Abstract
Introduction
Electrochemotherapy (ECT) is a new therapeutic method that is used in oncology as palliative treatment in patients with recurrent head and neck tumors and who are not candidates for standard therapeutic options. The aim of our study was to evaluate the cytoreductive effect of ECT in patients subjected to chemoradiotherapy for squamous cell carcinoma of the oral cavity. The primary endpoint of the study was to verify tumor debulking after ECT treatment as neoadjuvant, before conventional chemoradiotherapy. The secondary endpoint was to assess the safety and tolerability of ECT treatment.
Materials and methods
This experimental study was conducted at the Division of Otolaryngology, University of Catanzaro, Italy. From February 2013 to February 2014, four patients were enrolled, two males and two females, with a mean age of 56 years (range: 47–65 years), and with squamous cell carcinoma of the oral cavity in advanced stages of disease (T3–T4). All patients, with their informed consent, received ECT treatment in accordance with the Standard Operating Procedures defined in the European Standard Operating Procedures on Electrochemotherapy (ESOPE) study, followed by conventional chemoradiotherapy. Their response to ECT treatment was assessed after 30 days. For each patient, the following parameters were evaluated with the appropriate forms: local tumor control, control of pain (analgesia postsurgery scale [APS]), and quality of life (Short Form [36] Health Survey [SF-36]; v1).
Results
Three of four patients (75%) showed a partial response, whereas in one patient (25%), the disease remained stable. The treatment was well-tolerated by all patients, according to the APS and SF-36 results.
Conclusion
Although the study was conducted on a small number of cases, data from this study show that ECT represents a safe and effective treatment in terms of tumor cytoreduction and locoregional control of the disease. It also allows good control of postoperative pain and short hospitalization.
Keywords: neoadjuvant treatment, squamous cell carcinoma, oral cavity
Introduction
Research of new therapies to treat or oppose the growth of tumors has been studied in recent years in various academic fields. The idea of combining engineering technologies with medical and biological knowledge is the basis for the development of a new cancer treatment called electrochemotherapy (ECT).
ECT is a new therapeutic method that is used in oncology to facilitate the admission into the tumor cell of poorly permeant drugs that have high intrinsic toxicity. Through this method, the efficacy of chemotherapeutic drugs increases by the use of high-voltage electrical pulses, which encourage the phenomenon of electroporation (EP).1
Membrane EP is performed by applying pulses of appropriate frequency and intensity to biological tissue through a specific interface; the electrodes. The voltage and current applied to the tumor depend on the type and electrical characteristics of the biological tissue, such as electrical conductivity and permittivity. EP results in reversible permeabilization of cell membranes, which allows the penetration of chemotherapy into tumor cells, maximizing its effectiveness.2
The anticancer drugs most widely used with this method are bleomycin and cisplatin: preclinical studies have shown that antitumor activity increased by 8,000 times for bleomycin and by 80 times for cisplatin. This implies two important advantages: first, it is possible to achieve a significant antitumor effect with a minimum dose; and second, there is a strong reduction of systemic side effects.
Operating procedures have been developed and validated by the European Standard Operating Procedures for Electrochemotherapy (ESOPE) study, which described with precision the dosage, timing, and value of the electric field and the evaluation of the treatment response for various diseases.3 The ESOPE trial described the use of the electrical pulse generator Cliniporator™ produced by IGEA Ltd (Carpi, Modena, Italy).4
To date, ECT is used for the treatment of melanomas, sarcomas, and other types of skin cancer and subcutaneous malignant tumors.2,3 Regarding the head and neck, this procedure is indicated in locally advanced primary tumors that are not candidates for standard therapy, cutaneous metastases, local recurrence, and metastases, and secondary tumors of the anterior region of the oral cavity (tongue, lips, and palate).4–6
The objective of this study was to evaluate the neoadjuvant effect of ECT in patients with squamous cell carcinoma of the oral cavity in advanced stages and who are not candidates for surgery or have rejected it as highly demolitive, causing esthetic and functional deficits. The primary endpoint of the study was to verify tumor debulking after ECT treatment as neoadjuvant, before conventional chemoradiotherapy. The secondary endpoint was to assess the safety and tolerability of ECT treatment.
