Abstract
Objective: Previous studies suggest that the unexplained sudden and severe onset of obsessive-compulsive disorder (OCD) and/or tics may be infection or immune precipitated. Beta lactam antibiotics may be neuroprotective beyond their antimicrobial efficacy. We examine the preliminary safety and efficacy of cefdinir in reducing obsessive-compulsive and/or tic severity in children with new-onset symptoms.
Method: Twenty subjects were randomized to receive placebo or cefdinir for 30 days for the treatment of recent-onset OCD and/or tics. The placebo group received a comparable inactive treatment matched for taste, color, and consistency. The Children's Yale-Brown Obsessive-Compulsive Scale (CY-BOCS) and Yale Global Tic Severity Scale (YGTSS) were the primary outcome measures utilized.
Results: Subjects receiving cefdinir saw notable improvements in tic symptoms, with 44.4% showing at least a 25% reduction in YGTSS (mean decrease=9.5) scores compared with 9.1% of the placebo group (mean decrease=0.13). Despite improvements, significant group differences were not observed for YGTSS (F [1, 13]=4.03, p=0.066) although there were moderate differences between group treatment effects (d=0.72). For OCD symptoms, subjects receiving cefdinir saw improvements in OCD symptoms, with 33.3% showing at least a 25% reduction in CY-BOCS scores (mean decrease=7.8) compared with 27.3% of the placebo group (mean decrease=4.7), but there were also no significant differences for CY-BOCS (F [1, 13]=0.385, p=0.546; d=0.24).
Conclusions: Subjects assigned to cefdinir exhibited notable, albeit nonstatistically significant, improvements in tic symptoms, compared with the placebo group. There were also some improvements in OCD symptoms, although these were not significant. Overall, cefdinir was well tolerated. Given these preliminary results, a fully powered study is warranted to explore the efficacy of cefdinir as a therapeutic tool for new-onset pediatric neuropsychiatric symptoms, particularly those that appear to be precipitated by infection.
Introduction
Characterized by intrusive thoughts or impulses (obsessions), and repetitive behaviors or compulsions aimed at reducing distress, obsessive-compulsive disorder (OCD) often presents during childhood and early adolescence, with significant neuropsychiatric and neurological comorbidity. The etiology of OCD, like most neuropsychiatric disorders, remains largely unknown, although genetic predisposition and neurotransmitter dysfunction have been among the most prominent theories, with evidence of a familial association as well as abnormalities in serotonin, dopamine, and glutamate systems (Adams et al. 2005; Lazar et al. 2008; Pittenger et al. 2011).
Tic disorders, which also present during childhood with a predominantly prepubertal onset, have a particularly strong co-occurrence with OCD, with a reported 50% rate of comorbidity among those with the disorder (Bloch et al. 2006). Classified based on type (verbal and/or motor) and duration, tic disorders can range from provisional to chronic to Tourette's disorder. In patients with comorbid OCD/tics, OCD symptom onset often occurs when tics are most severe, and often present with distinct features, including greater rates of symmetry obsessions; and counting, repeating, ordering, and arranging compulsions compared with OCD, which presents without comorbid tic symptoms (Leckman et al. 1994). Tic disorders often follow an intermittent course, with periods of symptom exacerbations/flares, or increased intensity and frequency of tics, followed by periods of symptom waning. The presentation or onset of symptoms in OCD and tic disorders, as well as their course, can vary significantly among patients. The inciting “trigger” may also significantly impact symptom onset and course characteristics. In some patients, the onset of symptoms can be “acute,” signifying a dramatic and abrupt onset of symptoms reaching a level of significant impairment within 24–48 hours, whereas in others the onset is insidious. The etiology of tic disorders, like OCD, is unclear and surrounded by significant debate, although genetics, neurochemical abnormalities, and basal ganglia dysfunction appear to be the most likely mechanisms (Jijun et al. 2010; Lerner et al. 2012; Moya et al. 2013).
Recently, the role of infection and resulting immunological disruptions has been implicated in the pathology of tic disorders and OCD. This theory has been strengthened by findings from animal models and clinical studies suggesting that early life immune challenges can lead to later neuropathology. In schizophrenia, for example, perinatal insults resulting in immune activation have been linked to the occurrence of the disorder, particularly the neuropathological, behavioral, and immune abnormalities observed in these patients (Patterson 2009; Winter et al. 2009; Brown 2012).
Perhaps most convincing, however, have been observations made in Sydenham's chorea, a neurological disorder that occurs in a subset of individuals with rheumatic fever (RF), which presents with a high rate of OCD comorbidity (Swedo et al. 1989). Patients with Sydenham's chorea experience involuntary movements and behavioral changes thought to be the result of streptococcal-induced autoimmune reaction targeted at the basal ganglia (Bronze and Dale 1993; Kirvan et al. 2006). Similarly, a subset of children were observed to present with tics following acute infection (Kiessling et al. 1993). Originally described in a group of 50 patients by Swedo et al., and termed “pediatric autoimmune neuropsychiatric disorders associated with streptococcus” (PANDAS), patients were noted as experiencing a sudden onset or exacerbation of symptoms of OCD and/or tics following group A streptococcus (GAS) infection (Swedo et al. 1998). Additionally, these patients presented with deterioration in their previous level of functioning, including academic difficulties, changes in personality, and severe mood disturbances (Lewin et al. 2011). More recently, the PANDAS classification has been expanded to include infections from other agents such as mycoplasma pneumonia, influenza, and Lyme infections following observations of influenza, and Lyme disease triggering OCD and/or tics (Allen et al. 1995; Muller et al. 2004). The term “pediatric acute-onset neuropsychiatric syndrome” (PANS) accommodates these other “triggers,” and now emphasizes the acute and severe onset of OCD or food refusal (Swedo et al. 2012).
