Abstract
A variety of systemic inflammatory rheumatic diseases associate with an increased risk of atherosclerotic events and premature cardiovascular (CV) disease. Although this recognition has stimulated intense basic science and clinical research, the precise nature of the relationship between local and systemic inflammation, their interactions with traditional CV risk factors, and their role in accelerating atherogenesis remains unresolved. The individual rheumatic diseases have both shared and unique attributes that might impact CV events. Understanding of the positive and negative influences of individual anti-inflammatory therapies remains rudimentary. Clinicians need to adopt an evidence-based approach to develop diagnostic techniques to identify those rheumatologic patients most at risk of CV disease and to develop effective treatment protocols. Development of optimal preventative and disease-modifying approaches for atherosclerosis in these patients will require close collaboration between basic scientists, CV specialists, and rheumatologists. This interface presents a complex, important, and exciting challenge.
Keywords: Inflammation, Coronary artery disease, Atherosclerosis, Rheumatic disease, Rheumatoid arthritis, Systemic lupus erythematosus, Risk factors
Introduction
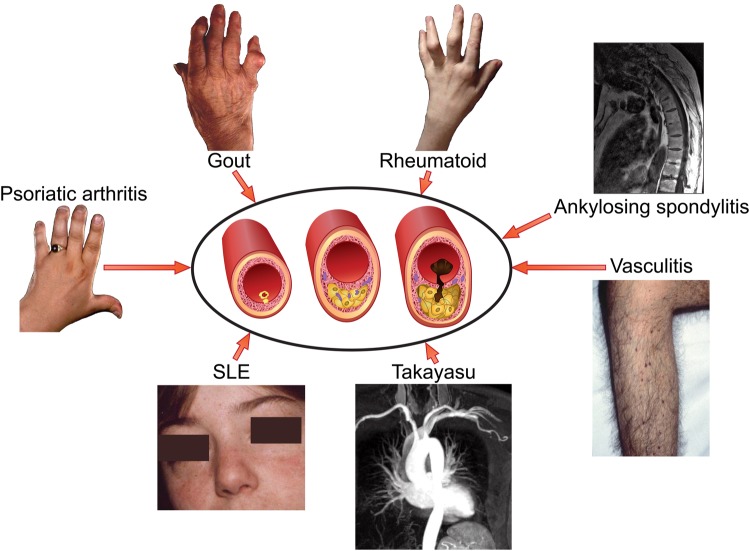
Recognition of the prominent role of inflammation at all stages of atherosclerotic plaque development highlighted the potential relationship between systemic inflammation and atherogenesis, and fuelled intense basic science and clinical research.1 The rheumatology community has long recognized that patients with rheumatoid arthritis (RA) have heightened risk of premature cardiovascular (CV) death.2 Indeed, a variety of systemic inflammatory diseases associate with increased risk of CV events including RA, systemic lupus erythematosus (SLE), ankylosing spondylitis, gout, psoriatic arthritis, and medium and large vessel vasculitides (Figure 1).3 Emerging data suggest that systemic sclerosis (SSc) and inflammatory myositis also associate with an increased risk of atherosclerotic CV events. A meta-analysis of SSc studies recording myocardial infarction (MI), angina, and coronary intervention found an 82% increased risk of coronary artery disease compared with matched controls.4 In dermatomyositis and polymyositis recent evidence also points towards an increased risk of coronary atherosclerosis-associated CV events.5
Figure 1.
Atherothrombosis in the rheumatic diseases. Systemic inflammatory rheumatic disorders of various types predispose to premature, accelerated atherosclerosis and increased cardiovascular morbidity and mortality.
Patients with inflammatory rheumatic disease have a heightened risk of premature coronary heart disease (recorded as angina, MI, coronary bypass grafting, and coronary angioplasty) and stroke that associates with the degree of inflammation.6 Although certain rheumatic conditions associate with coronary arteritis (Table 1),3,7,8 atherosclerosis likely underlies the majority of the coronary artery-related events.9 Notwithstanding, individuals with rheumatic diseases may not have accentuated atherosclerosis as estimated by angiography. Instead, autoimmune or other arteritides may aggravate lesional inflammation and so render plaques more vulnerable to rupture and thrombosis.10 Cardiovascular events in patients with rheumatic diseases do not arise solely from atherosclerosis, as myocarditis and other non-ischaemic causes of heart failure also contribute to this burden.10 Despite this recognition, the interactions of local vascular and systemic inflammation due to rheumatologic diseases with traditional coronary heart disease risk factors, and the degree to which these pathways contribute to adverse CV outcomes in this patient population remain unsettled.11 Moreover, some of the medications employed in management of patients with rheumatic diseases may aggravate CV risk (e.g. glucocorticoids), while other treatments may mitigate this risk [e.g. methotrexate (MTX), administration], as will be discussed below. These competing iatrogenic interventions render unravelling the pathophysiology of CV complications in patients with rheumatologic disease even more complex.
