Abstract
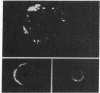
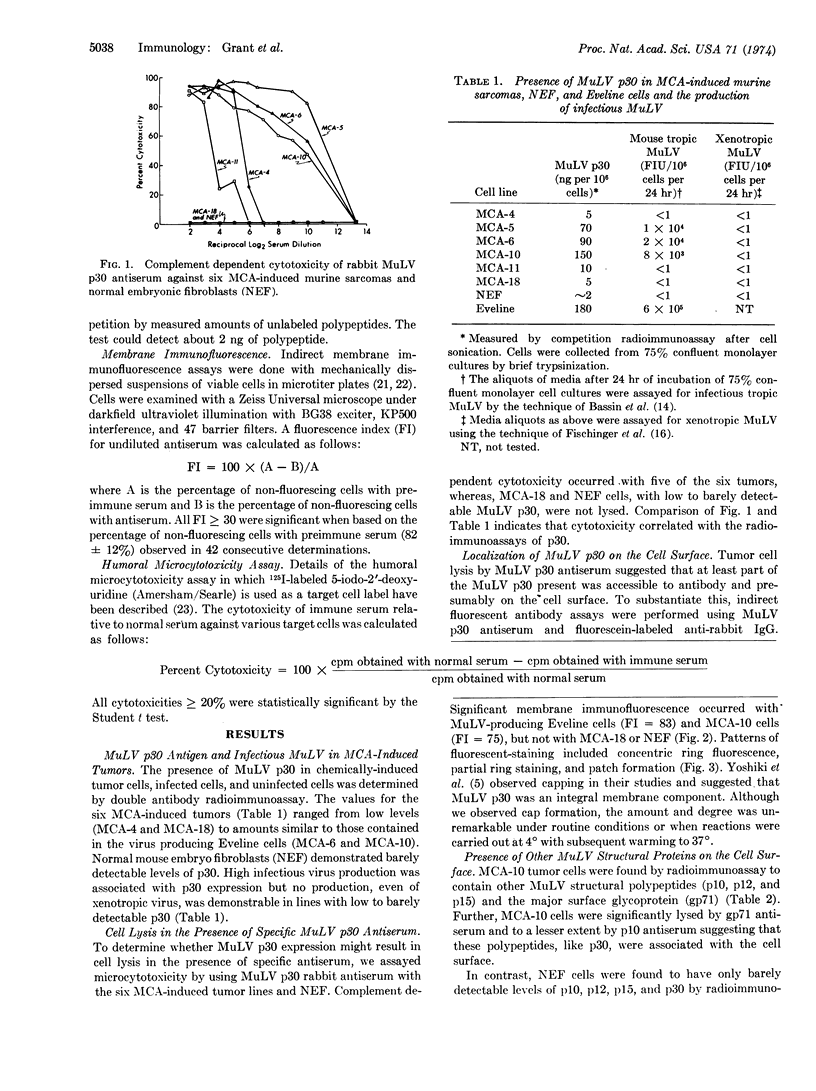
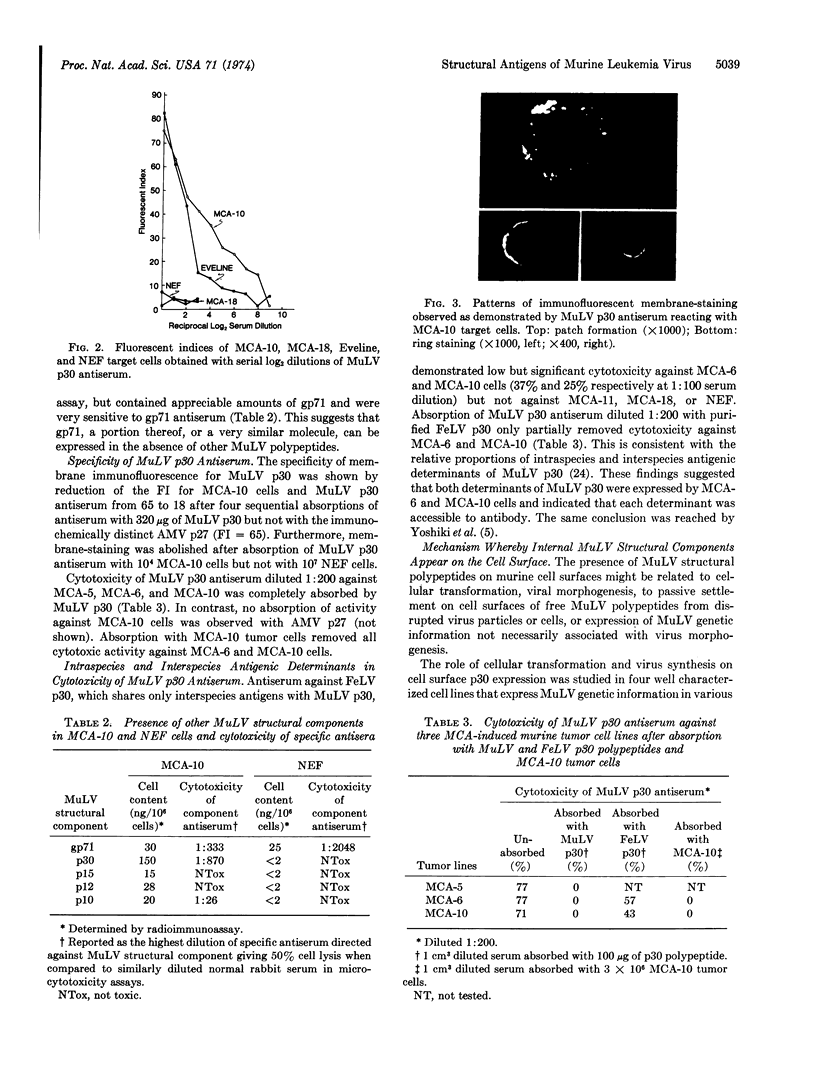
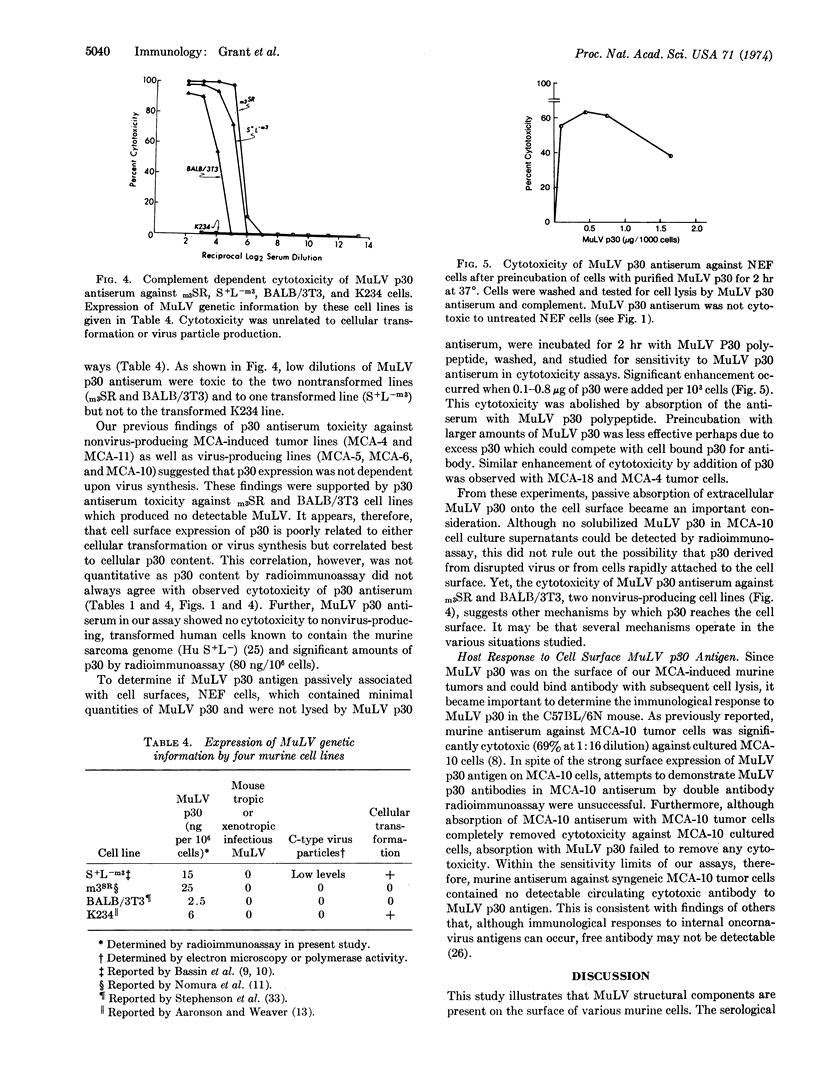
Cultured cells of different chemically-induced C57BL/6N murine sarcomas produced variable amounts of infectious murine leukemia virus (MuLV) and contained proportional amounts of MuLV structural components as determined by radioimmunoassay. Monospecific antisera directed against the major MuLV glycoprotein (gp71), the major internal antigen (p30), and the ribonucleoprotein (p10) were capable of mediating tumor cell lysis in the presence of complement, suggesting that these viral structural components were localized at least in part to the cell surface. Membrane immunofluorescence studies with MuLV p30 antiserum confirmed surface localization. Addition of MuLV p30 polypeptide to normal cells and tumor cells enhanced the cytotoxicity of MuLV p30 antiserum. Studies are presented which suggest that the presence of MuLV structural components on cell surfaces can be independent of virus production and cellular transformation.
Keywords: radioimmunoassay, humoral cytotoxicity, membrane immunofluorescence, viral antigens
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Baldwin R. W., Moore M. Rat hepatoma cell surface antigens: demonstration and isolation of membrane-associated isoantigens. Eur J Cancer. 1969 Nov;5(5):475–483. doi: 10.1016/0014-2964(69)90101-7. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Phillips L. A., Kramer M. J., Haapala D. K., Peebles P. T., Nomura S., Fischinger P. J. Transformation of mouse 3T3 cells by murine sarcoma virus: release of virus-like particles in the absence of replicating murine leukemia helper virus. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1520–1524. doi: 10.1073/pnas.68.7.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Dougherty R. M., Marucci A. A., Distefano H. S. Application of immunohistochemistry to study of avian leukosis virus. J Gen Virol. 