Current treatments for OCD rely primarily on antidepressant and cognitive-behavioral interventions, whereas treatment of tic disorders rely on α2 agonists, antipsychotics, and behavioral intervention, although the presence of comorbid conditions often makes treatment response variable. Patients with OCD and comorbid tics, for example, are likely to be less responsive to serotonin reuptake inhibitor (SSRI) treatment than their counterparts without tics (March et al. 2007; Pallanti et al. 2011), and those with PANDAS may be more prone to behavioral activation with antidepressants (Murphy et al. 2006). In PANS/PANDAS patients with sudden onset OCD and/or tics, in whom infection has been clinically confirmed, preliminary studies have reported symptom improvements following antibiotic intervention (Murphy and Pichichero 2002; Murphy et al. 2012). Although the validity of the PANDAS/PANS diagnosis remains heavily debated (Murphy et al. 2010), symptom flares or continued symptoms may be caused by undetected and untreated infections, as previously observed in RF, making antibiotic intervention a potentially useful therapeutic tool (Lee et al. 2000).
Beta-lactam antibiotics, such as the penicillins and cephalosporins, are first line options for eliminating GAS infections, and possess low levels of GAS resistance, with some studies suggesting that cephalosporins are more efficacious than the penicillins for GAS pharyngitis, with higher efficacy against β lactamase producing bacteria (Casey and Pichichero 2004; Pichichero and Casey 2006). Recent studies have highlighted the neuroprotective properties of β-lactam antibiotics beyond their antimicrobial efficacy, when found to promote the expression of glutamate transporter GLT1, a neurotransmitter system also implicated in OCD and tic disorder (Rothstein et al. 2005).
In this study, we investigated the feasibility, tolerability, and preliminary efficacy of cefdinir, a third generation, broad-spectrum cephalosporin, in the treatment of children with new-onset OCD and/or tics. We hypothesized that children receiving antibiotics would show greater overall improvement in symptom severity than children receiving placebo.
Methods
Participants
Youth between 4 and 13 years of age with a history of recent (but not necessarily sudden and severe) onset of OCD and/or tics and symptom duration≤6 months were included in the study. Subjects were recruited based on criteria previously described by Murphy et al. (2012). Briefly, all subjects met Diagnostic and Statistical Manual of Mental Disorders, 4th ed. criteria for OCD (American Psychiatric Association 1994), a tic disorder, or both, with diagnosis confirmed by clinical interview with the first author and a semistructured diagnostic interview conducted by a trained clinician. Youth were excluded if any of the following were present: Active psychosis, mania, current suicidal intent, a diagnosis of intellectual deficiency, or other psychiatric conditions (based on clinical interview with a child psychiatrist) that would limit their ability to participate in study-related procedures. Youth who were nonresponders to prior trials with antibiotic(s) for OCD/tics were also excluded from the study. Those on stable doses of psychotropic medication for their condition were not excluded. Table 1 details sample characteristics. The presiding institutional review board approved all study procedures. Subjects were screened for study eligibility over the phone or at their clinic visit by either a research coordinator or a study investigator. After being given a description of the study and providing the appropriate written consent and assent, parents and youth were given a battery of assessments to complete at a scheduled study visit.
Table 1.
Demographic Characteristics of Study Subjects
| All (n=20) | Placebo (n=11) | Cefdinir (n=9) | |
|---|---|---|---|
| Male, n (%) | 15 (75) | 7 (64) | 8 (89) |
| Female, n (%) | 5 (25) | 4 (36) | 1 (11) |
| Age at evaluation, mean (SD) | 7.55 (1.87) | 7.36 (1.90) | 7.79 (1.91) |
| Male | 7.55 (1.82) | 7.37 (1.68) | 7.87 (2.02) |
| Female | 7.29 (2.19) | 7.33 (2.53) | 7.14 (0) |
| Diagnosis | |||
| OCD only | 4 | 3 | 1 |
| Tics only | 4 | 1 | 3 |
| OCD/tics | 12 | 7 | 5 |
| Symptom course | |||
| Acute OCD | 2 | 1 | 1 |
| Episodic course | 2 | 1 | 1 |
| New and not dramatic | 4 | 2 | 2 |
| Dramatic flares | 9 | 6 | 3 |
| Wax/Wane w/illness | 0 | 0 | 0 |
| Stepwise progressive | 1 | 0 | 1 |
| Severe onset tics | 2 | 1 | 1 |
| Infectious trigger | 14 | 6 | 8 |
| Recent URI | 8 | 3 | 5 |
| Positive GAS (culture/rapid strep) | 6 | 3 | 3 |
| No evidence of infectious trigger | 6 | 5 | 1 |
| Comorbid diagnosis | |||
| Anxiety | 6 | 4 | 2 |
| Attention deficit/hyperactivity disorder | 5 | 2 | 3 |
| Depression | 2 | 2 | 0 |
| Psychosis | 2 | 1 | 1 |
| Previous speech disorder | 7 | 4 | 3 |
| Medical history | |||
| Adenoidectomy | 8 | 5 | 3 |
| Tonsillectomy | 3 | 1 | 2 |
| Myringotomy tubes | 3 | 1 | 2 |
| Frequent OM | 6 | 2 | 4 |
| Frequent URI/GAS | 13 | 5 | 6 |
| Allergy illness | 7 | 2 | 5 |
| Perinatal complications | 8 | 5 | 3 |
GAS, Group A Streptococcus; OCD, obsessive compulsive disorder; URI, upper respiratory infection; OM, otitis media.