Table 1.
| Premature atherosclerosis | Coronary arteritis |
|---|---|
| Systemic lupus erythematosus | Systemic lupus erythematosus |
| Rheumatoid arthritis | Takayasu arteritis |
| Ankylosing spondylitis | Kawasaki disease |
| Psoriatic arthritis | Giant cell arteritis |
| Gout | Polyarteritis nodosa |
| ANCA-associated vasculitis | Granulomatous polyangiitis |
| Takayasu arteritis | Churg–Strauss syndrome |
| Giant cell arteritis | Rheumatoid arthritis |
| Inflammatory myopathies |
Although the various diseases considered here all qualify as autoimmune and/or inflammatory in nature (Table 1), they have individual attributes that render lumping all rheumatic disorders into one bin with respect to their contribution to CV events a vast oversimplification.12 The differences among these diseases may provide important clues regarding their association with CV disease and to the aetiology of these complications. Yet, atherosclerosis may represent a common response to arterial injury generated by different disease-specific upstream insults. These questions have generated considerable interest, and launched quests to understand the pathophysiologic links between these rheumatologic conditions and CV diseases.12
In parallel, clinicians need tools to identify those rheumatology patients most at risk of CV disease, to deploy most appropriately diagnostic and therapeutic measures, and aid management. Identification and validation of CV risk biomarkers in patients with rheumatic diseases would aid this practical clinical concern. Finally, the magnitude of this clinical problem demands the development and evaluation of combined therapeutic approaches aimed at minimizing inflammation, and limiting atherosclerosis and CV events in patients with rheumatologic diseases. This review will consider progress in this field, with a focus predominantly on CV disease in RA and SLE, two more common conditions for which the most reliable data exist in this regard. The review also briefly discusses the vasculitides and gout, two important but contrasting conditions associated with CV disease.
Epidemiology of cardiovascular disease
Rheumatoid arthritis
The recognition that patients with RA die prematurely dates back >50 years.2,13 Cardiovascular disease causes >50% of these premature deaths.14 Rheumatoid arthritis and diabetes mellitus elevate CV risk to a similar extent.15 Risk factors associated with excess mortality include female sex, raised erythrocyte sedimentation rate, persistent synovitis, erosions, extra-articular features including rheumatoid nodules, vasculitis and lung disease, and seropositivity including the presence of rheumatoid factor (RhF) and/or anti-citrullinated peptide (CCP) antibodies.16–18 Despite transformation of RA drug therapy, the increased risk of CV mortality has not declined.16 This situation may reflect in part the increased incidence of valvular heart disease, non-ischaemic cardiac failure, myocarditis, and pericardial disease in RA.10
Patients with RA exhibit impaired endothelial vasodilator function in response to acetylcholine, in association with reduced circulating endothelial progenitors.19,20 Although these studies found no change in endothelial-independent vasodilation in response to glyceryl trinitrate, Bergholm et al. have reported attenuated responses to sodium nitroprusside.21 Contrary to the previous studies, this investigation initially involved untreated RA patients. Thus, early uncontrolled RA might involve an endothelial-independent defect in arterial smooth muscle cell relaxation.21 Patients with RA can also display augmented aortic stiffness.22 These findings indicate the presence of wider vascular abnormalities beyond endothelial dysfunction. Such impairments may in turn predispose to atherosclerosis which progresses most rapidly during the first 6 years after RA diagnosis and more slowly thereafter.23,24 Cardiovascular deaths increase 7–10 years following symptom onset.14,25
The increased risk of MI raises questions concerning the nature of the disease process in RA when compared with non-RA patients. The two groups share similar patterns of coronary disease angiographically. The clinical presentation, however, often has distinct features. Patients with RA may be more likely to exhibit silent or unrecognized ischaemia, to suffer MI, and to develop heart failure.26,27 Compared with the general population, patients with RA have two-fold excess in sudden cardiac death.28
The study of plaque morphology in coronary arteries from patients with RA and age- and sex-matched controls has proved informative. Overall, RA patients had a reduced prevalence of multi-vessel disease and less severe coronary atherosclerosis than age- and sex-matched controls. In a post-mortem series, although the overall burden of plaques appeared similar, 48% of plaques in the LAD of patients with RA were graded unstable by histologic criteria compared with 22% in non-RA controls. Moreover, medial and adventitial inflammation appeared more prominent in subjects with RA than controls.29 A recent study used ultrasound (US) to analyse carotid plaque in patients with active and inactive RA and non-RA controls. Measuring grey-scale median, patients with active RA had lower values, a characteristic ascribed to vulnerability to rupture and cause thrombosis.30 The potential role of arterial inflammation in promoting plaque instability in RA also received support from a study that used18F fluorodeoxyglucose positron emission tomography with CT co-registration (18F-FDG-CT PET). 18F-FDG-CT PET identified increased glucose uptake attributed to aortic inflammation and suggestive of sub-clinical vasculitis in patients with active RA, a finding not shared by non-RA patients with stable ischaemic heart disease (IHD).31
