype (−9.0 vs. −15.7%, P = 0.07). A similar gene-diet intervention interaction pattern was observed in the DIRECT, though it did not reach the significant level (P for interaction = 0.15) (Fig. 1D). In the combined data from these two trials, we observed a significant gene-diet interaction (P for interaction < 0.001), and the high-fat diet showed a more favorable effect on decrease in triglyceride levels than the low-fat diet among participants with the CETP CC genotype (−25.1 vs. −11.7%, P < 0.001) (Fig. 1E). After further adjustment for changes in body weight, the results were attenuated but remained significant in the combined data (P for interaction = 0.002).
We found no significant interaction between the CETP genotype and dietary fat intervention on changes in total cholesterol or LDL cholesterol levels in the POUNDS LOST or DIRECT (all P for interaction > 0.20).
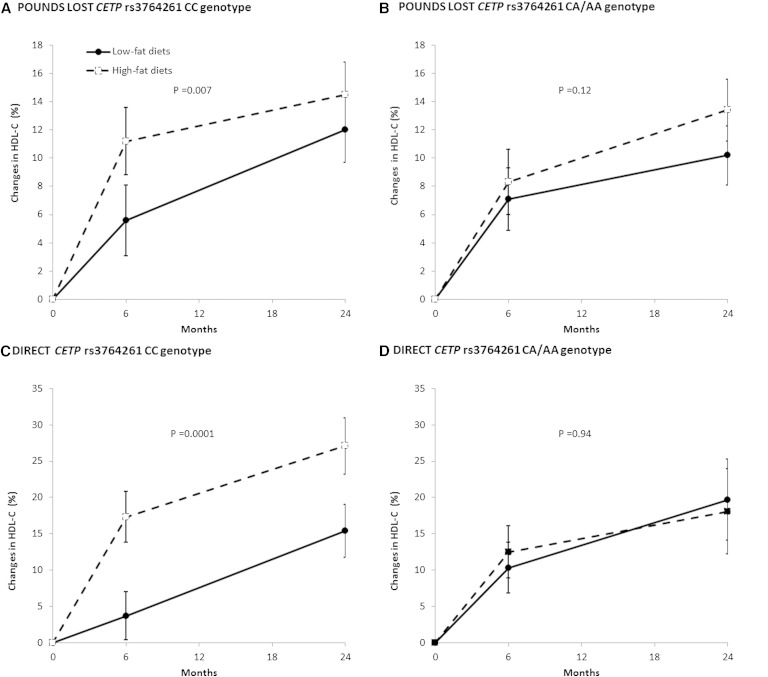
Trajectory of changes in HDL cholesterol and triglycerides
We then used generalized estimating equations to examine the trajectory of changes in blood lipids by CETP genotype groups over the 2 year intervention. In the POUNDS LOST, among participants with CETP CC genotype, HDL cholesterol levels increased significantly in the high-fat group as compared with the low-fat diet group (P = 0.007) (Fig. 2A). Similar results were replicated in the DIRECT (P = 0.0001) (Fig. 2C). There was no significant dietary effect among participants with CETP CA/AA genotype in the POUNDS LOST (P = 0.12) or DIRECT (P = 0.94) (Fig. 2B, D).
Fig. 2.
Percent changes in HDL cholesterol levels according to diet and genotype groups over the 2 year intervention. Data are means ± SE, adjusted for age, sex, ethnicity (POUNDS LOST only), baseline BMI, and lipid-lowering medication use.