Clinical assessments
Assessments consisted of comprehensive parent, child, and clinical ratings for OCD, tics, and attention-deficit/hyperactivity disorder (ADHD); neurological/physical examinations; and assessments of medication tolerability. Medical record reviews were also completed. Maternal and paternal family histories were collected, including history of autoimmune disorders, chronic conditions, and psychiatric history. As this study focused on the efficacy of cefdinir in reducing the symptoms of OCD/tics and did not require evidence of an infectious trigger, laboratory assessment of streptococcal antibodies was not required, but was noted if available in medical records.
All assessments were conducted or reviewed by the first author or, for the Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS) (Scahill et al. 1997), the Yale Global Tic Severity Scale (YGTSS) (Leckman et al. 1989), and the Clinical Global Impressions Scale (CGI) (Busner and Targum 2007), by a trained clinician with experience in pediatric OCD and tic disorders. Raters with demonstrated reliability with all instruments were utilized in this study. All study personnel, except the study pharmacist, were blinded to group status. Parent ratings for inattention, impulsivity, hyperactivity, and oppositionality were assessed by the Swanson, Nolan, and Pelham–IV Parent Scale (SNAP-IV) (Swanson 1992), whereas mood, ADHD, OCD, and tics were assessed using the Tourette's Disorder Scale (TODS), a parent-rated scale measuring symptom severity for irritability, motor and vocal tics, mood, and ADHD (Shytle et al. 2003).
For the CY-BOCS assessment, scores were analyzed for patients presenting with a clinical diagnosis of OCD or OCD/tics. Similarly, for the YGTSS assessment, scores were analyzed for patients presenting with a clinical diagnosis of tics or OCD/tics. Assessments were conducted at baseline to provide information on their utility in clinical practice and response prediction, and at the end of study to assess treatment efficacy. The onset and course characteristics (sudden and severe onset, recent but not sudden and severe onset, episodic, chronic), type of neuropsychiatric symptoms, and evidence of an infectious trigger were determined using a clinical interview and all available clinical documentation (see Table 1). Adverse events were carefully monitored. During the study, if a child developed a clinical infection that needed antibiotic therapy as recommended by the primary care provider, the study medication was held for the duration of that treatment, and study medication was resumed immediately upon completion of prescribed antibiotic.
Randomization and treatment
Subjects were randomized to the antibiotic group receiving 14 mg/kg per day in two daily doses (max 600 mg) or placebo for a total of 30 days. The placebo was matched for taste, color, and consistency to cefdinir suspension. Both cefdinir and placebo were dispensed in identical bottles, without manufacturer label or any other identifying information. For the purpose of this study, an important attribute of cefdinir was its increased palatability, with reports suggesting preferred taste and smell compared with other antibiotics, particularly for liquid formulations. As a large portion of our patients have difficulty swallowing tablets, necessitating the use of liquid formulations, the observed preference for cefdinir over other antibiotics makes this favorable in an effort to maintain the blinded nature of the study, avoiding the significant taste differences between cefdinir and placebo that may be encountered with other antibiotics.
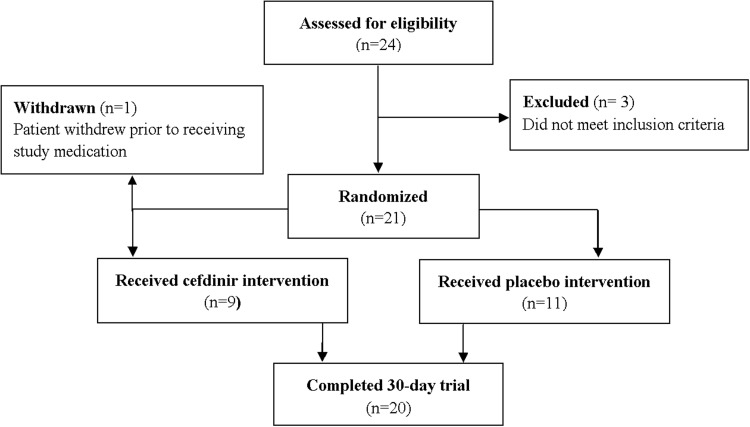
Of the 24 subjects consenting, 21 met study inclusion criteria to be randomized to receive either placebo or a standard treatment dose of cefdinir. Once randomized, the study medications were dispensed in a double-blind manner, with randomization conducted by a research pharmacy using a computer program 1:1 ratio stratification of cefdinir or placebo according to primary tic disorder versus non-tic disorder. Ten were randomized to receive cefdinir treatment (9 actually began the study medicine as one subject withdrew prior to beginning treatment), and 11 to receive placebo (Fig. 1). Subjects returned every 2 weeks for assessment of study compliance, symptom improvement, and adverse events, and were instructed not discuss the specifics such as taste or color of study medication with anyone on the study team, except the pharmacist dispensing the medication.
FIG. 1.
Consort diagram depicting recruitment and retention of subjects through the study.
Statistical analysis
Analysis of covariance (ANCOVA) was used to evaluate group differences in symptom severity between the cefdinir and placebo groups at posttreatment, controlling for the respective baseline variable. Cohen's d was used to determine within-group and between-group effect sizes, given the limited sample size employed in this study, with positive outcomes representative of symptom improvement. Cohen's d values of 0.2<d<0.5 were considered representative of a small treatment effect, values of 0.5<d<0.8 were considered representative of a moderate or medium effect, and values of d>0.8 were considered representative of a large effect. SPSS statistical software was used to analyze all data, and an α of 0.05 was used to define statistical significance, given the pilot nature of this trial; no other statistical correction procedures were implemented.