Systemic lupus erythematosus
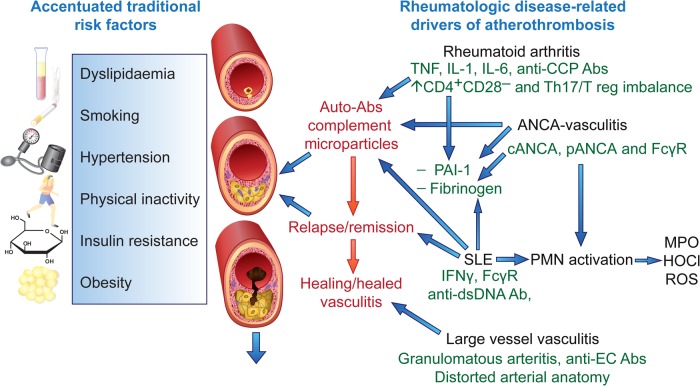
Evidence for a bi-modal mortality revealed the importance of CVD in SLE in the mid-1970s: early deaths reflected SLE disease activity, while the second peak associated principally with CVD, MI, or stroke.32 Since that time, improvements in the management of SLE have reduced mortality directly related to disease activity substantially, so that CVD and infection have emerged as the major cause of mortality.33 Although RA and SLE each predispose to premature atherosclerosis, the pathogenic mechanisms differ. While TNFα, interleukin (IL)-1, and IL-6 play a central role in RA pathogenesis, type I interferons (IFNs) predominate in SLE (Figure 2).34 The extent to which endothelial dysfunction, aortic stiffness, and atherosclerotic plaque instability seen in both of these diseases reflects increased traditional risk factors, common inflammatory mechanisms, or distinct disease-specific mediators remains unclear.
Figure 2.
Pathogenesis of atherosclerosis in inflammatory rheumatic diseases. Traditional risk factors play an important role in premature atherosclerosis and are typically more prevalent in patients with inflammatory rheumatic diseases. Both shared and disease-specific pathogenic mechanisms also contribute to accelerated atherogenesis, with rheumatoid arthritis, systemic lupus erythematosus and the vasculitides shown as examples. Ab, antibody; ANCA, anti-neutrophil cytoplasmic antibody; dsDNA, double-stranded DNA; CCP, cyclic citrullinated peptide; EC, endothelial cells; IFN, interferon; FcγR, Fc gamma receptor; HOCL, hypochlorite; MPO, myeloperoxidase; ROS, reactive oxygen species; T reg, regulatory T lymphocytes lymphocytes.
Despite the increased risk, the relatively modest absolute numbers of CV events in RA and SLE present a challenge to investigators.35 Hence, the need to rely on biomarkers of disease activity or burden to probe disease mechanisms or to inform the development of treatments. More than 50% of SLE patients have impaired endothelial vasodilator function quantified as flow-mediated dilatation of the brachial artery compared with unaffected controls.36 Likewise, cardiac PET revealed impaired microvascular blood flow and reduced coronary flow reserve following adenosine challenge in both RA and in SLE patients with angiographically normal-appearing epicardial arteries.37 Single photon emission tomography demonstrated myocardial perfusion defects in 40% of SLE patients.38 Carotid artery US revealed a marked increase in early plaque in patients with SLE compared with individually matched controls, and the plaque burden appeared related to SLE activity measured by the SLEDAI index.39 The overall relative risk of carotid plaque in SLE patients was 2.4, peaking at 5.6 in those <40 years.39,40 These non-invasive biomarkers of arterial abnormality generally indicate future CV events in non-SLE patients, but require further validation in this regard in patients with SLE or RA.
A recent study of 1874 SLE patients, accumulating 9485 person-years of follow-up, reported a 2.66-fold increased risk of total CV events (stroke, MI, angina, coronary intervention, and peripheral vascular disease) when compared with the general population based on Framingham risk scores.41 Events were most frequent in those <40 years and were related to average disease activity measured by the SELENA-SLEDAI index, but not in this study to disease duration. Those taking corticosteroids at the time of analysis demonstrated a dose-dependent increased risk for CV events, reaching five-fold in those receiving ≥20 mg/day.41 Other studies reveals broadly similar results, although the more recent have revised the overall rate of CV events downward, reflecting differences in study design, focus, and comparators.41–46 The reported risk of MI in SLE patients ranges from 2- to 10-fold greater than the general population, with a peak of 50-fold reported in women of 35–44 years.44 Relative risk values include 10.1 for non-fatal MI, 17 for death due to IHD, and 7.9 for stroke.42 These data are particularly striking given that the majority of patients are female and that 67% presenting with a first event are <55 years of age.44 Factors linked to the incidence of CV disease include disease duration, clinical activity, the titer of anti-dsDNA, the presence of lupus nephritis, and corticosteroid use.47
Biomarkers
The role of auto-antibodies in CV events seen in patients with rheumatic diseases, either as biomarkers of or in the pathogenesis of CV complications remains uncertain. In RA, the presence of RhF and/or anti-CCP antibodies associates with endothelial dysfunction and CVD, although a pathogenic link is unproven.34 Similarly, although laboratory experiments suggest that anti-phospholipid Abs accelerate plaque development, we lack convincing evidence for a causal role in atherosclerotic CV events in patients with SLE or the anti-phospholipid syndrome.3,34 Anti-apolipoprotein A-1 IgG associates with an increased systemic inflammatory response and major CV events, a finding that may reflect increased plaque vulnerability under these circumstances.48