1972 May;15(2):149–162. doi: 10.1099/0022-1317-15-2-149. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Nomura S. Simple, quantitative assay for both xenotropic murine leukemia and ecotropic feline leukemia viruses. J Virol. 1974 Jul;14(1):177–179. doi: 10.1128/jvi.14.1.177-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom H., Bauer H., Graf T. Cell-surface antigens induced by avian RNA tumor viruses: detection by immunoferritin technique. Virology. 1972 Feb;47(2):416–425. doi: 10.1016/0042-6822(72)90277-2. [DOI] [PubMed] [Google Scholar]
- Grant J. P., Tschang T. P., Wells S. A., Jr Similarities of fetal and tumor antigens of MCA-induced murine sarcomas. Surg Forum. 1974;25(0):104–106. [PubMed] [Google Scholar]
- Green R. W., Bolognesi D. P., Schäfer W., Pister L., Hunsmann G., De Noronha F. Polypeptides of mammalian oncornaviruses. I. Isolation and serological analysis polypeptides from murine and feline C-type viruses. Virology. 1973 Dec;56(2):565–579. doi: 10.1016/0042-6822(73)90058-5. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Aoki T., Kawai S., Miyamoto T., Wilsnack R. E. Presence of antigen common to avian tumor viral envelope antigen in normal chick embryo cells. Virology. 1973 Nov;56(1):22–32. doi: 10.1016/0042-6822(73)90284-5. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T., Fleissner E. Existence and expression of tumor virus genes in chick embryo cells. Virology. 1972 Feb;47(2):475–482. doi: 10.1016/0042-6822(72)90283-8. [DOI] [PubMed] [Google Scholar]
- Hanna M. G., Jr, Tennant R. W., Coggin J. H., Jr Suppressive effect of immunization with mouse fetal antigens on growth of cells infected with Rauscher leukemia virus and plasma-cell tumors. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1748–1752. doi: 10.1073/pnas.68.8.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Hanna M. G., Jr, Roberson L. E., Kenney F. T. Autogenous immunity to endogenous RNA tumor virus. Identification of antibody reactivity to select viral antigens. J Exp Med. 1974 Jun 1;139(6):1568–1581. doi: 10.1084/jem.139.