Results
Enrolled subjects were predominantly male (75%), with an average age of 7.55±1.87 years, with variable courses and co-occurring symptoms (Table 1). Notably, approximately one third had a prior speech disorder (phonological or stuttering or recovered delayed speech) and 55% had had adenoids and/or tonsils removed prior to onset.
Preliminary efficacy
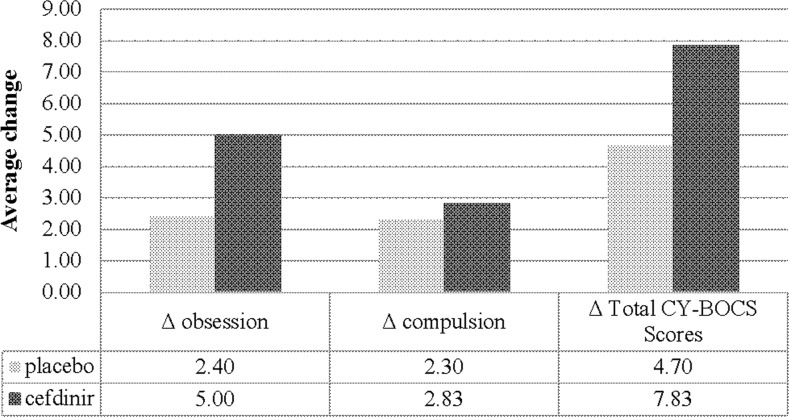
To determine the effectiveness of cefdinir treatment on symptoms of OCD, CY-BOCS scores were analyzed by symptom groups (OCD or tics) as determined by the treating clinician as outlined earlier. Patients presenting with symptoms of OCD and randomized to the cefdinir group (n=6), showed an average decrease in CY-BOCS score of 7.83 (Fig. 2). The placebo arm (n=10) by comparison, showed a CY-BOCS decrease of 4.70 (Fig. 2). There were also large within-group treatment effects (d=1.22) observed for the cefdinir group, compared with only moderate within-group effects for placebo treatment (d=0.51). These differences were not significant between the cefdinir and placebo groups (F [1, 13]=0.385, p=0.546, d=0.24 [weak between-group effect]). Similarly, for OCD CGI-Severity (CGI-S) scores, there were no significant between-group differences (F [1, 13]=0.241, p=0.632; d=0.20) (Table 2).
FIG. 2.
Average change in the Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS) scores for subjects receiving placebo or cefdinir treatment. Delta scores represent changes in CY-BOCS scores taken at baseline and end of study.
Table 2.
Descriptive Statistics for Baseline and 30 Day Outcomes for Placebo and Cefdinir Groups
| Placebo | Cefdinir | ||||||
|---|---|---|---|---|---|---|---|
| Baseline mean (SD) | 30 day mean (SD) | Within-group d | Baseline mean (SD) | 30 day mean (SD) | Within-group d | Between-group d | |
| CY-BOCS | 18.60 (9.00) | 13.90 (9.33) | 0.51 | 22.67 (4.41) | 14.83 (7.94) | 1.22 | 0.24 |
| YGTSS | 13.50 (9.78) | 13.38 (7.25) | 0.01 | 20.38 (9.69) | 10.88 (9.91) | 0.97 | 0.72 |
| CGI-S (OCD) | 2.60 (0.97) | 1.80 (1.14) | 0.76 | 2.33 (0.82) | 1.83 (0.98) | 0.55 | 0.20 |
| CGI-S (tic) | 2.82 (1.25) | 2.56 (1.13) | 0.22 | 3.50 (1.38) | 2.86 (0.69) | 0.54 | 0.53 |
CY-BOCS, Children's Yale-Brown Obsessive Compulsive Scale; YGTSS, Yale Global Tic Severity Scale; CGI-S, Clinical Global Impressions Scale – Severity; OCD, obsessive compulsive disorder.
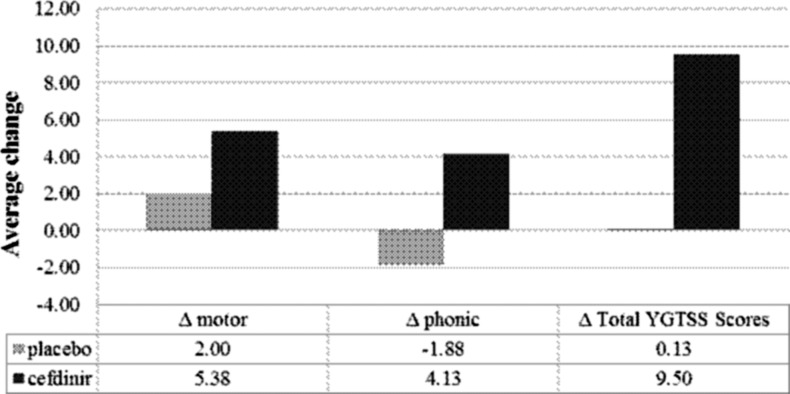
Next, we examined the efficacy of cefdinir in reducing the intensity and frequency of phonic and/or motor tics in patients presenting with a clinical diagnosis of tics or OCD/tics. Patients randomized to receive cefdinir treatment, who presented with symptoms of phonic and/or motor tics (n=8), showed improvement in tic symptoms relative to the placebo arm (n=8), with the cefdinir group showing an average decrease in YGTSS scores of 9.50 (Fig. 3). By contrast, patients randomized to the placebo group demonstrated an overall average decrease in YGTSS scores of 0.13 (Fig. 3). There were also large within-group treatment effects (d=0.97) observed for the cefdinir group, compared with very weak effect for placebo treatment (d=0.01). Despite the improvements within the cefdinir group, and a moderate between-group treatment effect (d=0.72), the between-group differences observed between cefdinir and placebo treatment were not statistically significant (F [1, 13]=4.030, p=0.066). Patients randomized to the cefdinir group who experienced symptoms of phonic and/or motor tics had reductions in tic CGI-S scores compared with those taking placebo, although between-group differences were not significant (F [1, 11]=2.890, p=0.117; d=0.53) (Table 2).