Materials and methods
Patient selection
This prospective study was conducted from February 2013 to February 2014 among patients with squamous cell carcinoma of the oral cavity in advanced stages.
The selection of patients was performed according to the following indications: life expectancy of more than 3 months, lesion size suitable for the application of electrical pulses, no treatment in the preceding 2 weeks, good performance status (Karnofsky index >70%), and appropriate renal function and blood indices.
The criteria for exclusion from treatment were: presence of progressive visceral disease, presence of known allergy or sensitivity to bleomycin or cisplatin, achievement of the cumulative dose of bleomycin due to previous therapies, presence of severe peripheral neuropathies, blood profile outside of the normal range, presence of previous renal disease, presence of cardiac arrhythmias or pacemaker, presence of epilepsy, and patients who are pregnant or breastfeeding.
Eight patients who had locally advanced primary disease of the oral cavity (T3–T4) were recruited at the Division of Otolaryngology, Magna Graecia University of Catanzaro, Italy. Four of the eight patients (cT3N1 [clinical TNM Classification of Malignant Tumours staging: tumor with maximum size >3 cm; metastasis in a single ipsilateral lymph node maximum size ≤3 cm]) underwent surgical excision of the tumor and selective laterocervical lymph node dissection because of the exclusion criteria. The other four patients, two males and two females, aged 47–65 years (median: 56 years) were within the inclusion criteria of the study and were treated with ECT and functional laterocervical lymph node dissection because they were not candidates for radical surgery (cT3-4N2) or had refused it because of demolitive esthetic and functional outcomes.
Before ECT treatment, all patients were subjected to otolaryngology (ear, nose, and throat) examination and oncologic and radiologic evaluation with computed tomography/magnetic resonance imaging (CT/MRI) to measure the maximum diameter of the lesion to be treated and to complete the staging process.
The patients completed the Short Form (36) Health Survey (SF-36) questionnaire (v1) before and after ECT treatment.7 All lesions were documented by photos and videos for evaluation of the treatment results in terms of reduction in the local extension of the disease.
The study was performed with the approval of the Institutional Review Board of Magna Graecia University of Catanzaro, Italy. All patients gave their informed consent to participate in the study.
Treatment
ECT was performed as local treatment which, via cell membrane permeabilizing electric pulses, potentiated the cytotoxicity of the anticancer drug at the site of the electric pulse application. EP transiently permeabilized tumor cell membranes, thus enabling diffusion of the anticancer drug into the cells and increasing its cytotoxicity. According to existing studies, in this study, the anticancer drug used was bleomycin at individualized dosage, in the following manner.2
The ECT treatment was performed under general anesthesia to reduce the pain and muscle spasms induced by the technique. Immediately after anesthesia induction, all patients received an intravenous bolus of 15,000 IU/m2 of bleomycin. Eight minutes after the infusion, the electrical impulses generated by the Cliniporator™ were sent to the tumoral lesions with the use of various types of electrodes (flat, hexagonal, or finger), which were chosen according to the site, volume, and shape of the lesions to be treated. The pulses were completed within 30 minutes after injection of the chemotherapeutic drug.
Evaluation of treatment response
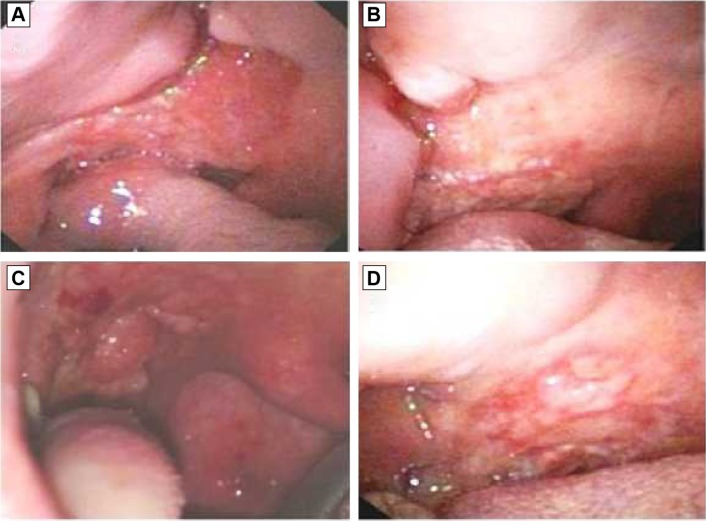
The evaluation of the responses was done after 4 weeks. All patients were clinically observed and the observations documented with photos (Figure 1). The patients were classified based on their treatment response according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria.8
Figure 1.
