Abstract
To demonstrate monoacylglycerol acyltransferase 2 (MGAT2)-mediated enzyme activity in a cellular context, cells of the murine secretin tumor cell-1 line of enteroendocrine origin were used to construct human MGAT2-expressing recombinant cell lines. Low throughput and utilization of radiolabeled substrate in a traditional TLC technique were circumvented by development of a high-resolution LC/MS platform. Monitoring incorporation of stable isotope-labeled D31-palmitate into diacylglycerol (DAG) allowed selective tracing of the cellular DAG synthesis activity. This assay format dramatically reduced background interference and increased the sensitivity and the signal window compared with the TLC method. Using this assay, several MGAT2 inhibitors from different chemotypes were characterized. The described cell-based assay adds a new methodology for the development and evaluation of MGAT2 inhibitors for the treatment of obesity and type 2 diabetes.
Keywords: monoacylglycerol acyltransferase, diacylglycerol acyltransferase, triglycerides, liquid chromatography/mass spectrometry, cell-based assay
Monoacylglycerol acyltransferase-2 (MGAT2) is a membrane-bound lipid acyltransferase that is an emerging molecular target for the treatment of obesity and type 2 diabetes (1). MGAT2 is highly and selectively expressed in the small intestine, where it exerts an important role in the monoacylglycerol pathway of triacylglycerol (TAG) synthesis for the absorption of dietary fat (2, 3). When dietary fat is ingested, pancreatic lipase digests TAG into FFAs and monoacylglycerol, which are absorbed by intestinal epithelial enterocytes. Once inside enterocytes, FFAs and monoacylglycerol are used as building blocks to resynthesize TAG through two sequential acylation steps. The first acylation step is performed by MGAT, followed by diacylglycerol acyltransferase (DGAT) enzyme reactions. TAGs are then incorporated into chylomicrons and secreted into lymph to be used as an energy supply for the body (4).
MGAT2 KO mice exhibit a multitude of healthy metabolic phenotypes relative to WT controls, including resistance to high-fat diet-induced obesity, improvement in insulin sensitivity, and decreased fat accumulation in liver and adipose tissue (1). Under a high-fat diet regimen for 8–9 months, WT mice became diabetic, with fasting glucose levels of approximately 220 mg/dl. In contrast, littermate MGAT2 KO mice exposed to the same high-fat diet regimen exhibited significantly lower fasting glucose levels (∼120 mg/dl). In addition, mice with genetic deletion of MGAT2 appeared to absorb fat at a more distal site in the intestine than WT controls, suggesting delayed TG absorption. Further, there is evidence that glucagon-like peptide-1 is elevated in MGAT2 KO mice. The mouse genetic data therefore support the concept of using MGAT2 inhibition for the treatment of type 2 diabetes through weight loss and gut hormone regulation (1).
Multiple pharmaceutical companies have initiated campaigns to identify MGAT2 inhibitors as therapeutic agents to combat obesity and type 2 diabetes. Indeed, recently in the medicinal chemistry field many papers and patents describing a number of structurally diverse small molecules as inhibitors of MGAT2 have been published (5–8). In most reports, the structure-activity relationship was primarily evaluated based on results obtained using in vitro biochemical assays. Because MGAT2 is an intracellular enzyme that resides in the endoplasmic reticulum, a functional assay that takes into consideration both cellular penetrance and inhibitory potency in a cellular context is highly desirable. We present here the development of a cellular assay to evaluate functional inhibition of MGAT2 inhibitors. A recombinant cell line was constructed by transfecting full-length cDNA of human MGAT2 in the secretin tumor cell (STC)-1 cell line (designated STC-1/Human MGAT2 cell line). Initial development of the assay used radiolabeled substrate and TLC to measure diacylglycerol (DAG) and TAG products. To increase sensitivity and throughput, the assay was adapted to a high-resolution LC/MS platform. Incorporation of stable isotope-labeled substrates drastically reduced background interference and increased the sensitivity and the signal window of the assay. Monitoring of the incorporation of stable isotope-labeled residues into DAG and TAG products allowed us to selectively trace the cellular DAG synthesis activity. Using this assay, several MGAT2 inhibitors from different chemotypes were characterized.