FIG. 3.
Average change in Yale Global Tic Severity Scale (YGTSS) scores for subjects receiving placebo or cefdinir treatment. Delta scores represent changes in YGTSS scores taken at baseline and end of study. Negative signs denote an increase in scores.
Although improvements in OCD and tic symptomology, as assessed by the CY-BOCS and YGTSS, were utilized as the main measures of cefdinir efficacy, we also employed additional neuropsychiatric measures to assess symptom improvement for symptoms of ADHD and mood. For ADHD, which is often observed within this population, two out of nine subjects randomized to the cefdinir group met criteria at baseline and at the end of 30 days treatment for SNAP-IV subscales of inattention and hyperactivity/impulsivity, and one also met criteria for oppositional defiant disorder (ODD). Among the placebo group, one subject met criteria for hyperactivity/impulsivity at baseline but not at 30 days, whereas another met criteria for ODD at the end of 30 days (but not at baseline). Although there were improvements in symptomology and decreased scores for the cefdinir group, there were no significant group differences in SNAP-IV ratings at post-treatment (F [1, 15]=0.031, p=0.862). Similarly, there were no significant group differences for symptoms of mood, tics, OCD, and ADHD, as assessed by the TODS rating scale (F [1, 15]=0.234, p=0.635) (Table 3).
Table 3.
Average Change in SNAP and TODS Scores for Subjects Receiving Placebo or Cefdinir Treatment
| Rating scale | Placebo (n=11) | Cefdinir (n=9) | F test | p value |
|---|---|---|---|---|
| SNAP-IV | 4.50 | 11.92 | (1, 15)=0.03 | 0.86 |
| Inattention | 0.14 | 0.26 | ||
| Hyperactivity/Impulsivity | 0.04 | 0.12 | ||
| ODD | 0.17 | −0.03 | ||
| TODS | 6.20 | 5.75 | (1, 15)=0.23 | 0.64 |
| Mood | 1.71 | −1.25 | ||
| Tics | 1.70 | 4.63 | ||
| OCD | 1.91 | −0.63 | ||
| ADHD | 1.26 | 3.00 |
SNAP-IV, Swanson, Nolan, and Pelham–IV Parent Scale; TODS, Tourette's Disorder Scale; ODD, oppositional defiant disorder; OCD, obsessive compulsive disorder; ADHD, attention-deficit-hyperactivity disorder.
Post-hoc examination of youth showing large reduction in symptoms
Two subjects in the cefdinir group had a large improvement (>50%) in OCD symptoms (average CY-BOCS change of 12.5), as did two subjects in the placebo group (average CY-BOCS change of 16.5). It is of note that one of the two subjects receiving cefdinir with large reductions in OCD severity presented with recent, but not dramatic, onset, and the other had an episodic course. One youth on placebo presented with an initial sudden and severe symptom onset, whereas another youth displayed episodic course. In addition, two subjects receiving cefdinir had dramatic improvements in tic severity (YGTSS average tic score improvement of 20); one had an onset that was not sudden and severe but with sudden and severe flare, and the other subject had episodic tics. All of these subjects with large symptom reductions had both OCD and tics, with one subject having large reductions in both OCD and tics.
Safety
There were few adverse events associated with cefdinir treatment, with most common adverse events being gastrointestinal disturbance such as abdominal pain and diarrhea. However, adverse events were reported in both cefdinir (n=6) and placebo groups (n=8) (Table 4). These events were transient and not serious. During the randomized controlled trial, two subjects in the placebo group were treated with antibiotics for infections (pneumonia and otitis, respectively) and one in the cefdinir group was treated (otitis). Neither of these subjects were the four with very much improved OCD and/or tics, who were described previously.
Table 4.
Adverse Events Experienced by Subjects Randomized to Receive Cefdinir or Placebo
| Adverse event | Placebo (n=11) | Cefdinir (n=9) |
|---|---|---|
| Gastrointestinal | 4 | 1 |
| Fatigue | 1 | 0 |
| Headache | 2 | 0 |
| Difficulty sitting still | 2 | 2 |
| Increased appetite | 3 | 0 |
| Sleep disturbances | 2 | 2 |
| Dry lips | 0 | 1 |
| Tinnitus | 0 | 1 |
| Moodiness/oppositional | 0 | 2 |
| Rash | 0 | 1 |
| Vertigo/dizziness | 0 | 1 |
Discussion
Although the etiology of tic disorders and OCD remains unclear, research continues to suggest an infectious or immune-based etiology in a subset of individuals. Observations from disorders sharing similar clinical characteristics, such as Sydenham's chorea, with evidence of antibody-mediated neuropathology, and clinical observations from patients presenting with abrupt symptom manifestations following acute infections, have all indicated aberrant immunological responses to infection as a key disease mechanism. Infection and the resulting immune responses are proposed to trigger a cascade of events, including antibody cross- reactivity, that culminates in the clinical presentation. Serological studies further support this theory, showing atypical streptococcal antibody responses from the sera of tic patients (Bombaci et al. 2009). In a study by Snider and colleagues, in which patients presenting with a sudden onset of OCD and/or tic symptom were giving prophylactic antibiotic treatment, a significant improvement in the frequency of neuropsychiatric flares was observed, further suggesting an infection-mediated and/or immune-related etiology (Snider et al. 2005).