Complement components and the adipocytokines leptin and resistin have also engendered interest as pro-inflammatory injurious factors, while adiponectin may exert anti-inflammatory effects on vascular endothelium.49 In a variety of rheumatic diseases, leukocytes, platelets, and endothelial cells can release extracellular vesicles including exosomes and microparticles. Their ability to transport micro-RNA, auto-antigens, damage-associated molecular patterns, pro-inflammatory cytokines, and matrix metalloproteases may contribute to increased atherosclerotic plaque inflammation and vulnerability in patients with systemic inflammatory disease.50 Thus, extracellular vesicles have the potential to act as biomarkers of endothelial injury and modifying their content and release may prove to be therapeutically important.50,51
Traditional risk factors and atherogenesis in rheumatoid arthritis and systemic lupus erythematosus
As expected, traditional CV risk factors appear to contribute to atherogenesis in patients with systemic inflammatory diseases (Figure 2). Yet, the relative contribution of specific risk factors remains uncertain. One recent study of micro- and macrovascular function in RA has suggested that traditional CV risk factors influence endothelial function more than disease-related inflammation.52 Adjustment for traditional risk factors revealed a significant disease-specific effect in the pathogenesis of accelerated atherosclerosis in both RA and SLE.42,53 The systemic and vascular inflammation may act synergistically with traditional risk factors to promote atherosclerosis in patients with RA or SLE (Figure 2).
Patients with RA or SLE have a higher burden of traditional risk factors than the general population. Tobacco smoking associates with both CV risk and the development of RA. Disability caused by RA can limit the ability to exercise. Elevated TNFα levels in RA patients may promote insulin resistance, which together with physical inactivity can favour development of the ‘metabolic syndrome’ risk factor cluster.34 However, the metabolic syndrome may not influence CV risk beyond its individual components. Patients with RA may have dyslipidaemia characterized by increased triglycerides, decreased total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C).54 Moreover, in both RA and SLE, a putative pro-inflammatory form of HDL-C (piHDL) increases.55 The pro-inflammatory form of HDL-C reportedly promotes LDL oxidation and the development of foam cells. Patients with SLE commonly have hypertension, likely contributed to by renal involvement and the use of corticosteroid therapy. The heightened prevalence of insulin resistance and the metabolic syndrome cluster in SLE patients may also relate to renal impairment and higher corticosteroid doses.56
Outcomes
Optimizing CV prevention and care in this patient population should involve close liaison between cardiologists and rheumatologists.57 Currently, RA patients with MI may less likely receive acute reperfusion therapy and secondary prevention measures and have worse outcomes than other MI patients.58 Furthermore, although patients with RA have increased risk for heart failure, which may result in part from diastolic dysfunction, they typically receive less aggressive investigation and management.59
In SLE, despite a similar anatomic distribution of atherosclerosis to non-SLE patients, those with SLE may harbour more inflamed plaques considered more likely to cause thrombotic complications. Indeed, in experimental atherosclerosis, systemic or remote inflammation elicits an ‘echo’ of increased inflammation in the arterial lesions.60,61 Rheumatoid arthritis or SLE increases the risk of mortality post MI.62 Patients with SLE have poorer outcomes post percutaneous coronary interventions according to registry data, yielding a significantly increased risk of subsequent MI.63 The poor CV prognosis in SLE may also reflect late diagnosis and a reluctance to treat immunosuppressed patients aggressively.
Treatment
The last 15 years has witnessed a remarkable transformation in drug therapy for many systemic inflammatory diseases. The use of combination disease-modifying anti-rheumatic drug (DMARD) therapy has increased, and a variety of biologic agents have become available.64 Although these treatments produce clear benefits with respect to primary disease complications including rheumatoid erosions and lupus nephritis, they have shown a less dramatic impact on CV disease (Table 2). The limited long-term data have not conclusively demonstrated reduced CV events,34,65 save for low-dose MTX.66 This lack of evidence may reflect in part the low incidence of events and hence the need for large, long-term studies. Registries of patients treated with biologic agents in a variety of countries may prove informative in this regard. With respect to anti-TNFα and anti-IL-6R strategies, an aggravation of dyslipidaemia may mitigate the anti-inflammatory effect of these agents with respect to atherosclerosis. In addition, anti-TNFα agents did not prove beneficial in patients with heart failure in large clinical trials. Indeed, some data indicate an increase in CV events in heart failure patients treated with anti-TNFα drugs.67,68
Table 2.
