Abstract
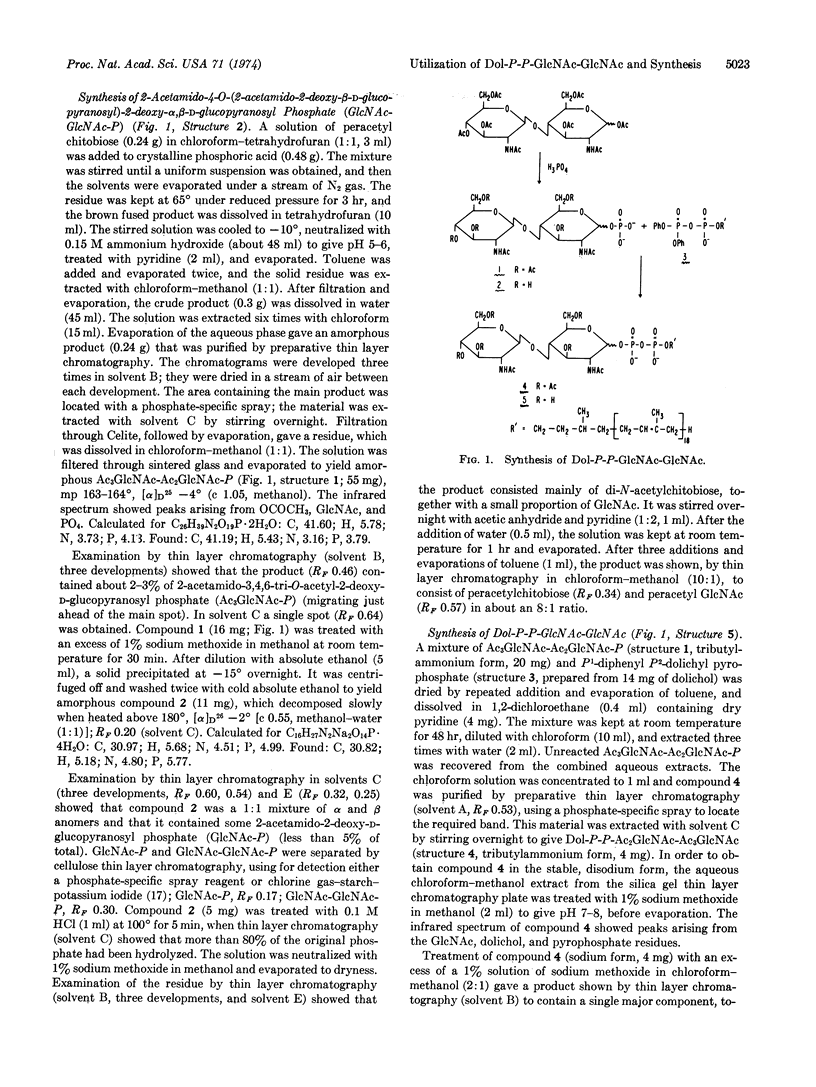
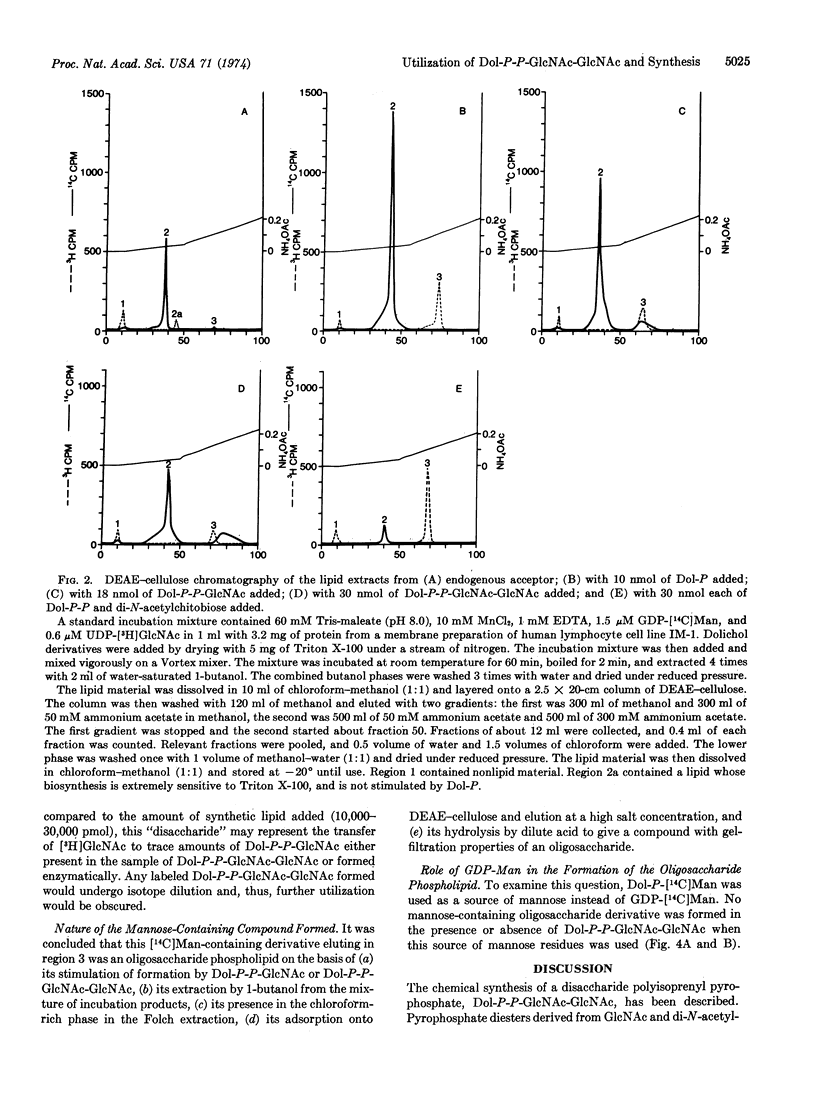
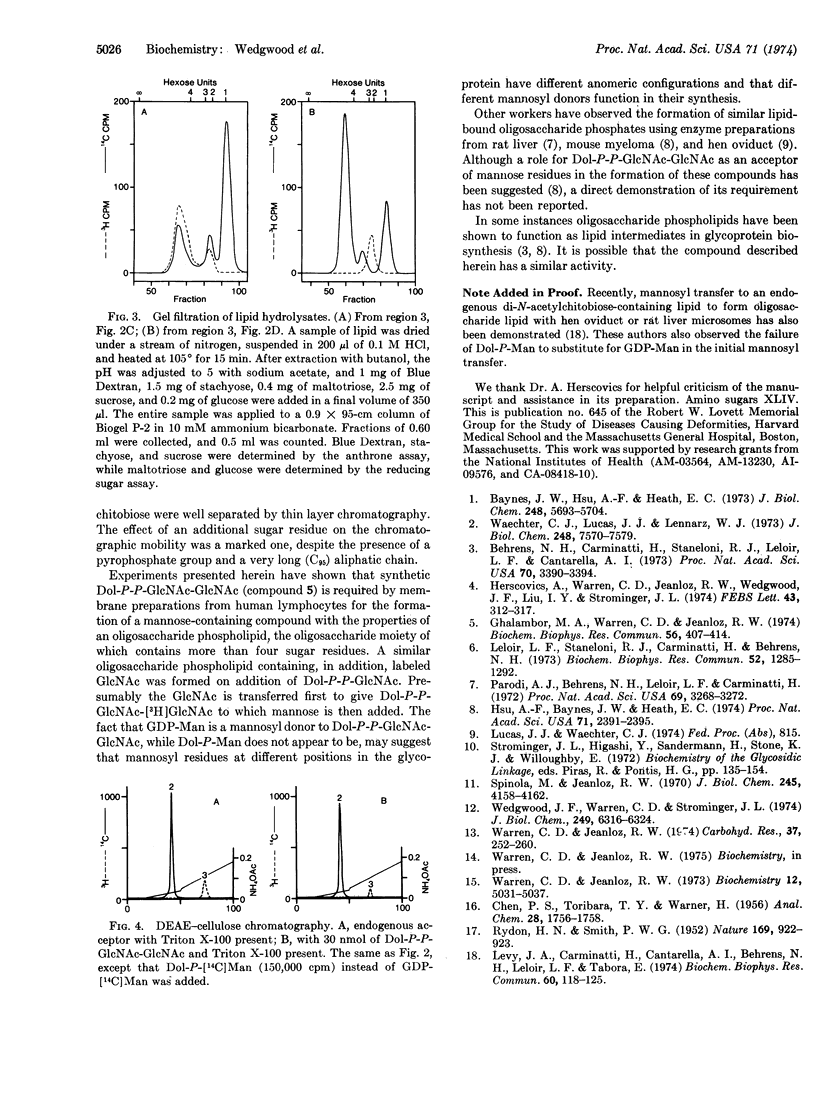
Fully acetylated chitobiose was treated with phosphoric acid to give a mixture of products from which 2-acetamido-4-O-(2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β- D-glucopyranosyl)-3,6-di-O-acetyl-2-deoxy-α,β- D-glucopyranosyl phosphate (Ac3GlcNAc-Ac2GlcNAc-P) was isolated by preparative thin layer chromatography. Treatment of this compound with P1-diphenyl P2-dolichyl pyrophosphate gave an acetylated pyrophosphate diester, which was purified chromatographically and deacetylated. The product, P1-di-N-acetylchitobiosyl P2-dolichyl pyrophosphate (Dol-P-P-GlcNAc-GlcNAc), was readily separated from P1-2-acetamido-2-deoxy-α-D-glucopyranosyl P2-dolichyl pyrophosphate (Dol-P-P-GlcNAc) by thin layer chromatography in several solvent systems. Addition of this compound to human lymphocyte membrane preparations led to the formation of a mannose-containing derivative which appears to be an oligosaccharide phospholipid, as judged by its behavior on DEAE-cellulose chromatography and by its hydrolysis to give an oligosaccharide containing more than four monosaccharide units.