Materials and Methods
Materials
The STC-1 cell line was obtained from Grant et al. at Cold Spring Harbor Laboratory (9). For tissue culture, we obtained DMEM from Life Technologies (#11960), FBS from Hyclone (#SH30071.03, lot# ATD31921), and Geneticin from Life Technologies (#10131-035). For isotope label-based MGAT2 cellular assay, we obtained D31-palmitate from Cambridge Isotope (DLM-215-1), glyceryl-tri-pentadecanoate-D29 from CDN Isotope (D-5265), monopalmitoyl glycerol from Fluka (#76184), and cholate and deoxycholate from Sigma (#C1254 and #D6750). For LC/MS, formic acid (>95%), water, and methanol (both Chromasolv®Plus for HPLC) were from Sigma-Aldrich.
Cell line construction and maintenance
STC-1 cells were maintained in complete growth medium (DMEM supplemented with 10% FBS) at 37°C in a humidified 5% CO2 atmosphere. The cells were passaged by trypsinization twice a week and reseeded at a ratio of 1:5. To construct a STC-1/Human MGAT2 stable cell line, cDNA of human MGAT2 in the mammalian expression vector, pcDNA3, was transfected into STC-1 cells. The selection medium was prepared by adding 1 mg/ml Geneticin to the complete growth medium. Geneticin-resistant colonies were identified by local trypsinization, expanded, and maintained in selection medium, passaged by trypsinization twice a week and reseeded at a ratio of 1:5.
STC-1/Human MGAT2 cell-based assays
STC1/Human MGAT2 cells were plated at 4 × 104 per well (or otherwise specified density) in 24-well Poly-D-Lysine coated plates (BD # 356414) in complete growth medium supplemented with 1 mg/ml Geneticin. After an overnight culture, cells were washed with PBS and incubated with serum-free DMEM for 1 h. After the incubation, the cells were labeled with serum-free DMEM supplemented with 1.2 mM D31-palmitate, 0.19 mM monopalmitoylglycerol, 0.14 mM cholate, and 0.15 mM deoxycholate with compounds of interest for 90 min. The labeling recipe was designed according to Cheng et al. (10). Cells were washed with PBS, and the samples (in a 96-deep-well plate) were prepared for LC/MS by protein precipitation with cold methanol containing the internal standard glyceryl-tri-pentadecanoate-D29 (1 µg/ml) and butylated hydroxytoluene at 100 μg/ml. The samples were stored frozen at −20°C until LC/MS analysis. On the day of analysis the samples were removed from the freezer, vortexed vigorously for 2 min, and centrifuged at 4,000 RPM for 10 min, and the supernatants (containing the lipids of interest) were robotically transferred to a clean 96-well plate. The supernatants were then dried under heated (∼55°C) nitrogen and reconstituted in 225 µl of methanol. After vigorous vortexing (2 min) the samples were centrifuged at 4,000 RPM for 10 min, and 120 µl of supernatant was transferred to a shallow 96-well PCR plate. The plate was placed into the autosampler (chilled to 5°C), and 10 µl was injected for LC/MS analysis as described below.
LC/MS and data analyses
LC/MS was performed on a ThermoFisher (San Jose, CA) Exactive high-resolution mass spectrometer fitted with an atmospheric pressure chemical ionization (APCI) source and coupled to an Accela μHPLC pump and a Pal CTC autosampler with a DLW wash station. LC was performed on a Waters BEHC8 50 × 2.1 mm, 1.7 µm particle size column using a column temperature of 65°C, a mobile phase consisting of solvent A = 0.1% formic acid in water and solvent B = 0.1% formic acid in methanol, and a flow rate of 0.6 ml/min. The LC gradient used was 80–100% solvent B for 1 min and held at 100% B for 1.5 min. Products were monitored using positive ionization mode. The ion monitored for the product D31-dipalmitin was m/z 582.6985 (M-H2O+H)+. Ions monitored for TAG products of interest were summed and consisted of m/z 855.9653 (containing one D31-palmitate label), 887.4599 (containing two D31-palmitate labels), and 582.6985 (most abundant ion: nonspecific ion deriving from any TAG containing a fatty acyl residue plus a palmitate and D31-palmitate residue). The ion monitored for the internal standard (tri-pentadecanoate-D29) was m/z 581.8351. Data analysis was through Xcalibur® software, and peak areas were obtained using a 7 ppm mass window. Formation of stable isotope-labeled DAG and TAG products was determined qualitatively by calculating the peak area ratio (PAR) of D31-dipalmitin to internal standard (glyceryl-tri-pentadecanoate-D29). Percent inhibition of DAG and TAG synthesis at each concentration of inhibitor was defined as the PAR obtained from the titration of inhibitor/ PAR of the control using the following equation:
where A = minimal Y value (activity level of inhibited sample), B = maximal Y value (activity level of uninhibited sample), C = LogIC50, D = hill slope, and x = concentration of inhibitor.