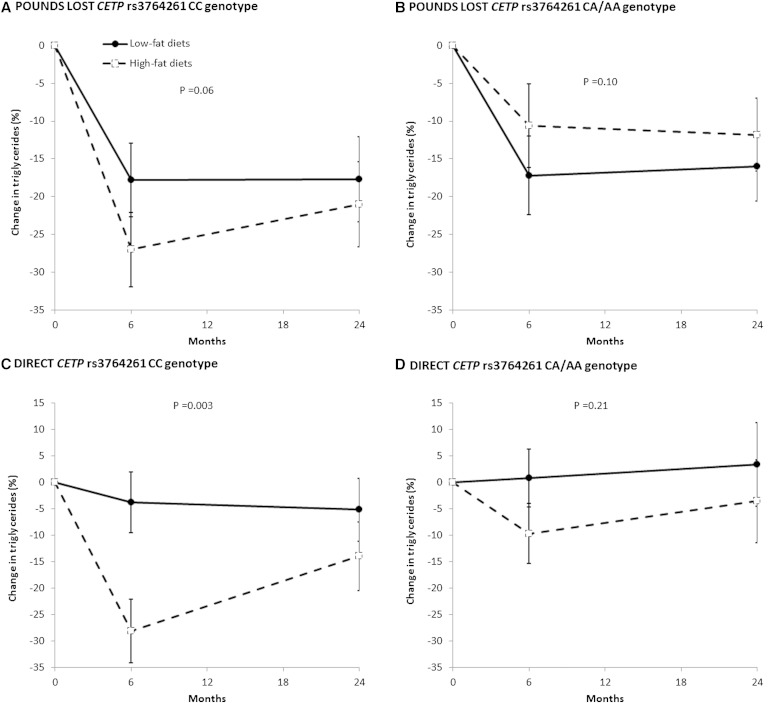
For changes in triglycerides, there was a marginally significant difference between the high-fat and the low-fat diet groups (P = 0.06) among participants with CETP CC genotype in the POUNDS LOST (Fig. 3A). The results were replicated in the DIRECT, where triglyceride levels increased significantly in the high-fat group as compared with the low-fat diet group (P = 0.003) among participants with CETP CC genotype (Fig. 3C). There was no significant dietary effect on triglycerides among participants with the CETP CA/AA genotype in the POUNDS LOST (P = 0.10) or DIRECT (P = 0.21) (Fig. 3B, D).
Fig. 3.
Percent changes in triglyceride levels according to diet and genotype groups over the 2-year intervention. Data are means ± SE, adjusted for age, sex, ethnicity (POUNDS LOST only), baseline BMI, and lipid-lowering medication use.
DISCUSSION
In a 2 year randomized weight-loss intervention trial (POUNDS LOST), we observed significant interactions between the CETP rs3764261 variant and diet intervention in relation to changes in HDL cholesterol and triglyceride levels. Among participants with the CETP rs3764261 CC genotype, high-fat diets showed greater effects on increasing HDL cholesterol levels and reducing triglyceride levels compared with low-fat diets. The findings were replicated in an independent 2 year randomized weight-loss intervention trial (DIRECT). Our data, for the first, time provide reproducible evidence for CETP-diet interaction in relation to long-term changes in blood lipids (HDL cholesterol and triglycerides) in randomized intervention trials.
Previous studies of the interactions between CETP gene and dietary fat intake on lipid levels have focused on the TaqIB SNP [rs708272, moderate linkage disequilibrium with rs3764261 (r2 = 0.44, D′ = 0.89)]. In an observational study, Li et al. (15) reported an interaction between the CETP TaqIB and dietary fat intake on plasma HDL cholesterol levels in US diabetic men, but the result was not replicated in a US biracial community study (20) or in a Mediterranean population study (16). In two small 4 week diet intervention trials, there was no significant modulatory effect of the CETP TaqIB on lipid levels in response to changes in dietary fat intake (21, 22). The inferences from these previous studies were limited by observational study design, short-term intervention, small sample size, and genetic variants that might not be instrumental in determining lipid levels (32). In the current study, we found significant interactions between the CETP variant rs3764261, an established lipid-associated SNP identified by GWAS (27), and dietary fat intake on changes in HDL cholesterol and triglyceride levels in two independent long-term diet intervention trials. A recent study reported that the SNP rs3764261 was associated with the CETP mRNA expression in human livers, suggesting a possible functional role of this variant in regulating CETP gene expression, while no association was observed for the TaqIB SNP (33).
Changes in blood lipids occurred rapidly in the weight-loss phase in early months and became slow after 6 months when most participants regained body weight (28, 29). However, we did not find interaction between CETP genotype and dietary intervention on weight loss and the gene-diet interactions on changes in HDL cholesterol and triglycerides remained significant after further adjustment for changes in body weight. This suggests that observed gene-diet interactions on changes in HDL cholesterol and triglycerides are independent of weight loss. Interestingly, the gene-diet interactions and dietary effects on changes in HDL cholesterol were less attenuated at 2 years compared with those on changes in triglycerides. It should be noted that HDL cholesterol levels displayed a continuing change in the same direction throughout the 2 year intervention, while triglyceride levels increased after 6 months in both the POUNDS LOST and DIRECT. Indeed, Blüher et al. (34) have reported two different dynamic patterns of HDL cholesterol and triglyceride levels along with weight loss and weight regain in the DIRECT. The continued long-term effect on raising HDL cholesterol levels despite partial weight regain may likely reflect either a delayed effect of the initial weight loss or a continuous beneficial response to dietary interventions (34).