Keywords: glycoprotein biosynthesis, lipid intermediate, mannose-containing oligosaccharide, di-N-acetylchitobiosyl phosphate
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREITENBACH J. W., DERKOSCH J., WESSELY F. Energetics of peptide formation. Nature. 1952 May 31;169(4309):922–922. doi: 10.1038/169922a0. [DOI] [PubMed] [Google Scholar]
- Baynes J. W., Hsu A. F., Heath E. C. The role of mannosyl-phosphoryl-dihydropolyisoprenol in the synthesis of mammalian glycoproteins. J Biol Chem. 1973 Aug 25;248(16):5693–5704. [PubMed] [Google Scholar]
- Behrens N. H., Carminatti H., Staneloni R. J., Leloir L. F., Cantarella A. I. Formation of lipid-bound oligosaccharides containing mannose. Their role in glycoprotein synthesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3390–3394. doi: 10.1073/pnas.70.12.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalambor M. A., Warren C. D., Jeanloz R. W. Biosynthesis of a P1-2-acetamido-2-deoxy-D-glucosyl P2-polyisoprenyl pyrophosphate by calf pancreas microsomes. Biochem Biophys Res Commun. 1974 Jan 23;56(2):407–414. doi: 10.1016/0006-291x(74)90857-2. [DOI] [PubMed] [Google Scholar]
- Herscovics A., Warren C. D., Jeanloz R. W., Wedgwood J. F., Liu I. Y., Strominger J. L. Occurrence of a beta-D-mannopyranosyl phosphate residue in the polyprenyl mannosyl phosphate formed in calf pancreas microsomes and in human lymphocytes. FEBS Lett. 1974 Sep 1;45(1):312–317. doi: 10.1016/0014-5793(74)80869-0. [DOI] [PubMed] [Google Scholar]
- Hsu A. F., Baynes J. W., Heath E. C. The role of a dolichol-oligosaccharide as an intermediate in glycoprotein biosynthesis. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2391–2395. doi: 10.1073/pnas.71.6.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloir L. F., Staneloni R. J., Carminatti H., Behrens N. H. The biosynthesis of a N,N'-diacetylchitobiose containing lipid by liver microsomes. A probable dolichol pyrophosphate derivative. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1285–1292. doi: 10.1016/0006-291x(73)90640-2. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Carminatti H., Cantarella A. I., Behrens N. H., Leloir L. F., Tábora E. Mannose transfer to lipid linked di-N-acetylchitobiose. Biochem Biophys Res Commun. 1974 Sep 9;60(1):118–125. doi: 10.1016/0006-291x(74)90180-6. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Behrens N. H., Leloir L. F., Carminatti H. The role of polyprenol-bound saccharides as intermediates in glycoprotein synthesis in liver. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3268–3272. doi: 10.1073/pnas.69.11.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinola M., Jeanloz R. W. The synthesis of a di-N-acetylchitobiose asparagine derivative, 2-acetamido-4-O-(2-acetamido-2-deoxy-beta-D-glucopyranosyl)-1-N-(4-L-aspartyl)-2-deoxy-beta-D-glucopyranosylamine. J Biol Chem. 1970 Aug 25;245(16):4158–4162. [PubMed] [Google Scholar]
- Waechter C. J., Lucas J. J., Lennarz W. J. Membrane glycoproteins. I. Enzymatic synthesis of mannosyl phosphoryl polyisoprenol and its role as a mannosyl donor in glycoprotein synthesis. J Biol Chem. 1973 Nov 10;248(21):7570–7579. [PubMed] [Google Scholar]
- Warren C. D., Jeanloz R. W. Chemical synthesis of ficaprenyl alpha-D-mannopyranosyl phosphate. Biochemistry. 1973 Dec 4;12(25):5031–5037. doi: 10.1021/bi00749a001. [DOI] [PubMed] [Google Scholar]
- Wedgwood J. F., Strominger J. L., Warren C. D. Transfer of sugars from nucleoside diphosphosugar compounds to endogenous and synthetic dolichyl phosphate in human lymphocytes. J Biol Chem. 1974 Oct 10;249(19):6316–6324. [PubMed] [Google Scholar]