In this pilot study, we aimed to determine the safety and efficacy of the antibiotic cefdinir in children with recent onset OCD/tics. This study did not require acute and severe onset of OCD/tics nor did it require evidence of infectious trigger, but rather focused on recent-onset pediatric neuropsychiatric disorder, as many children may have subclinical infections or non-GAS infections. In patients randomized to the cefdinir group, symptoms of OCD and tics improved following 30 day cefdinir treatment, with moderate treatment effects observed with tic symptoms, suggesting that cefdinir might be a useful therapeutic tool in treating the symptoms of OCD and tics for youth with recent onset of symptoms. Although we did not detect significant group differences between the cefdinir and placebo groups, we did observe large within-group treatment effects for the CY-BOCS and YGTSS for the cefdinir group but not the placebo group, suggesting improvement with cefdinir, especially for the youth with tics. Moderate between- group treatment effects for YGTSS further supported the benefit for tic symptoms. With further investigation, including a fully powered study, we may also be able to identify certain characteristics such as age, duration of neuropsychiatric illness, symptom presentation, type of infection, immune risks, family history, and comorbid disorders that may influence treatment response. These factors may aid in defining persons who may respond optimally antibiotic intervention.
Cefdinir, like other cephalosporins, exerts its effects through disruption of bacterial protein synthesis. In addition to their antibacterial activity, this group of antibiotics has immunomodulatory effects, an attribute that may help explain its potential therapeutic effects (Ramos-Sevillano et al. 2012). Interestingly, other cephalosporins, such as ceftriaxone, have been shown to exhibit antidepressant properties, via increasing glutamate uptake in a mouse model of depression (Mineur et al. 2007; Thone-Reineke et al. 2008). Although the exact mechanism of action is still largely unknown, these properties may help explain the mechanism by which cefdinir may improve OCD and tics in the earliest stages of these disorders. Furthermore, it may help further support the possible link among infection, immune disruptions, and the neurological manifestations seen clinically in disorders such as OCD/tics.
Although cefdinir was well tolerated in this study, risks versus benefits of this treatment warrant very careful consideration. Risks of long-term use of certain antibiotics as it pertains to promoting the resistance of certain microbes, risk of allergic reaction, risk of intestinal overgrowth presentations, and cardiovascular complications are possible. Cephalosporin antibiotics have also been shown to increase the risk of seizures, particularly in patients with renal impairments (Grill and Maganti 2008). Although we did not encounter this within our study, the potential for neurotoxicity must be carefully examined when considering implementing this treatment regimen.
Limitations
As mentioned, this study was a small pilot to investigate the use of cefdinir in the treatment of symptoms of recent onset OCD and tics. There are a few limitations of the current study that may impact the interpretation of outcomes. The most notable limitation was the small sample size utilized, which limits the amount of statistical significance that we can attribute to the study. Additionally, there were baseline differences in OCD and tic symptom severity; specifically, patients randomized to the cefdinir group presented with higher ratings, indicative of more severe symptomology. Although statistical analyses were performed to account for baseline differences, the potential exists for confounding the outcome. There were also limitations attributed to the study medication. Because of the liquid formulation of cefdinir, the integrity of the double blind may have been impacted more than would have been the case with tablet formulations, as taste, color, and consistency are more difficult to match to placebo. However, we were only aware of this occurring on one occasion. Additionally, subjects who had intercurrent infections requiring an antibiotic during the course of the study limited the total number of subjects receiving no antibiotics in the placebo arm during the course of the study. Lastly, the retrospective nature of parent report of initial symptom onset and course needs to be considered, because of the inherent recall bias associated with retrospective reporting.
Clinical Significance
Infections and immunological disruptions have recently gained momentum, with increasing evidence of the linkage between immune disruptions and neurological disturbances. Although other factors such as genetics, behavioral theory, and abnormalities in neurotransmitter systems should always be considered, the idea of an immune etiology opens the door for the utilization of known pharmaceuticals, including antibiotics.
Conclusion
In this study, we showed the potential for cephalosporin to reduce symptoms of OCD and tics in patients with a recent onset of symptoms. This study is preliminary, but results suggest that further investigation is warranted in order to establish relevance in standard clinical care. Additionally, this study highlights important areas in need of further investigation, in particular, the effects of comorbidity on treatment response, and potential predictors of disease course and treatment response. For example, because of this small sample size, it is unclear if those with symptoms with more dramatic onset fared better than those with a less dramatic onset of symptoms. Subjects with large symptom improvement did appear to have a dramatic onset, flares, or an episodic course. This was observed in the placebo arm as well, suggesting that some children with these types of courses may improve spontaneously. Armed with this information, we may be better able to improve diagnosis and treatment of these individuals by creating more tailored interventions that address their unique clinical presentations.