The mechanism underlying the observed gene-diet interaction on changes in HDL cholesterol and triglyceride levels is unclear. A number of animal studies have suggested that the quantity and quality of dietary fat may regulate CETP activity, mass, and gene expression (35–40). For example, plasma CETP activity, CETP mass, and hepatic CETP mRNA levels were significantly higher in transgenic mice expressing human CETP (CETP-TG mice) fed a high-fat diet compared with CETP-TG mice fed a low-fat diet (40). In human studies, it has been reported that high-saturated fat diets may increase CETP activity and concentrations as compared with high-monounsaturated and high-polyunsaturated fat diets (41–43). Moreover, the CETP genetic variant rs3764261 is associated with CETP gene expression (33). Thus, it is plausible that interactions between the CETP genetic variant and dietary fat intake in relation to changes in lipid levels are through their effects on CETP gene expression. More experimental studies are needed to further explore how the genetic variant rs3764261 may interact with dietary fat in determining blood lipid levels, especially HDL cholesterol and triglycerides.
It was difficult to tease out the effects of dietary fat and carbohydrate in our study because the high-fat diets actually have low carbohydrate content, and vice versa, to maintain energy balance. It should be noted that diets varying in fat content (low-fat diets or high-fat diets) are not emphasized to reduce CVD risk in the current American College of Cardiology/American Heart Association dietary guideline, but limiting intake of saturated fat and trans fat is recommended (44). In the POUNDS LOST, saturated fat intake was restricted to 8% of energy intake in the high-fat diet groups (28). In the DIRECT, although saturated fat intake was not specifically restricted, participants in the high-fat (low-carbohydrate) diet group were counseled to choose plant sources of fat to avoid saturated and trans fats (29).
The strengths of our study include data from two of the largest and longest-term randomized weight-loss trials, with relatively high completion rates (≥80%) and comparable study designs. More importantly, the consistent findings of the gene-diet interactions in two randomized trials minimize the potential false positive errors and indicate the robustness of our results. Because the study conditions are prescribed and the confounding effects are maximally reduced, randomized dietary intervention trials may provide reliable evidence for gene-diet interactions.
There are several limitations to our study. Participants did not completely reach the goals for macronutrient intake of their assigned group in the POUNDS LOST, but mean differences in fat and carbohydrate intake between the diet groups were significant (28). The power to detect long-term gene-diet interaction effects on changes in blood lipids at 2 years was reduced due to diminished adherence after 6 months. However, the adherence to diets was strict in the DIRECT, as this trial was conducted in a workplace, which permitted a closely monitored dietary intervention for 2 years. Strategies to improve adherence to dietary interventions in long-term are warranted. In addition, CETP activity, CETP concentrations, and CETP expression were not measured in our study. This limited our analysis to further investigate the functional changes related to CETP genetic variant. Finally, most of the participants were whites (∼80% in the POUNDS LOST and all in the DIRECT) and it remains to be determined whether our findings can be generalized in other ethnic groups.
In summary, our consistent results from two independent randomized trials indicate that individuals with the CETP rs3764261 CC genotype might be more responsive to low-carbohydrate/high-fat weight-loss diets in raising HDL cholesterol and lowering triglyceride levels compared with those without this genotype. Our findings provide novel information to the development of effective strategies for dietary interventions and supportive evidence for the notion of a personalized dietary intervention based on genetic background.
Acknowledgments
The authors are particularly grateful to all participants in the trials for their dedication and contribution to the research.
Footnotes
Abbreviations:
- CETP
- cholesteryl ester transfer protein
- DIRECT
- Dietary Intervention Randomized Controlled Trial
- GWAS
- genome-wide association study
- POUNDS LOST
- Preventing Overweight Using Novel Dietary Strategies
This study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718), the National Cancer Institute (CA155626), the General Clinical Research Center (RR-02635), the Boston Obesity Nutrition Research Center (DK46200), the Israel-US Binational Science Foundation (BSF) (2011036), and the Dr. Robert C. and Veronica Atkins Research Foundation.