Anti-rheumatic drugs and cardiovascular risk
| Agent | Effect on risk biomarkers | Effect on CV outcome |
|---|---|---|
| Glucocorticoids | ↑BP, ↑TG, ↑glucose, ↓CRP | Prolonged high dose: worsen40,41,47 Suppression of active SLE protective39 |
| NSAIDs/COXIBs | ↑BP, ↑thrombosis risk, ↓renal function | Worsen. May improve in RA83–85 |
| MTX | ↓CRP, ↑adenosine | ↓Risk in observational studies66 |
| Mycophenolate | ↓CRP and plaque inflammation98 | Minimal data47 |
| Hydroxychloroquine | ↓LDL, ↓thrombosis risk | Reduced risk in RA and SLE64 |
| Anti-TNFα | ↓CRP, ↑LDL, ↑TG54 | Worsens cardiac failure. ↓May MI risk67,68,93,94 |
| Anti-IL-6 | ↓CRP, ↓FN, ↑LDL, ↑TG54 | No data64 |
| Anti-IL-1 | ↓CRP, ↓FN, ↓IL-664 | No data, study in progress |
| B-cell depletion | Long-term treatment may ↓LDL64 | No data |
| Cyclosporine | ↑BP, ↑LDL, ↓renal function | Worsen |
BP, blood pressure; TG, triglycerides; LDL, low-density lipoprotein; CRP, C-reactive protein; NSAIDs, traditional non-steroidal anti-inflammatory drugs; COXIBs, COX-2 selective anti-inflammatory drugs; RA, rheumatoid arthritis; MTX, Methotrexate; SLE, systemic lupus erythematosus; FN, fibrinogen; IL-6, interleukin-6.
Advances in therapy would benefit from further understanding of disease-specific pathways involved in vascular injury and accelerated atherosclerosis. Moreover, the development and use of novel imaging and biomarker approaches could facilitate the identification of patients most at risk of CV disease and hasten the development and evaluation of new therapeutic strategies.
Traditional risk factors
Acknowledging that we lack sufficiently powered intervention trials in patients with RA or SLE, given their heightened CV risk they should receive aggressive management of conventional CV risk factors including cessation of smoking, control of weight and blood pressure, prevention/treatment of diabetes mellitus, and encouragement to engage in physical activity consistent with ability. Hypertension commonly complicates many rheumatic diseases including SLE, Takayasu arteritis (TA), SSc, and anti-neutrophil cytoplasmic antibody (ANCA) vasculitis and should receive aggressive treatment. In the absence of renal artery stenosis and provided the renal function allows, angiotensin converting enzyme inhibitors or receptor blockers are favoured, and many patients require additional therapy including vasodilators such as calcium channel antagonists. Some current CVD guidelines support the use of prophylactic anti-platelet medication in rheumatic disease patients.69 Effective treatment of the primary inflammatory disease may mitigate dyslipidaemia, and some have proposed inclusion of the total cholesterol to HDL-C ratio as an appropriate measure for CV risk assessment in this population.54 Ideally, disease-specific prediction tools should guide CV risk management. The availability and validation of such instruments, however, remain limited. Current CV risk models appear to underestimate risk in the RA population.70 The EULAR guidelines for inflammatory arthritis have suggested adding a 1.5× multiplier to standard CV risk calculations (mSCORE).71 Some have suggested the addition of carotid artery US analysis,57 particularly in those with a moderate mSCORE.72 Endothelial dysfunction and aortic stiffness also predict CV risk and may merit inclusion.73 As in the case of all risk prediction calculators, such instruments should undergo rigorous prospective validation.
Despite the overwhelming evidence for the benefit of statins for CV risk reduction in broad patient categories, we lack specific clinical trial evidence to support the routine use of statins in RA and SLE. In RA, recommended indications include an LDL-C ≥190 mg/dL (a somewhat conservative measure), a long history of RA, a family history of IHD or hyperlipidaemia, older age at disease onset and the presence of any other CV risk factor.74 In SLE, some have proposed an LDL-C target of <100 mg/dL, with statins the first choice therapy.75 Nonetheless, statins have proved disappointing for CV event prevention in SLE, showing no benefit in primary or secondary endpoints in adult patients,76 and no reduction in carotid intima-media thickness in a paediatric trial.77
Treatment approaches in rheumatoid arthritis
Methotrexate is the most frequently used DMARD in the treatment of RA and has had remarkable impact since becoming widely used in the 1990s. The efficacy of MTX against RA-related CV disease remains undefined. An initial retrospective cohort study found that RA patients with known CV disease who subsequently started MTX had a higher risk of death during follow-up.78 Despite this, subsequent clinical data and current opinion suggests that anti-inflammatory actions of MTX reduce the risk of CV disease and associated mortality.66,79–81 Mechanistically, MTX may alleviate the dyslipidaemic profile associated with RA and in vitro limits foam cell development through promotion of macrophage cholesterol efflux.54 A large-scale CV outcome study, the Cardiovascular Inflammation Reduction Trial, funded by the US National Institutes of Health, is evaluating the efficacy of weekly low-dose MTX in CV event reduction in MI survivors already receiving standard of care medication including high-dose statins but with residual features of inflammation indicated by the presence of elements of the metabolic syndrome cluster. Although this study will not enrol patients with RA or SLE, its results may nonetheless provide insight into the role of anti-inflammatory therapy in the prevention of recurrent CV events.82
The role of non-steroidal anti-inflammatory drugs (NSAIDs) and cyclo-oxygenase-2 selective antagonists (COXIBs) in RA has diminished. These agents cause a dose-dependent risk of CV complications. A recent network meta-analysis suggested that no traditional NSAID or COX-2 inhibitor is entirely safe and that naproxen has the best CV profile due to its anti-platelet effects.83 Of note, in inflammatory arthritis, use of NSAIDs was not associated with an increased risk of mortality, and in fact reduces CV risk and mortality.84,85 An ongoing large-scale critical trial is evaluating the CV safety of various NSAIDs in patients with rheumatoid and osteoarthritis.86
Corticosteroid therapy and accelerated atherosclerosis have a complex relationship. Corticosteroids increase insulin resistance, the risk of metabolic syndrome and hypertension, disturb the lipid profile and may promote CV disease in RA.16 In contrast, in RA patients with pre-existing IHD, corticosteroid therapy associated with a reduced risk of CV death.87 Insufficient use of corticosteroids and persistent disease activity may increase the risk of CV disease. Current ‘treat to target’ paradigms, employing combination DMARDs and biologic therapy in those with persistent disease activity, should minimize corticosteroid requirement in the treatment of RA. Nonetheless, the impact of this approach on CV complications remains uncertain.