Cytoreductive effect observed in two patients before (A and C) and after 30 days (B and D) of ECT.
Abbreviation: ECT, electrochemotherapy.
The occurrence of complications, such as bleeding, edema of the lesions and surrounding tissues, and impaired swallowing and respiratory function, was also considered. Postoperative pain was assessed in all patients by using the analgesia post-surgery (APS) card, which included both the visual analog scale (VAS) and the verbal rating scale (VRS).9
The VAS allows the patient to mark the intensity of pain along a 100 mm line, indicating ‘no pain’ as 0 to ‘severe pain’ as 100. The VRS defines the intensity of pain as absent, mild, moderate, severe, and very severe, corresponding to values ranging from 0 to 5, respectively. Both analgesic pain scales were assessed at rest and in motion before surgery, after surgery, and then at regular intervals until 48 hours postsurgery.10
All patients completed the SF-36 questionnaire, which is a generic questionnaire on quality of life that is not specific to disease, age, or therapy. It is used to assess the subjective perception of health concepts related to activity levels and well-being. The questionnaire is articulated through 36 questions that assemble eight different scales. The 36 questions inquire about eight domains of health: physical activity (FA, ten questions); role limitations due to physical health (RP, four questions); role limitations due to emotional problems (RE, three questions); physical pain (BP, two questions); perception of general health (GH, five questions); vitality (VT, four questions); social activities (SF, two questions); and mental health (MH, five questions); it also includes one question about change in health status. All SF-36 questions, except one, refer to the 4 weeks prior to completing the questionnaire. High scores indicate good well-being; low scores indicate the opposite.7 After 4 weeks, all patients were subjected to chemoradiotherapy.
Results
For each of the four patients included in the study, clinical-anamnestic data were collected regarding the location of the primary tumor, the clinical staging by the American Joint Committee on Cancer (AJCC) tumor staging system, and the pathologic and histopathologic staging. Regarding tumor site, one patient had a tumor of the tongue, two patients had tonsillar cancer, and one patient had a tumor of the oral cavity floor. The T staging classified three patients as T3 and one patient as T4; the pathologic grading classified two tumors as mildly differentiated and two as poorly differentiated. The pathologic examination recognized lymph node metastases in three of the four patients (75%). Furthermore, data on alcohol-drinking habits were registered, with 12 g of alcohol considered as a standard drink (one beer, 330 mL; one glass of wine, 125 mL; and one shot of whiskey, 40 mL). The subjects were then divided into those who consumed more than two drinks per day (50%) and those who did not consume alcohol or who consumed less than two drinks per day (50%). Regarding smoking habits, we divided the subjects according to the number of cigarettes smoked in 24 hours. Those who smoked >20 cigarettes per day were considered as heavy smokers, those who smoked ≤20 cigarettes per day as moderate smokers, and those who had never smoked or had stopped for more than 10 years as nonsmokers. Of the four patients, one was a heavy smoker, two were moderate smokers, and one had never smoked. Patient demographic and clinical-pathological details are summarized in Table 1.
Table 1.
Demographic data and risk factors and clinical features for cases
| Parameters | Patients N (%) |
|---|---|
| Sex | |
| Male | 2 (50) |
| Female | 2 (50) |
| Smoking | |
| Heavy (>20 cigarettes/day) | 1 (25) |
| Moderate (≤20 cigarettes/day) | 2 (50) |
| Never or ex-smokers (>10 years) | 1 (25) |
| Drinking | |
| >2 drink/day | 2 (50) |
| ≤2 drink/day | 2 (50) |
| Site | |
| Tongue | 1 (25) |
| Tonsil | 2 (50) |
| Oral cavity floor | 1 (25) |
| Pathological grading | |
| Well-differentiated | 0 (0) |
| Mildly differentiated | 2 (50) |
| Poorly differentiated | 2 (50) |
| Tumor size | |
| T1 | 0 (0) |
| T2 | 0 (0) |
| T3 | 3 (75) |
| T4 | 1 (25) |
| Lymph nodes status | |
| pN0 | 1 (25) |
| pN+ | 3 (75) |
| Clinical stage | |
| Early (I–II) | 0 (0) |
| Advanced (III–IV) | 4 (100) |
| Total | 4 (100) |
The overall results of the treatment performed on the four patients in the study showed good subjective tolerance in all cases (100%). None of the four patients had postoperative bleeding or swelling of the treated area that could cause difficulty in breathing. Specifically, as a precaution, the first patient in the series was subjected to an elective tracheotomy before ECT application because the tumor site (base of the tongue/mouth floor) resulted in possible dangerous obstructive complications. The remaining three patients did not undergo tracheotomy because small tissue and perilesional edemas were observed during the intra- and postoperative periods. Regarding toxicity, no side effects related to bleomycin (used in low doses) were observed during the study, and no serious adverse events associated with ECT were reported.