REFERENCES
- 1.Lewington S., Whitlock G., Clarke R., Sherliker P., Emberson J., Halsey J., Qizilbash N., Peto R., Collins R. 2007. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 370: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 2.Sarwar N., Danesh J., Eiriksdottir G., Sigurdsson G., Wareham N., Bingham S., Boekholdt S. M., Khaw K. T., Gudnason V. 2007. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 115: 450–458. [DOI] [PubMed] [Google Scholar]
- 3.Di Angelantonio E., Sarwar N., Perry P., Kaptoge S., Ray K. K., Thompson A., Wood A. M., Lewington S., Sattar N., Packard C. J., et al. 2009. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 302: 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costanza M. C., Cayanis E., Ross B. M., Flaherty M. S., Alvin G. B., Das K., Morabia A. 2005. Relative contributions of genes, environment, and interactions to blood lipid concentrations in a general adult population. Am. J. Epidemiol. 161: 714–724. [DOI] [PubMed] [Google Scholar]
- 5.Ordovas J. M., Shen A. H. 2002. Genetics, the environment, and lipid abnormalities. Curr. Cardiol. Rep. 4: 508–513. [DOI] [PubMed] [Google Scholar]
- 6.Tall A. R. 1986. Plasma lipid transfer proteins. J. Lipid Res. 27: 361–367. [PubMed] [Google Scholar]
- 7.Brown M. L., Inazu A., Hesler C. B., Agellon L. B., Mann C., Whitlock M. E., Marcel Y. L., Milne R. W., Koizumi J., Mabuchi H., et al. 1989. Molecular basis of lipid transfer protein deficiency in a family with increased high-density lipoproteins. Nature. 342: 448–451. [DOI] [PubMed] [Google Scholar]
- 8.Inazu A., Brown M. L., Hesler C. B., Agellon L. B., Koizumi J., Takata K., Maruhama Y., Mabuchi H., Tall A. R. 1990. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N. Engl. J. Med. 323: 1234–1238. [DOI] [PubMed] [Google Scholar]
- 9.Brousseau M. E., Schaefer E. J., Wolfe M. L., Bloedon L. T., Digenio A. G., Clark R. W., Mancuso J. P., Rader D. J. 2004. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N. Engl. J. Med. 350: 1505–1515. [DOI] [PubMed] [Google Scholar]
- 10.Thompson A., Di Angelantonio E., Sarwar N., Erqou S., Saleheen D., Dullaart R. P., Keavney B., Ye Z., Danesh J. 2008. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 299: 2777–2788. [DOI] [PubMed] [Google Scholar]
- 11.Boekholdt S. M., Sacks F. M., Jukema J. W., Shepherd J., Freeman D. J., McMahon A. D., Cambien F., Nicaud V., de Grooth G. J., Talmud P. J., et al. 2005. Cholesteryl ester transfer protein TaqIB variant, high-density lipoprotein cholesterol levels, cardiovascular risk, and efficacy of pravastatin treatment: individual patient meta-analysis of 13,677 subjects. Circulation. 111: 278–287. [DOI] [PubMed] [Google Scholar]
- 12.Fumeron F., Betoulle D., Luc G., Behague I., Ricard S., Poirier O., Jemaa R., Evans A., Arveiler D., Marques-Vidal P., et al. 1995. Alcohol intake modulates the effect of a polymorphism of the cholesteryl ester transfer protein gene on plasma high density lipoprotein and the risk of myocardial infarction. J. Clin. Invest. 96: 1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsujita Y., Nakamura Y., Zhang Q. S., Tamaki S., Nozaki A., Amamoto K., Kadowaki T., Kita Y., Okamura T., Horie M., et al. 2007. The association between high-density lipoprotein cholesterol level and cholesteryl ester transfer protein TaqIB gene polymorphism is influenced by alcohol drinking in a population-based sample. Atherosclerosis. 191: 199–205. [DOI] [PubMed] [Google Scholar]
- 14.Jensen M. K., Mukamal K. J., Overvad K., Rimm E. B. 2008. Alcohol consumption, TaqIB polymorphism of cholesteryl ester transfer protein, high-density lipoprotein cholesterol, and risk of coronary heart disease in men and women. Eur. Heart J. 29: 104–112. [DOI] [PubMed] [Google Scholar]
- 15.Li T. Y., Zhang C., Asselbergs F. W., Qi L., Rimm E., Hunter D. J., Hu F. B. 2007. Interaction between dietary fat intake and the cholesterol ester transfer protein TaqIB polymorphism in relation to HDL-cholesterol concentrations among US diabetic men. Am. J. Clin. Nutr. 86: 1524–1529. [DOI] [PubMed] [Google Scholar]