Disclosures
Dr. Tanya Murphy has received research support in the past 3 years from All Children's Hospital Research Foundation, Centers for Disease Control, International OCD Foundation (IOCDF), National Institutes of Health, Ortho McNeil Scientific Affairs, Otsuka, Pfizer Pharmaceuticals, Roche Pharmaceuticals, Shire, Tourette Syndrome Association, and Transcept Pharmaceuticals, Inc. Dr. Murphy is on the Medical Advisory Board for Tourette Syndrome Association and on the Scientific Advisory Board for IOCDF. She receives a textbook honorarium from Lawrence Erlbaum. Dr. Ellisa C. Parker-Athill has no financial relationships to disclose. Dr. Eric Storch has received grant funding in the last 3 years from Agency for Healthcare Research and Quality, All Children's Hospital Research Foundation, Centers for Disease Control, IOCDF, National Alliance for Research on Schizophrenia and Affective Disorders, the National Institutes of Health, Ortho McNeil Scientific Affairs, and Tourette Syndrome Association. He receives a textbook honorarium from American Psychological Association, Lawrence Erlbaum, and Springer publishers. Dr. Storch has been an educational consultant for Rogers Memorial Hospital. He is a consultant for CroNos, Inc. and Prophase, Inc., and is on the Speaker's Bureau and Scientific Advisory Board for IOCDF. Dr. Adam Lewin receives grant funding from Agency for Healthcare Research and Quality, Centers for Disease Control, IOCDF, Joseph Drown Foundation, National Alliance for Research on Schizophrenia and Affective Disorders, National Institutes of Health, and University of South Florida Research Council. He is a consultant for Prophase, Inc., and has received speaker's honoraria from the Tourette Syndrome Association. He received travel reimbursement from Roche Pharmaceuticals. P. Jane Mutch, Ph.D. has no financial relationships to disclose.
References
- Adams KH, Hansen ES, Pinborg LH, Hasselbalch SG, Svarer C, Holm S, Bolwig TG, Knudsen GM: Patients with obsessive-compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei. Int J Neuropsychopharmacol 8:391–401, 2005 [DOI] [PubMed] [Google Scholar]
- Allen AJ, Leonard HL, Swedo SE: Case study: A new infection-triggered, autoimmune subtype of pediatric OCD and Tourette's syndrome. J Am Acad Child Adolesc Psychiatry 34:307–311, 1995 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Bloch MH, Peterson BS, Scahill L, Otka J, Katsovich L, Zhang H, Leckman JF: Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med 160:65–69, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombaci M, Grifantini R, Mora M, Reguzzi V, Petracca R, Meoni E, Balloni S, Zingaretti C, Falugi F, Manetti AG, Margarit I, Musser JM, Cardona F, Orefici G, Grandi G, Bensi G: Protein array profiling of tic patient sera reveals a broad range and enhanced immune response against Group A Streptococcus antigens. PloS one 4:e6332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronze MS, Dale JB: Epitopes of streptococcal M proteins that evoke antibodies that cross-react with human brain. J Immunol 151:2820–2828, 1993 [PubMed] [Google Scholar]
- Brown AS: Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol 72:1272–1276, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busner J, Targum SD: The Clinical Global Impressions scale: Applying a research tool in clinical practice. Psychiatry 4:28–37, 2007 [PMC free article] [PubMed] [Google Scholar]
- Casey JR, Pichichero ME: Meta-analysis of cephalosporin versus penicillin treatment of group A streptococcal tonsillopharyngitis in children. Pediatrics 113:866–882, 2004 [DOI] [PubMed] [Google Scholar]
- Grill MF, Maganti R: Cephalosporin-induced neurotoxicity: Clinical manifestations, potential pathogenic mechanisms, and the role of electroencephalographic monitoring. Ann Pharmacother 42:1843–1850, 2008 [DOI] [PubMed] [Google Scholar]
- Jijun L, Zaiwang L, Anyuan L, Shuzhen W, Fanghua Q, Lin Z, Hong L: Abnormal expression of dopamine and serotonin transporters associated with the pathophysiologic mechanism of Tourette syndrome. Neurol India 58:523–529, 2010 [DOI] [PubMed] [Google Scholar]
- Kiessling L, Marcotte A, Benson M, Kuhn C, Wrenn D: Relationship between GABHS and childhood movement disorders. Pediatric Res 33:12a, 1993 [Google Scholar]
- Kirvan CA, Swedo SE, Kurahara D, Cunningham MW: Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham's chorea. Autoimmunity 39:21–29, 2006 [DOI] [PubMed] [Google Scholar]
- Lazar A, Walitza S, Jetter A, Gerlach M, Warnke A, Herpertz–Dahlmann B, Grundemann D, Grimberg G, Schulz E, Remschmidt H, Wewetzer C, Schomig E: Novel mutations of the extraneuronal monoamine transporter gene in children and adolescents with obsessive-compulsive disorder. Int J Neuropsychopharmacol 11:35–48, 2008 [DOI] [PubMed] [Google Scholar]
- Leckman JF, Grice DE, Barr LC, de Vries AL, Martin C, Cohen DJ, McDougle CJ, Goodman WK, Rasmussen SA: Tic-related vs. non-tic-related obsessive compulsive disorder. Anxiety 1:208–215, 1994 [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ: The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 28:566–573, 1989 [DOI] [PubMed] [Google Scholar]
- Lee LH, Ayoub E, Pichichero ME: Fewer symptoms occur in same-serotype recurrent streptococcal tonsillopharyngitis. Arch Otolaryngol Head Neck Surg 126:1359–1362, 2000 [DOI] [PubMed] [Google Scholar]