Dysphagia or difficulty in oral feeding were not observed postoperatively in any of the cases, although a nasogastric tube was placed in all patients as a preventive measure; all tubes were removed immediately during the postoperative period.
Generally, the intensity of pain reported was higher in the immediate postoperative period for all patients, according to the VAS and VRS. Based on the VAS, the level of pain intensity was greater at 2 hours after ECT, with a VAS-rest score of 70 mm and a VAS-functional score of 76 mm (range: 0–100 mm). The level decreased dramatically after 18 hours and during the second day of hospitalization, with a VAS-rest score of 4 mm and VAS-functional score of 6 mm at 2 hours after ECT. The VRS score showed the same trend: a VRS-rest score of 2 and VRS-functional score of 4.4 (range: 0–5) at 2 hours after ECT, and a VRS-rest score of 1 and VRS-functional score of 1.5 at 18 hours after ECT and during the second day of hospitalization (Figures 2 and 3).9,10
Figure 2.
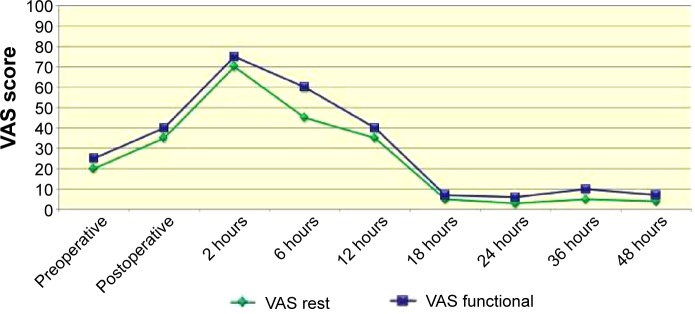
VAS score.
Abbreviation: VAS, visual analog scale.
Figure 3.
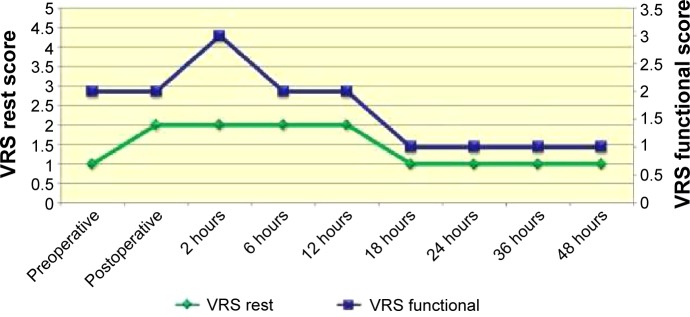
VRS score.
Abbreviation: VRS, verbal rating scale.
Referred pain was limited mainly to the treated tumor area and surrounding tissue. According to these data, the level of pain control during ECT was low and acceptable to all patients.
Probably the best indicator that ECT is a procedure not too stressful or painful is the fact that patients treated with ECT would accept being subjected to a new ECT treatment, if indicated.
After ECT, a partial response (PR) was achieved in three patients (75%), whereas the disease remained stable in one patient (25%) according to the RECIST criteria.study was 60±10 days; follow-up is currently in progress. The antitumor effects of ECT were evaluated as tumor volume reduction at 4 weeks after the treatment; this was evident in three tumor lesions (Figures 1A–D).