- 16.Corella D., Carrasco P., Fito M., Martinez-Gonzalez M. A., Salas-Salvado J., Aros F., Lapetra J., Guillen M., Ortega-Azorin C., Warnberg J., et al. 2010. Gene-environment interactions of CETP gene variation in a high cardiovascular risk Mediterranean population. J. Lipid Res. 51: 2798–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corella D., Saiz C., Guillen M., Portoles O., Mulet F., Gonzalez J. I., Ordovas J. M. 2000. Association of TaqIB polymorphism in the cholesteryl ester transfer protein gene with plasma lipid levels in a healthy Spanish population. Atherosclerosis. 152: 367–376. [DOI] [PubMed] [Google Scholar]
- 18.Chen J., Yokoyama T., Saito K., Yoshiike N., Date C., Tanaka H. 2002. Association of Human Cholesteryl Ester Transfer Protein-Tagl Polymorphisms with Serum HDL Cholesterol Levels in a Normolipemic Japanese Rural Population. J. Epidemiol. 12: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talmud P. J., Hawe E., Robertson K., Miller G. J., Miller N. E., Humphries S. E. 2002. Genetic and environmental determinants of plasma high density lipoprotein cholesterol and apolipoprotein AI concentrations in healthy middle-aged men. Ann. Hum. Genet. 66: 111–124. [DOI] [PubMed] [Google Scholar]
- 20.Nettleton J. A., Steffen L. M., Ballantyne C. M., Boerwinkle E., Folsom A. R. 2007. Associations between HDL-cholesterol and polymorphisms in hepatic lipase and lipoprotein lipase genes are modified by dietary fat intake in African American and White adults. Atherosclerosis. 194: e131–e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmena-Ramón R., Ascaso J. F., Real J. T., Njera G., Ordovs J. M., Carmena R. 2001. Association between the TaqIB polymorphism in the cholesteryl ester transfer protein gene locus and plasma lipoprotein levels in familial hypercholesterolemia. Metabolism. 50: 651–656. [DOI] [PubMed] [Google Scholar]
- 22.Aitken W. A. E., Chisholm A. W. A. H., Duncan A. W., Harper M. J., Humphries S. E., Mann J. I., Murray Skeaff C., Sutherland W. H. F., Wallace A. J., Williams S. M. 2006. Variation in the cholesteryl ester transfer protein (CETP) gene does not influence individual plasma cholesterol response to changes in the nature of dietary fat. Nutr. Metab. Cardiovasc. Dis. 16: 353–363. [DOI] [PubMed] [Google Scholar]
- 23.Willer C. J., Sanna S., Jackson A. U., Scuteri A., Bonnycastle L. L., Clarke R., Heath S. C., Timpson N. J., Najjar S. S., Stringham H. M., et al. 2008. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 40: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kathiresan S., Melander O., Guiducci C., Surti A., Burtt N. P., Rieder M. J., Cooper G. M., Roos C., Voight B. F., Havulinna A. S., et al. 2008. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 40: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kathiresan S., Willer C. J., Peloso G. M., Demissie S., Musunuru K., Schadt E. E., Kaplan L., Bennett D., Li Y., Tanaka T., et al. 2009. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 41: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aulchenko Y. S., Ripatti S., Lindqvist I., Boomsma D., Heid I. M., Pramstaller P. P., Penninx B. W., Janssens A. C., Wilson J. F., Spector T., et al. 2009. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat. Genet. 41: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teslovich T. M., Musunuru K., Smith A. V., Edmondson A. C., Stylianou I. M., Koseki M., Pirruccello J. P., Ripatti S., Chasman D. I., Willer C. J., et al. 2010. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 466: 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacks F. M., Bray G. A., Carey V. J., Smith S. R., Ryan D. H., Anton S. D., McManus K., Champagne C. M., Bishop L. M., Laranjo N., et al. 2009. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 360: 859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shai I., Schwarzfuchs D., Henkin Y., Shahar D. R., Witkow S., Greenberg I., Golan R., Fraser D., Bolotin A., Vardi H., et al. 2008. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 359: 229–241. [DOI] [PubMed] [Google Scholar]