- Lerner A, Bagic A, Simmons JM, Mari Z, Bonne O, Xu B, Kazuba D, Herscovitch P, Carson RE, Murphy DL, Drevets WC, Hallett M: Widespread abnormality of the gamma-aminobutyric acid-ergic system in Tourette syndrome. Brain 135:1926–1936, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin AB, Storch EA, Mutch PJ, Murphy TK: Neurocognitive functioning in youth with pediatric autoimmune neuropsychiatric disorders associated with streptococcus. J Neuropsychiatry Clin Neurosci 23:391–398, 2011 [DOI] [PubMed] [Google Scholar]
- March JS, Franklin ME, Leonard H, Garcia A, Moore P, Freeman J, Foa E: Tics moderate treatment outcome with sertraline but not cognitive-behavior therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry 61:344–347, 2007 [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR, Sanacora G: Antidepressant-like effects of ceftriaxone in male C57BL/6J mice. Biol Psychiatry 61:250–252, 2007 [DOI] [PubMed] [Google Scholar]
- Moya PR, Wendland JR, Rubenstein LM, Timpano KR, Heiman GA, Tischfield JA, King RA, Andrews AM, Ramamoorthy S, McMahon FJ, Murphy DL: Common and rare alleles of the serotonin transporter gene, SLC6A4, associated with Tourette's disorder. Mov Disord 28:1263–1270, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Riedel M, Blendinger C, Oberle K, Jacobs E, Abele–Horn M: Mycoplasma pneumoniae infection and Tourette's syndrome. Psychiatry Res 129:119–125, 2004 [DOI] [PubMed] [Google Scholar]
- Murphy M, Pichichero M: Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS). Arch Pediatr Adolesc Med 156:356–361, 2002 [DOI] [PubMed] [Google Scholar]
- Murphy TK, Kurlan R, Leckman J: The immunobiology of Tourette's disorder, pediatric autoimmune neuropsychiatric disorders associated with Streptococcus, and related disorders: A way forward. J Child Adolesc Psychopharmacol 20:317–331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TK, Storch EA, Lewin AB, Edge PJ, Goodman WK: Clinical factors associated with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Pediatr 160:314–319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TK, Storch EA, Strawser MS: Selective Serotonin reuptake inhibitor-induced behavioral activation in the PANDAS subtype. Prim Psychiatry 13:87–89, 2006 [Google Scholar]
- Pallanti S, Grassi G, Sarrecchia ED, Cantisani A, Pellegrini M: Obsessive-compulsive disorder comorbidity: clinical assessment and therapeutic implications. Front Psychiatry 2:70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH: Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav Brain Res 204:313–321, 2009 [DOI] [PubMed] [Google Scholar]
- Pichichero M, Casey J: Comparison of European and U.S. results for cephalosporin versus penicillin treatment of group A streptococcal tonsillopharyngitis. Eur J Clin Microbiol Infect Dis 25:354–364, 2006 [DOI] [PubMed] [Google Scholar]
- Pittenger C, Bloch MH, Williams K: Glutamate abnormalities in obsessive compulsive disorder: Neurobiology, pathophysiology, and treatment. Pharmacol Ther 132:314–332, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos–Sevillano E, Rodriguez–Sosa C, Cafini F, Gimenez MJ, Navarro A, Sevillano D, Alou L, Garcia E, Aguilar L, Yuste J: Cefditoren and ceftriaxone enhance complement-mediated immunity in the presence of specific antibodies against antibiotic-resistant pneumococcal strains. PloS one 7:e44135, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB: Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433:73–77, 2005 [DOI] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin–Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF: Children's Yale-Brown Obsessive Compulsive Scale: Reliability and validity. J Am Acad Child Adolesc Psychiatry 36:844–852, 1997 [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Sheehan KH, Wilkinson BJ, Newman M, Sanberg PR, Sheehan D: The Tourette's Disorder Scale (TODS): Development, reliability, and validity. Assessment 10:273–287, 2003 [DOI] [PubMed] [Google Scholar]
- Snider LA, Lougee L, Slattery M, Grant P, Swedo SE: Antibiotic prophylaxis with azithromycin or penicillin for childhood-onset neuropsychiatric disorders. Biol Psychiatry 57:788–792, 2005 [DOI] [PubMed] [Google Scholar]
- Swanson JM: School-Based Assessments and Interventions for ADD Students. Irvine, CA: K C Publishing; 1992 [Google Scholar]
- Swedo SE, Leckman JF, Rose NR: From research subgroup to clinical syndrome: Modifying the PANDAS criteria to describe PANS (pediatric acute-onset neuropsychiatric syndrome). Pediatr Ther 2: 113, doi: 10.4172/2161-0665.1000113, 2012 [DOI] [Google Scholar]
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Lougee L, Dow S, Zamkoff J, Dubbert BK: Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: Clinical description of the first 50 cases. Am J Psychiatry 155:264–271, 1998 [DOI] [PubMed] [Google Scholar]
- Swedo SE, Rapoport JL, Cheslow DL, Leonard HL, Ayoub EM, Hosier DM, Wald ER: High prevalence of obsessive-compulsive symptoms in patients with Sydenham's chorea. Am J Psychiatry 146:246–249, 1989 [DOI] [PubMed] [Google Scholar]
- Thone–Reineke C, Neumann C, Namsolleck P, Schmerbach K, Krikov M, Schefe JH, Lucht K, Hortnagl H, Godes M, Muller S, Rumschussel K, Funke–Kaiser H, Villringer A, Steckelings UM, Unger T: The beta-lactam antibiotic, ceftriaxone, dramatically improves survival, increases glutamate uptake and induces neurotrophins in stroke. J Hypertens 26:2426–2435, 2008 [DOI] [PubMed] [Google Scholar]
- Winter C, Djodari–Irani A, Sohr R, Morgenstern R, Feldon J, Juckel G, Meyer U: Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int J Neuropsychopharmacol 12:513–524, 2009 [DOI] [PubMed] [Google Scholar]