Results
To demonstrate MGAT2-mediated enzyme activity in a cellular context, murine STC-1 cells were used to construct MGAT2-expressing recombinant cell lines. STC-1 was chosen because it lacks detectable enzymatic and cellular MGAT activities. In addition, it is an intestinal cell line that can survive the exposure to deoxycholate and cholate; these detergents act as lipid carriers that mimic intestinal lumenal lipid substrate delivery. Human MGAT2 cDNA was transfected into STC-1 cells, and quantitative immunoblot analyses were conducted to isolate the lines demonstrating similar MGAT2 expression compared with endogenous enterocytes isolated from mouse small intestinal mucosa (data not shown). The selected cell line was designated STC-1/Human MGAT2 and was used for all subsequent studies.
We first used TLC [as described in Cheng et al. (10)] to measure diacylglycerol synthesis in STC1/Human MGAT2 after introduction of the radioisotope labeled substrate [14C]oleate. Using the STC1/Human MGAT2-TLC assay, MGAT2-driven diacylglycerol synthesis and inhibition by MGAT2 selective inhibitors (supplementary Fig. 1) was successfully demonstrated. However, the labor-intensive nature of TLC combined with the undesirable use of radiolabels led us to pursue an assay better suited to screening inhibitors in a discovery mode.
LC/MS analysis of lipids such as TAG and DAG using various instrument platforms and methods of ionization is well established [for selected reviews describing APCI of DAG/TAGs, see Byrdwell and Cai et al. (11, 12)]. Due to the high sensitivity of LC/MS, a common problem encountered when analyzing lipids in complex matrixes is the extremely high background of endogenous lipid components. Often, changes in a targeted lipid population upon perturbation of the pathways of interest are masked due to high baseline levels of natural lipids. In these cases, the use of stable isotope-labeled lipids as surrogate tracers offers a solution through which subtle changes in a targeted lipid can be monitored that would otherwise be lost in the crowded native lipid population. In our case, D31-palmitate was used as a stable isotope-labeled fatty acid tracer to track MGAT2-driven DAG synthesis. Using high-resolution MS, preliminary experiments were done to determine the most abundant D31-palmitate containing DAG and TAG products formed in the STC1/Human MGAT2 cellular assay. The most abundant stable isotope-labeled DAG and TAG products are shown in Fig. 1A. D31-dipalmitin consisted of one naturally occurring palmitate residue and one D31-palmitate residue (the position of the stable isotope-labeled residue cannot be determined by high-resolution MS). The most abundant TAG consisted of one naturally occurring palmitate residue, one D31-palmitate residue, and one unidentified acyl residue. APCI results in fragmentation of TAG residues such that the major ion observed corresponds to the TAG minus one of its acyl residues. For this reason, the fragmented acyl group cannot be identified. As determined using dipalmitin and tripalmitin purchased standards, the stable isotope-labeled DAG and TAG products are readily distinguishable by chromatographic separation (Fig. 1B) (a representative total ion chromatogram can be found in supplementary Fig. 2).
Fig. 1.
Outline of MGAT2 cell-based reaction scheme using stable isotope-labeled substrate. A: Schematic depicting incorporation of stable isotope-labeled fatty acid (D31-palmitate) into palmitoyl-monoacylglycerol by the STC-1/Human MGAT2 cell line to form stable isotope-labeled products containing DAG and TAG. The ion monitored (see Materials and Methods) for 1 does not distinguish the position of the D31-palmitate label on the glycerol backbone. Similarly, the ions monitored for 2 (see Materials and Methods) could be formed for any TAG containing one palmitate residue, one D31-palmitate residue, and any third acyl residue. B: Extracted ion chromatograms for D31-dipalmitin (extracted mass m/z 582.6985; 5 ppm mass window) and the internal standard tri(pentadecanoate-D29) (extracted mass m/z 581.8351 + 870.2699; 5 ppm mass window). The unidentified peak eluting before D31-dipalmitin was observed in cell-based assay samples generated using both STC-1 mock-transfected and STC-1/Human MGAT2 cell lines. Baseline separation of the unidentified peak from D31-dipalmitin ensured no interference with peak integration.