Biologic agents
The introduction of TNFα antagonists transformed the management of RA and provided a catalyst for the development of further targeted biologics. Tumour necrosis factor α blockade improves endothelial function20 and reduces aortic stiffness,22,88 although effects on these biomarkers vary89 and may have limited durability.90 Long-term data demonstrating a reduction in CV events are sparse, and some studies reported no significant effect.91,92 Registry data show that RA patients responding to anti-TNFα therapy have a lower risk of future MI than non-responders.93 A meta-analysis of both observational cohorts and randomized controlled trials suggests that TNFα blockade reduces the risk of MI, congestive cardiac failure, and stroke, while noting that studies lack sufficient power and include CV events as secondary endpoints only.94 A retrospective review of 2000 patients suggests patients starting TNFα inhibitors have a reduced risk of CV events for 6 months when compared with those prescribed a conventional DMARD,92 while a survey of 7704 patients revealed a significant reduction in risk of acute coronary syndrome in anti-TNFα-treated patients vs. the biologic naive group.95 Abatacept, rituximab, tocilizumab, and the janus kinase inhibitor tofacitinib are now all licensed for the treatment of RA. The rigorous determination of the effects of all agents on CV outcomes will require randomized controlled trials with defined and adjudicated CV endpoints.
Treatment approaches in systemic lupus erythematosus
Despite significant improvements in SLE therapies, their impact on CV risk remains uncertain. Hydroxychloroquine, currently widely prescribed, has lipid-lowering effects, reduces the risk of thrombovascular events, and associates with reduced plaque burden and improved survival.47 Although under-treatment of SLE must be avoided as this increases the risk of CV events,39 EULAR recommends that the minimal dose of corticosteroids possible should be used. High cumulative doses may associate with raised total cholesterol and increased atherosclerosis (Table 2).47 Current use however seems to confer the maximal risk.41
Of the immunosuppressant drugs used in SLE, mycophenolate mofetil (MMF), a purine biosynthesis antagonist, has received most attention with respect to potential CV benefits. In mice with experimental SLE and atherosclerosis, MMF reduced oxidative stress, CD4+ T-cell recruitment and attenuated atherogenesis.96,97 Of note, atorvastatin failed to do this in the same animals, indicating that statin treatment alone may not provide optimum CV protection.97 In clinical studies, short-term treatment with MMF in patients with primary atherosclerosis and carotid artery stenosis, reduced inflammatory gene expression and T-cell activation in plaques.98 Although, a 2-year longitudinal cohort study in SLE using carotid IMT and coronary calcification as endpoints did not demonstrate an effect of MMF, only 25 patients received the drug and at variable doses.99 The available data do not permit conclusions concerning the effects of azathioprine and MTX on CV events in SLE. The results of prospective studies with dedicated CV endpoints are awaited with interest and these include trials of IFN-α antagonists and new B-cell-targeted therapies.