The results of the SF-36 questionnaire are shown in Figure 4. In areas related to emotional (RE) and social activities (SF), the subjective perceptions of the four patients on health concepts related to well-being levels were almost comparable during the 4 weeks before ECT and in the month after the treatment. Their subjective perceptions of well-being levels decreased slightly in areas related to general health (GH), physical activity (FA), and vitality (VT). In areas regarding the status of organic health, the subjective perceptions of health of the four patients were lower after ECT than in the month before the treatment (role limitations due to physical health [RP] and physical pain [BP]), especially in the area concerning mental health (likely due to increased emotional awareness of the state of disease).
Figure 4.
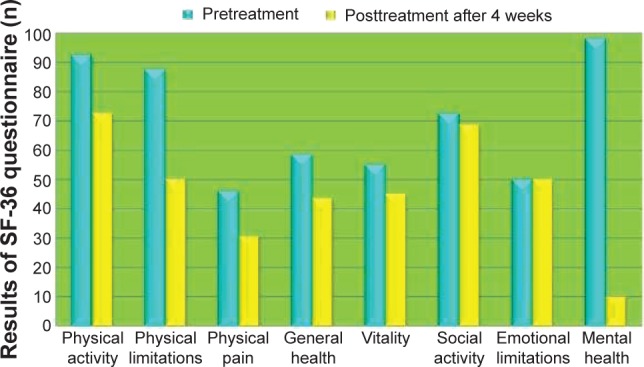
Results of the generic quality of life questionnaire (SF-36). Abbreviation: SF-36, Short Form (36) Health Survey.
Discussion
The treatment of head and neck cancer that has spread or is relapsing can be difficult for the surgeon and debilitating for patients, especially when important anatomic structures are involved.5 The surgical treatment of lesions can be disfiguring for patients and can result in functional impairment. For these reasons, therapies that maintain organ functionality combined with effective local tumor control are of great interest.
In recent years, ECT has been proposed as a new therapeutic method for the control of neoplastic subcutaneous or mucosal lesions of the skin.6 The literature contains few data about the efficacy of this procedure in the treatment of head and neck cancer, and the objective response rates seem promising, ranging from 56% to 100%.11,12 Our data seem to be comparable with those reported in the literature; in fact, we found an objective response rate of 75%.
Regarding the methodology, the electrical pulses generated by the device used can be emitted locally by using electrodes of different shapes and sizes. The choice of electrode depends on the volume and site of the lesions to be treated. To achieve an optimal response, ECT must be applied when the chemotherapeutic agent is present in the tissues at an appropriate concentration. Many authors have shown that the time window for applying ECT in head and neck tumors is 8–28 minutes after administration of bleomycin (15,000 IU/m2). The chemotherapeutic drugs may be given in three different ways: intravenous, intra-arterial, and intratumoral. Intratumoral injection is the best way for poorly vascularized lesions; in the case of multiple tumors or extensive lesions, intravenous drug administration is more convenient.3
In the literature, the efficacy of ECT has been reported to be influenced only by the size of the tumor and not by the histologic nature of the lesions.3,13
ECT is still considered as palliative treatment that should be reserved for patients in advanced stages of disease; however, some authors have emphasized the role of ECT not only as palliative treatment but as a curative and neoadjuvant therapy, especially in the case of limited lesions.14 Many potential benefits are associated with the use of ECT, including tissue preservation, short hospitalization, repeatability, and low cost.15
We chose to treat our patients under general anesthesia, as have other authors, because the muscle spasms induced by the electrical impulses can be very painful and psychologically traumatic if the patients are not pharmacologically paralyzed.16 However, the technique needs to undergo improvements, in particular regarding the technical devices used. In fact, the areas actually treated are limited. The depth and manageability of the electrodes need to be improved because the areas treated include crevices in anatomic regions. Most of the cases reported in the literature concern injuries that are easily treatable with currently available devices.17 The requirements for oral, pharyngeal, or intranasal localization necessitate studying other forms of electrodes, in particular for endoscopic application.
Conclusion
ECT offers considerable advantages: in terms of time of hospitalization, the treatment can be completed within 30 minutes, can be performed at a day hospital, and does not require long-term hospitalization. It is also possible to repeat several ECT cycles without precluding other types of treatment.
The limitation of this technique comes from the fact that it is not possible to treat all types of tumors: thus far, the electrodes can reach only the subcutaneous layer or, at most, by means of long needles, connective tissue and muscle.