Figure 2 shows the results of an experiment comparing diacylglycerol synthesis between STC-1/Human MGAT2 and mock-transfected STC-1 cells. Approximately 3 × 104 STC-1/Human MGAT2 cells or mock-transfected STC-1 cells were plated in 24-well Poly-D-Lysine-coated plates. A time course was designed by labeling the cells with D31-palmitate for 30, 60, 90, and 120 min. DAG synthesis was increased in a time-dependent manner in the STC-1/Human MGAT2 cell line (Fig. 2A). In contrast, mock-transfected STC-1 cells failed to exhibit DAG synthesis. To further establish assay linearity, cell number was titrated between 0.016–0.25 million/well in 24-well plates, and cells were incubated with labeled substrate for 90 min. Formation of product was clearly differentiated between STC-1/Human and mock-transfected cells. The optimal signal-to-noise ratio was achieved at a cell density of 0.016–0.125 cells/well (Fig. 2B).
Fig. 2.
Development and optimization of a cell-based assay using the STC-1/Human MGAT2 cell line. A: Time course (0, 30, 60, 90, and 120 min) comparing formation of the product D31-dipalmitin in STC-1/Human MGAT2-transfected cells versus mock-transfected cells. Approximately 3 × 104 cells/well were plated in 24-well plates and labeled with stabled isotope-labeled substrate as described in Materials and Methods. Cells were quenched with methanol containing internal standard at the indicated time points, and the product D31-dipalmitin was measured by LC/MS. The optimal time for assay completion was 90 min. B: Comparison of product (D31-dipalmitin) formation at 90 min using different cell numbers. Titration of cell number was accomplished by plating (in a 24-well plate) 0.016–0.25 million cells per well followed by labeling of the cells with stable isotope-labeled substrate as described in Materials and Methods. Cells were quenched with methanol containing internal standard at the indicated time points, and the product D31-dipalmitin was measured by LC/MS. The optimal cell density was 0.04 million cells per well.
The results shown in Fig. 2 demonstrate that native STC-1 cells do not have endogenous DAG synthesis machinery. A superior signal window of DAG synthesis (as reflected by formation of the stable isotope-labeled DAG surrogate product) was measured in the MGAT2-transfected cells. When cells were plated between 1.6 × 104 and 6.3 × 104 and labeling time was less than 120 min, the cellular assay demonstrated linear product formation. Therefore, in subsequent studies 4 × 104 cells per well and a labeling time of 90 min were used as the standard protocol.
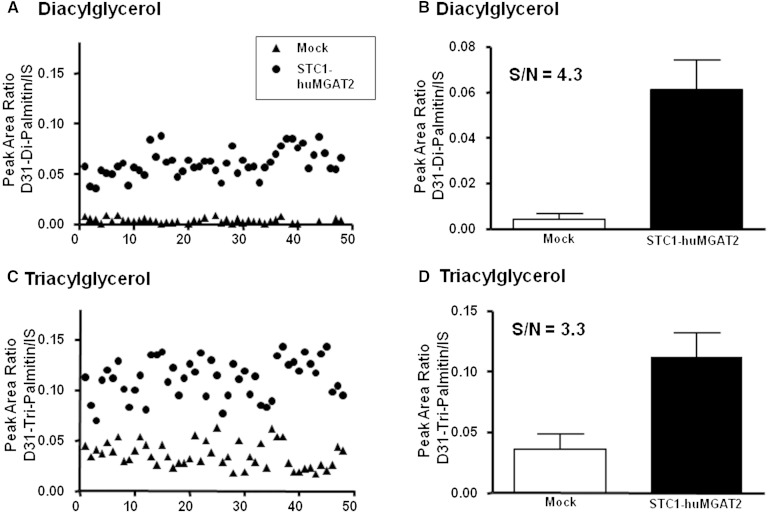
To test the reproducibility and robustness of the cell-based assay, STC-1/Human MGAT2 and mock-transfected cells were each plated in 48 wells at 4 × 104 cells/well. Cells were labeled with stable isotope-labeled substrate for 90 min, and the products D31-dipalmitin (Fig. 3A, B) and D31-tripalmitin (Fig. 3C, D) were measured by LC/MS. Measurement of the stable isotope-labeled DAG product provided a superior signal-to-noise window compared with measurement of stable isotope-labeled TAG products (for D31-DAG product: PAR mock vs. MGAT2 transfected = 0.004 ± 0.002 vs. 0.061 ± 0.013; for D31-TAG product: PAR mock vs. MGAT2 transfected = 0.036 ± 0.01 vs. 0.112 ± 0.02). The signal-to-noise ratios were 4.3 for DAG and 3.3 for TAG. Mock-transfected cells had a much higher background D31-tripalmitin signal, suggesting MGAT2-independent TAG synthesis at the basal level. For this reason, we focused on the analysis of D31-dipalmitin signal to reflect MGAT2-driven DAG synthesis.