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMevel B. P., Wells S. A., Jr A microassay for the guantitation of cytotoxic antitumor antibody: use of 125 I-iododeoxyuridine as a tumor cell label. J Natl Cancer Inst. 1973 Mar;50(3):803–806. doi: 10.1093/jnci/50.3.803. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Moennig V., Frank H., Hunsmann G., Schneider I., Schafer W. Properties of mouse leukemia viruses. VII. The major viral glycoprotein of friend leukemia virus. Isolation and physicochemical properties. Virology. 1974 Sep;61(1):100–111. doi: 10.1016/0042-6822(74)90245-1. [DOI] [PubMed] [Google Scholar]
- Nomura S., Fischinger P. J., Mattern C. F., Peebles P. T., Bassin R. H., Friedman G. P. Revertants of mouse cells transformed by murine sarcoma virus. I. Characterization of flat and transformed sublines without a rescuable murine sarcoma virus. Virology. 1972 Oct;50(1):51–64. doi: 10.1016/0042-6822(72)90345-5. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Peters E. D. Cell surface antigens associated with murine leukemia virus: definition of the GL and GT antigenic systems. J Virol. 1973 Nov;12(5):1104–1117. doi: 10.1128/jvi.12.5.1104-1117.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Aoki T., Dixon F. J. The antibody response of mice to murine leukemia virus in spontaneous infection: absence of classical immunologic tolerance (AKR mice-complement-fixing antibodies-lymphocytic choriomeningitis virus-immunofluorescence-glomerular deposits of antigen-antibody complexes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):134–138. doi: 10.1073/pnas.69.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles P. T., Fischinger P. J., Bassin R. H., Papageorge A. G. Isolation of human amnion cells transformed by rescuable murine sarcoma virus. Nat New Biol. 1973 Mar 28;242(117):98–101. doi: 10.1038/newbio242098a0. [DOI] [PubMed] [Google Scholar]