The primary endpoint of the study was to verify tumor debulking after ECT treatment as neoadjuvant, before conventional chemoradiotherapy. Our study of ECT as neoadjuvant treatment has given promising results, although for a small group of patients with oral carcinoma in the advanced stages; the cytoreductive effect of ECT (evaluated as tumor volume reduction at 4 weeks after treatment) was evident in three of the four tumor lesions.
The secondary endpoint was to assess the safety and tolerability of ECT treatment. The overall results of the treatment performed on the four patients in the study showed good subjective tolerance in all cases (100%) without important adverse effects.
Obviously, the use of ECT as neoadjuvant, adjuvant, or palliative treatment in patients with head and neck tumors needs further study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sersa G, Miklavcic D, Cemazar M, Rudolf Z, Pucihar G, Snoj M. Electrochemotherapy in treatment of tumours. Eur J Surg Oncol. 2008;34(2):232–240. doi: 10.1016/j.ejso.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Mir LM. Bases and rationale of the electrochemotherapy. Eur J Cancer. 2006;4(11):38–44. [Google Scholar]
- 3.Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy – an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer. 2006;4(11):3–13. [Google Scholar]
- 4.Fumanti C, Berardi A, Bertino G, et al. Electrochemotherapy (ECT) in head and neck tumors: an alternative to conventional treatments in advanced. Bull Med Surg Soc Pavia. 2011;124(2):245–248. [Google Scholar]
- 5.Landström FJ, Nilsson CO, Crafoord S, Reizenstein JA, Adamsson GB, Löfgren LA. Electroporation therapy of skin cancer in the head and neck area. Dermatol Surg. 2010;36(8):1245–1250. doi: 10.1111/j.1524-4725.2010.01617.x. [DOI] [PubMed] [Google Scholar]
- 6.Gargiulo M, Moio M, Monda G, Parascandolo S, Cubicciotti G. Electrochemotherapy: actual considerations and clinical experience in head and neck cancers. Ann Surg. 2010;251(4):773. doi: 10.1097/SLA.0b013e3181d64b81. [DOI] [PubMed] [Google Scholar]
- 7.Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998;51(11):1025–1036. doi: 10.1016/s0895-4356(98)00094-8. [DOI] [PubMed] [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–2404. doi: 10.1016/j.pain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP) Arthritis Care Res. 2011;63(Suppl 11):S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 11.Bloom DC, Goldfarb PM. The role of intratumour therapy with electroporation and bleomycin in the management of advanced squamous cell carcinoma of the head and neck. Eur J Surg Oncol. 2005;31(9):1029–1035. doi: 10.1016/j.ejso.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Burian M, Formanek M, Regele H. Electroporation therapy in head and neck cancer. Acta Otolaryngol. 2003;123(2):264–268. doi: 10.1080/00016480310001114. [DOI] [PubMed] [Google Scholar]
- 13.Panje WR, Hier MP, Garman GR, Harrell E, Goldman A, Bloch I. Electroporation therapy of head and neck cancer. Ann Otol Rhinol Laryngol. 1998;107(9 Pt 1):779–785. doi: 10.1177/000348949810700908. [DOI] [PubMed] [Google Scholar]
- 14.Sersa G. The state-of-the-art of electrochemotherapy before the ESOPE study; advantages and clinical uses. Eur J Cancer. 2006;4(11):52–59. [Google Scholar]
- 15.Colombo GL, Matteo SD, Mir LM. Cost-effectiveness analysis of electrochemotherapy with the Cliniporator™ vs other methods for the control and treatment of cutaneous and subcutaneous tumors. Ther Clin Risk Manag. 2008;4(2):541–554. doi: 10.2147/tcrm.s2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mevio N, Bertini G, Occhini A, et al. Electrochemotherapy for the treatment of recurrent head and neck cancers: preliminary results. Tumori. 2012;98(3):308–313. doi: 10.1177/030089161209800305. [DOI] [PubMed] [Google Scholar]
- 17.Testori A, Tosti G, Martinoli C, et al. Electrochemotherapy for cutaneous and subcutaneous tumor lesions: a novel therapeutic approach. Dermatol Ther. 2010;23(6):651–661. doi: 10.1111/j.1529-8019.2010.01370.x. [DOI] [PubMed] [Google Scholar]