Fig. 3.
Reproducibility of cell-based assay. Reproducibility of the cell-based assay was conducted in mock and STC-1/Human MGAT2 cell lines (n = 48). Briefly, cells were plated at a density of 0.04 million cells/well and labeled with stable isotope-labeled substrate as described in Materials and Methods. Cells were quenched at 90 min, and the product D31-dipalmitin was measured by LC/MS. A: Scatter plot of D31-dipalmitin formation versus n in mock versus STC-1/Human MGAT2 cell line. B: Bar chart showing mean (±SD) D31-dipalmitin formation in mock versus STC-1/Human MGAT2 cells. C: Scatter plot of D31-tripalmitin formation versus n in mock versus STC-1/Human MGAT2 cells. D: Bar chart showing mean (±SD) D31-tripalmitin formation in mock versus STC-1/Human MGAT2 cells. Measurement of the stable isotope-labeled DAG product provided a superior signal-to-noise window compared with measurement of stable isotope-labeled TAG products (S/N DAG = 4.3; S/N TAG = 3.3).
Compound A (Fig. 4A) is a potent and selective MGAT2 inhibitor derived from a BMS in-house MGAT2 high-throughput screening campaign and structure activity relationship evolution (manuscript in preparation). The IC50 values of Compound A against recombinant human, rat, and mouse MGAT2 were 4.0 ± 2.9 nM, 4.0 ± 3.4 nM, and 23 ± 17 nM, respectively. The IC50 values against other membrane lipid acyltransferases MGAT3, acyl-CoA wax alcohol acyltransferase 2, and DGAT1 were 14 ± 3.8 μM, 6.5 ± 3.3 μM, and 6.3 ± 0.5 μM, respectively, indicating that Compound A is highly selective. We applied Compound A to STC-1/Human MGAT2 cells and characterized its inhibitory activity using both the radiolabeled TLC protocol and the stable isotope-labeled LC/MS protocol. Fig. 4B shows a representative inhibitory curve using the TLC method. The IC50 values were 12.4 ± 7.7 nM (n = 4). Fig. 4C shows a representative inhibitory curve using the LC/MS assay. The IC50 values were 2.3 ± 1.2 nM (n = 6). Due to the labor-intensive nature of the TLC assays, a limit of nine point titrations were routinely used for IC50 determinations. However, because of the throughput improvement of the LC/MS assay, we were able to routinely implement a 12 point titration. As a result, the assay variation was considerably smaller using the LC/MS assay.
Fig. 4.
Comparison of TLC and LC/MS IC50 curves using a representative compound. A: Structure of Compound A. B: Representative IC50 curve generated using TLC assay (for details, see Materials and Methods). C: Representative IC50 curve generated using cell-based LC/MS assay (for details, see Materials and Methods).
Table 1 summarizes the results from a series of structurally diverse acyltransferase inhibitors that were assessed in the STC-1/Human MGAT2 cell-based assay and in the in vitro enzyme assay. XP-620 is a potent and selective DGAT1 inhibitor (13). When XP-620 was tested using monoacylglycerol as a substrate in an in vitro assay not described here, the IC50 against human DGAT1 was∼16 nM. However, the IC50 values were >30,000 nM for human MGAT2, MGAT3, and DGAT2 (10). As expected, XP-620 had no inhibitory activity on DAG synthesis, further confirming the specificity of the STC-1/Human MGAT2 cell-based assay. The lack of inhibitory activity by XP-620 is not due to a lack of cell penetrance because XP-620 was shown to be highly potent for inhibiting the synthesis of TAG in mouse primary enterocytes (Ching-Hsuen Chu, Dong Cheng, unpublished results).