The vasculitides
Accelerated atherosclerosis and premature CV death has also been associated with the large, medium, and small vessel systemic vasculitides, the classification of which has recently undergone revision.100 These conditions associate with accentuated traditional risk factors including hypertension, dyslipidaemia, and insulin resistance, as well as with indices of vascular dysfunction.8 Both humoral and cellular immune mechanisms likely contribute to vasculitis pathogenesis. Hence, the vasculitides may involve multiple mechanisms of vascular injury, both distinct from and shared with those implicated in RA and SLE (Figure 2).101
The ANCA-associated vasculitides (AAV) comprise granulomatous polyangiitis (Wegener granulomatosis), microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome). Anti-neutrophil cytoplasmic antibodies bind proteinase-3 or myeloperoxidase and identify distinct autoimmune syndromes.102 These antibodies may directly activate TNFα-primed neutrophils leading to a respiratory burst, generation of reactive oxygen species and subsequent endothelial damage. Immune complexes contribute to pathogenesis by fixing complement and by binding to neutrophil Fcγ receptors and activating neutrophils.101,103 The resultant systemic inflammatory response associates with endothelial dysfunction and increased aortic stiffness, and immunosuppression or TNFα blocker treatment may improve these biomarkers.104–106 Associated vasculitides may follow a bimodal mortality pattern, with the second peak due to CV disease and malignancy. The risk of coronary heart disease increases up to four-fold and the relapsing and remitting nature of the AAV's may accelerate atherogenesis.107,108
The predominant large vessel vasculitides in the adult are giant cell arteritis (GCA), most common in the 6th decade and beyond, and TA that usually presents in those <40 years. Accelerated atherosclerosis and increased premature mortality may occur in TA.109,110 Patients with TA have increased aortic stiffness,111 early plaque and evidence of silent MI.112 In addition to direct vasculitic injury to the arterial wall and pro-atherogenic effects of glucocorticoid therapy, the accelerated atherosclerosis may reflect distorted arterial anatomy leading to disturbed arterial blood flow shear stress patterns associated with pro-inflammatory changes in vascular endothelium.113 Plaque in common carotid arteries, a site normally protected against atherosclerosis, was only seen in those TA patients with documented common carotid arteritis and increased intima-medial thickness.110 Up to 40% of patients have demonstrable coronary artery abnormalities. These lesions comprise coronary arteritis with stenosis, typically ostial and non-calcified, and secondary atherosclerotic plaques typically with an irregular angiographic appearance and often calcified.7
In GCA, although not associated with increased long-term mortality,114,115 an observational cohort study has identified a short-term increased risk of CV disease, when compared with the age-matched general population.116 The predominant risk was within the first 2 months of diagnosis, with a two-fold risk of MI sustained up to 2 years. Likewise, a case–control study revealed increased early mortality.117 Giant cell arteritis does not associate with accelerated atherosclerosis, and the increased short-term mortality seen likely relates to active arteritis, ischaemic complications of GCA, and adverse effects of glucocorticoid therapy.118
Gout
Another rheumatologic condition, gout, affects up to 2% of individuals. Classical observational studies implicated gout and hyperuricemia as risk factors for atherosclerosis. In the Framingham Study gout associated with a 60% excess in coronary heart disease in men, but not in women, independent of traditional risk factors or diuretic use.119 Interrogation of the US National Health and Nutrition Examination Survey showed an ∼60% increase in risk for CV mortality in those with a history of gout.120 This analysis also found a stepwise increase in CV mortality with uric acid concentrations in blood. A prospective examination of the Health Professionals Follow-up Study confirmed an ∼60% increase in risk of fatal CHD coronary heart disease in men with a history of gout and a prior history of CV disease.121 Several recent meta-analyses have confirmed an independent association between gout and increased CV risk.122,123 The relationship of gout with the risk of CV events in women requires further study.124,125 Uric acid crystals activate the NLRP3 inflammasome, a supramolecular complex within cells that generates the active form of the prominent pro-inflammatory cytokine IL-1β.126 Thus, a strong pathophysiologic underpinning provides biologic plausibility for the association of gout and hyperuricemia with increased CV risk.
Conclusion
Increasing scientific and clinical appreciation of the roles of inflammation and immunity in atherosclerosis and myocardial disease furnishes a mechanistic connection between the heightened risk of arteriosclerotic CV events and rheumatic diseases. Study of extreme cases of systemic inflammatory vascular disease (e.g. TA) may provide novel windows into the pathophysiology of atherosclerotic CV disease in non-rheumatologic populations. In a similar manner, study of the mechanisms that provoke accelerated arteriosclerosis in solid organ allografts has illuminated the role of the adaptive immunity in usual atherosclerosis. Various pathophysiologic concepts originating from the study of rheumatic diseases have inspired pathophysiologic studies in the CV arena. For example, the role of matrix metalloproteinases in connective tissue breakdown in atherosclerotic plaques and in the remodelling of the left ventricle after MI has fundamental similarities with mechanisms often invoked in the joint destruction of RA. Anti-inflammatory therapies under exploration in CV patients have received inspiration from pioneering efforts in patients with rheumatologic diseases, for example, the attempt to lower atherosclerotic risk with MTX.
From a clinical perspective, gaps exist which require the attention of the medical community at large and rheumatologists and CV specialists in particular. The findings summarized in this review remind us that CV disease causes much morbidity and mortality in patients with rheumatic diseases, and that these individuals often do not receive appropriate management to lower vascular risk. This recognition requires aggressive treatment of conventional CV risk factors, and calls for additional investigation of ways to mitigate excess risk. The extent to which the anti-inflammatory therapies used to treat rheumatic diseases will improve CV outcomes requires further study. Whether we need to develop and validate novel biomarkers of risk to inform the intensity of therapy required to lower the CV risk of patients with rheumatologic diseases requires rigorous examination in clinical trials. Development and validation of optimal strategies for monitoring CV disease markers in patients with rheumatic diseases also requires an evidence-based approach. These strategies should strive to avoid complications associated with unnecessary invasive evaluation, prevent over-testing, and minimize imaging radiation exposure, as well as conserve healthcare resources.