- Reiner J., Southam C. M. Evidence of common antigenic properties in chemically induced sarcomas of mice. Cancer Res. 1967 Jul;27(7):1243–1247. [PubMed] [Google Scholar]
- Reiner J., Southam C. M. Further evidence of common antigenic properties in chemically induced sarcomas of mice. Cancer Res. 1969 Oct;29(10):1814–1820. [PubMed] [Google Scholar]
- Rowe W. P., Lowy D. R., Teich N., Hartley J. W. Some implications of the activation of murine leukemia virus by halogenated pyrimidines. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1033–1035. doi: 10.1073/pnas.69.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Tronick S. R., Reynolds R. K., Aaronson S. A. Isolation and characterization of C-type viral gene products of virus-negative mouse cells. J Exp Med. 1974 Feb 1;139(2):427–438. doi: 10.1084/jem.139.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of mammalian oncogenic RNA viruses: multiple antigenic determinants of the major internal protein and envelope glycoprotein. J Virol. 1974 Jan;13(1):171–180. doi: 10.1128/jvi.13.1.171-180.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of oncogenic ribonucleic acid viruses. Interspec II, a new interspecies antigen. J Biol Chem. 1973 Aug 25;248(16):5627–5633. [PubMed] [Google Scholar]
- Todaro G. J., Huebner R. J. N.A.S. symposium: new evidence as the basis for increased efforts in cancer research. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1009–1015. doi: 10.1073/pnas.69.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiki T., Mellors R. C., Hardy W. D., Jr Common cell-surface antigen associated with murine and feline C-type RNA leukemia viruses. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1878–1882. doi: 10.1073/pnas.70.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiki T., Mellors R. C., Hardy W. D., Jr, Fleissner E. Common cell surface antigen associated with mammalian C-type RNA viruses. Cell membrane-bound gs antigen. J Exp Med. 1974 Apr 1;139(4):925–942. doi: 10.1084/jem.139.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]