TABLE 1.
Comparison of IC50 values of MGAT2 inhibitors obtained using the cell-based assay and the in vitro enzyme assay
| Compound | IC50 (nM) | Fold Difference Cell-Based Assay/In Vitro Assay | |
| Cell-Based Assay | In Vitro Enzyme Assay | ||
| XP-620 | >10,000 | >10,000 | |
| Compound B | 0.6 | 0.9 | 0.7 |
| Compound C | 1.5 | 1.9 | 0.8 |
| Compound D | 235 | 179 | 1.3 |
| Compound E | 99 | 45 | 2.2 |
| Compound F | 112 | 28 | 4.0 |
| Compound G | 33 | 3.7 | 8.9 |
| Compound H | 624 | 19 | 32.8 |
| Compound I | 166 | 4.9 | 33.9 |
| Compound J | 1,646 | 43 | 38.3 |
Compounds B–J are representative structurally diverse MGAT2 inhibitors that cover a range of potencies in the in vitro assays. The rank order of potencies obtained from the STC-1/Human MGAT2 cell-based assay compared with the in vitro enzyme assay generally correlated well (Compounds B–G). In contrast, compounds H–J were ∼30-fold more potent in the in vitro enzyme assay compared with the cell-based assay. This discrepancy suggests that some compounds, although potent in an in vitro scenario, lack cell penetration or do not perform as an MGAT2 inhibitor in an intracellular environment. Compounds whose cellular potencies track well with in vitro enzyme assays would be of high interest for further in vivo physiological investigation.
Conclusions
We implemented a high-resolution LC/MS platform to monitor DAG synthesis in a recombinant STC-1/Human MGAT2 cell line. Monitoring of the incorporation of stable isotope-labeled D31-palmitate into DAG allowed us to selectively trace cellular DAG synthesis activity. This assay format dramatically reduced background interference and increased the sensitivity and the signal window as compared with the traditional TLC method.
At least three lines of evidence indicate that this assay specifically monitors MGAT2-driven DAG synthesis. 1) Stable isotope-labeled product signal levels produced in the linear range of the STC-1/Human MGAT2 cell line were 4-fold greater compared with signal produced in mock-transfected STC-1 cells; 2) selective DGAT1 inhibitors that do not cross react with MGAT2 in in vitro enzyme assays, such as XP-620, also do not inhibit DAG synthesis in the STC-1/Human MGAT2 cell line; and 3) the rank order of potencies for a diverse set of MGAT2 inhibitors tracks well between the cell-based assay and the in vitro MGAT2 assay.
One caveat of a cell-based assay is that IC50 values could reflect inhibition of substrate uptake by the cells as opposed to inhibition of intracellular enzyme activity. However, if this were the case, the cellular IC50 values would be expected to appear more potent than in vitro values. This is not the case in compounds evaluated in Table 1, suggesting that cellular uptake was not affected. Further, representative compounds were evaluated using the cellular MGAT2-TLC assay. As shown in supplementary Fig. 1, in contrast to the TAG and DAG bands, the intensity of the FFA band was not altered by MGAT2 inhibitors, further supporting the notion that cellular uptake was not affected.
The use of stable isotope-labeled substrate coupled with LC/MS is easily adaptable to the study of fatty acid metabolism in other cellular model systems, such as intestinal Caco-2 cell lines. In such model systems, however, we could not discern if the MGAT and/or DGAT activity was due to MGAT2, MGAT3, or DGAT1 because the expression of all these enzymes is upregulated during the differentiation of Caco-2 (Ching-Hsuen Chu, Dong Cheng, unpublished results). This was further evidence that creation of an MGAT2-expressing cell line was necessary to ensure that MGAT activity was strictly derived from transfected MGAT2.