- 30.Qi Q., Bray G. A., Hu F. B., Sacks F. M., Qi L. 2012. Weight-loss diets modify glucose-dependent insulinotropic polypeptide receptor rs2287019 genotype effects on changes in body weight, fasting glucose, and insulin resistance: the Preventing Overweight Using Novel Dietary Strategies trial. Am. J. Clin. Nutr. 95: 506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi Q., Bray G. A., Smith S. R., Hu F. B., Sacks F. M., Qi L. 2011. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation. 124: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klerkx A. H. E. M., Tanck M. W. T., Kastelein J. J. P., Molhuizen H. O. F., Jukema J. W., Zwinderman A. H., Kuivenhoven J. A. 2003. Haplotype analysis of the CETP gene: not TaqIB, but the closely linked-629C -> A polymorphism and a novel promoter variant are independently associated with CETP concentration. Hum. Mol. Genet. 12: 111–123. [DOI] [PubMed] [Google Scholar]
- 33.Papp A. C., Pinsonneault J. K., Wang D., Newman L. C., Gong Y., Johnson J. A., Pepine C. J., Kumari M., Hingorani A. D., Talmud P. J., et al. 2012. Cholesteryl ester transfer protein (CETP) polymorphisms affect mRNA splicing, HDL levels, and sex-dependent cardiovascular risk. PLoS ONE. 7: e31930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blüher M., Rudich A., Klöting N., Golan R., Henkin Y., Rubin E., Schwarzfuchs D., Gepner Y., Stampfer M. J., Fiedler M., et al. 2012. Two patterns of adipokine and other biomarker dynamics in a long-term weight loss intervention. Diabetes Care. 35: 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Son Y. S., Zilversmit D. B. 1986. Increased lipid transfer activities in hyperlipidemic rabbit plasma. Arteriosclerosis. 6: 345–351. [PubMed] [Google Scholar]
- 36.McPherson R., Lau P., Kussie P., Barrett H., Tall A. R. 1997. Plasma kinetics of cholesteryl ester transfer protein in the rabbit. Effects of dietary cholesterol. Arterioscler. Thromb. Vasc. Biol. 17: 203–210. [DOI] [PubMed] [Google Scholar]
- 37.Quinet E., Tall A., Ramakrishnan R., Rudel L. 1991. Plasma lipid transfer protein as a determinant of the atherogenicity of monkey plasma lipoproteins. J. Clin. Invest. 87: 1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fusegawa Y., Kelley K. L., Sawyer J. K., Shah R. N., Rudel L. L. 2001. Influence of dietary fatty acid composition on the relationship between CETP activity and plasma lipoproteins in monkeys. J. Lipid Res. 42: 1849–1857. [PubMed] [Google Scholar]
- 39.Gupta S. V., Yamada N., Fungwe T. V., Khosla P. 2003. Replacing 40% of dietary animal fat with vegetable oil is associated with lower HDL cholesterol and higher cholesterol ester transfer protein in cynomolgus monkeys fed sufficient linoleic acid. J. Nutr. 133: 2600–2606. [DOI] [PubMed] [Google Scholar]
- 40.Cheema S. K., Agarwal-Mawal A., Murray C. M., Tucker S. 2005. Lack of stimulation of cholesteryl ester transfer protein by cholesterol in the presence of a high-fat diet. J. Lipid Res. 46: 2356–2366. [DOI] [PubMed] [Google Scholar]
- 41.Groener J. E., van Ramshorst E. M., Katan M. B., Mensink R. P., van Tol A. 1991. Diet-induced alteration in the activity of plasma lipid transfer protein in normolipidemic human subjects. Atherosclerosis. 87: 221–226. [DOI] [PubMed] [Google Scholar]
- 42.Schwab U. S., Maliranta H. M., Sarkkinen E. S., Savolainen M. J., Kesaniemi Y. A., Uusitupa M. I. 1996. Different effects of palmitic and stearic acid-enriched diets on serum lipids and lipoproteins and plasma cholesteryl ester transfer protein activity in healthy young women. Metabolism. 45: 143–149. [DOI] [PubMed] [Google Scholar]
- 43.Jansen S., Lopez-Miranda J., Castro P., Lopez-Segura F., Marin C., Ordovas J. M., Paz E., Jimenez-Pereperez J., Fuentes F., Perez-Jimenez F. 2000. Low-fat and high-monounsaturated fatty acid diets decrease plasma cholesterol ester transfer protein concentrations in young, healthy, normolipemic men. Am. J. Clin. Nutr. 72: 36–41. [DOI] [PubMed] [Google Scholar]
- 44.Eckel R. H., Jakicic J. M., Ard J. D., de Jesus J. M., Houston Miller N., Hubbard V. S., Lee I. M., Lichtenstein A. H., Loria C. M., Millen B. E., et al. 2014. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 129: S76–S99. [DOI] [PubMed] [Google Scholar]