The described MGAT2 cell-based assay represents a novel technique for the assessment of MGAT2 inhibitors that takes into account molecule cell penetrance and intracellular behavior. It may prove a useful tool for bridging in vitro enzyme assays and in vivo efficacy assessment in the development of therapeutic MGAT2 inhibitors for the treatment of metabolic disorders such as obesity and type 2 diabetes.
Supplementary Material
Acknowledgments
The authors thank Petia Shipkova, Jean Whaley, Mary Ann Pelleymounter, Jeff Robl, and Adrienne Tymiak for their support and Pratik Devasthale for critical review of the manuscript.
Footnotes
Abbreviations:
- APCI
- atmospheric pressure chemical ionization
- DAG
- diacylglycerol
- DGAT
- diacylglycerol acyltransferase
- MGAT
- monoacylglycerol acyltransferase
- PAR
- peak area ratio
- SCT
- secretin tumor cell
- TAG
- triacylglycerol
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures.
REFERENCES
- 1.Yen C. L., Cheong M. L., Grueter C., Zhou P., Moriwaki J., Wong J. S., Hubbard B., Marmor S., Farese R. V., Jr. 2009. Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat. Med. 15: 442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yen C. L., Farese R. V., Jr. 2003. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 278: 18532–18537. [DOI] [PubMed] [Google Scholar]
- 3.Cao J., Lockwood J., Burn P., Shi Y. 2003. Cloning and functional characterization of a mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J. Biol. Chem. 278: 13860–13866. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y., Cheng D. 2009. Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism. Am. J. Physiol. Endocrinol. Metab. 297: E10–E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez M. C., Gonzalez-Garcia M. R., Liu B., Pfeifer L. A. 2013. Phenyl methanesulfonamide derivatives useful as MGAT - 2 inhibitors. Eli Lilly and Company, Indianapolis, IN.
- 6.Huji T., Hangeland J., Lawrence R. M., Cheng D., Ahmad S., Meng W., Brigance P. R., Devasthale P. 2013. Aryl dihydropyridinone and piperidinone as MGAT2 inhibitors. Bristol-Myers Squibb Company, Princeton, NJ.
- 7.Scott J. S., Berry D. J., Brown H. S., Buckett L., Clarke D. S., Goldberg K., Hudson J. A., Leach A. G., MacFaul P. A., Raubo P., Robb G. 2013. Achieving improved permeability by hydrogen bond donor modulation in a series of MGAT2 inhibitors. Med. Chem. Commun. 4: 1305–1311. [Google Scholar]
- 8.Barlind J. G., Buckett L. K., Crosby S. G., Davidsson O., Emtenas H., Ertan A., Jurva U., Lemurell M., Gutierrez P. M., Nilsson K., et al. 2013. Identification and design of a novel series of MGAT2 inhibitors. Bioorg. Med. Chem. Lett. 23: 2721–2726. [DOI] [PubMed] [Google Scholar]
- 9.Grant S. G., Seidman I., Hanahan D., Bautch V. L. 1991. Early invasiveness characterizes metastatic carcinoid tumors in transgenic mice. Cancer Res. 51: 4917–4923. [PubMed] [Google Scholar]
- 10.Cheng D., Iqbal J., Devenny J., Chu C. H., Chen L., Dong J., Seethala R., Keim W. J., Azzara A. V., Lawrence R. M., et al. 2008. Acylation of acylglycerols by acyl coenzyme A:diacylglycerol acyltransferase 1 (DGAT1). Functional importance of DGAT1 in the intestinal fat absorption. J. Biol. Chem. 283: 29802–29811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrdwell W. C. 2001. Atmospheric pressure chemical ionization mass spectrometry for analysis of lipids. Lipids. 36: 327–346. [DOI] [PubMed] [Google Scholar]
- 12.Cai S. S., Syage J. A. 2006. Comparison of atmospheric pressure photoionization, atmospheric pressure chemical ionization, and electrospray ionization mass spectrometry for analysis of lipids. Anal. Chem. 78: 1191–1199. [DOI] [PubMed] [Google Scholar]
- 13.Orland M. D., Anwar K., Cromley D., Chu C. H., Chen L., Billheimer J. T., Hussain M. M., Cheng D. 2005. Acyl coenzyme A dependent retinol esterification by acyl coenzyme A: diacylglycerol acyltransferase 1. Biochim. Biophys. Acta. 1737: 76–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.