Ultimately, close coordination between care-providers for patients with rheumatologic diseases should improve CV outcomes for this important population. The convergence of mechanisms shared by rheumatic and CV diseases should continue to shed light on fundamental mechanistic as well as therapeutic advances and help to address the unmet medical need for these patients.
Funding
J.C.M. acknowledges funding from the Imperial College London National Institute for Health Research Biomedical Research Centre funding scheme and the British Heart Foundation. P.L. acknowledges funding from the US National Institutes of Health grant HL080472.
Conflict of interest: none declared.
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Cobb S, Anderson F, Bauer W. Length of life and cause of death in rheumatoid arthritis. N Engl J Med. 1953;249:553–556. doi: 10.1056/NEJM195310012491402. [DOI] [PubMed] [Google Scholar]
- 3.Hollan I, Meroni PL, Ahearn JM, Cohen Tervaert JW, Curran S, Goodyear CS, Hestad KA, Kahaleh B, Riggio M, Shields K, Wasko MC. Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev. 2013;12:1004–1015. doi: 10.1016/j.autrev.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Ungprasert P, Charoenpong P, Ratanasrimetha P, Thongprayoon C, Cheungpasitporn W, Suksaranjit P. Risk of coronary artery disease in patients with systemic sclerosis: a systematic review and meta-analysis. Clin Rheumatol. 2014;33:1099–1104. doi: 10.1007/s10067-014-2681-4. [DOI] [PubMed] [Google Scholar]
- 5.Van Gelder H, Charles-Schoeman C. The heart in inflammatory myopathies. Rheum Dis Clin North Am. 2014;40:1–10. doi: 10.1016/j.rdc.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation. 2014;130:837–844. doi: 10.1161/CIRCULATIONAHA.114.009990. [DOI] [PubMed] [Google Scholar]
- 7.Soto ME, Melendez-Ramirez G, Kimura-Hayama E, Meave-Gonzalez A, Achenbach S, Herrera MC, Guering EL, Alexanderson-Rosas E, Reyes PA. Coronary CT angiography in Takayasu arteritis. JACC Cardiovasc Imaging. 2011;4:958–966. doi: 10.1016/j.jcmg.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Tervaert JWC. Cardiovascular disease due to accelerated atherosclerosis in systemic vasculitides. Best Prac Res Clin Rheum. 2013;27:33–44. doi: 10.1016/j.berh.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Evans MR, Escalante A, Battafarano DF, Freeman GL, O'Leary DH, del Rincon I. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211–1220. doi: 10.1002/art.30265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitas GD, Gabriel SE. Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis. 2011;70:8–14. doi: 10.1136/ard.2010.142133. [DOI] [PubMed] [Google Scholar]
- 11.Libby P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am J Med. 2008;121:S21–S31. doi: 10.1016/j.amjmed.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Mason JC. Rheumatic diseases and the cardiovascular system. In: Mann DL, Zipes DP, Libby P, editors. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 10th ed. Philadelphia: Elsevier; 2014. [Google Scholar]
- 13.Meune C, Touze E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2009;48:1309–1313. doi: 10.1093/rheumatology/kep252. [DOI] [PubMed] [Google Scholar]
- 14.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 15.Stamatelopoulos KS, Kitas GD, Papamichael CM, Chryssohoou E, Kyrkou K, Georgiopoulos G, Protogerou A, Panoulas VF, Sandoo A, Tentolouris N, Mavrikakis M, Sfikakis PP. Atherosclerosis in rheumatoid arthritis versus diabetes: a comparative study. Arterioscler Thromb Vasc Biol. 2009;29:1702–1708. doi: 10.1161/ATVBAHA.109.190108. [DOI] [PubMed] [Google Scholar]
- 16.Gabriel SE. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am J Med. 2008;121:S9–S14. doi: 10.1016/j.amjmed.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodson NJ, Wiles NJ, Lunt M, Barrett EM, Silman AJ, Symmons DP. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum. 2002;46:2010–2019. doi: 10.1002/art.10419. [DOI] [PubMed] [Google Scholar]
- 18.Wallberg-Jonsson S, Johansson H, Ohman ML, Rantapaa-Dahlqvist S. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis. A retrospective cohort study from disease onset. J Rheumatol. 1999;26:2562–2571. [PubMed] [Google Scholar]
- 19.Herbrig K, Haensel S, Oelschlaegel U, Pistrosch F, Foerster S, Passauer J. Endothelial dysfunction in patients with rheumatoid arthritis is associated with a reduced number and impaired function of endothelial progenitor cells. Ann Rheum Dis. 2006;65:157–163. doi: 10.1136/ard.2005.035378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O, Bechir M, Spieker LE, Neidhart M, Michel BA, Gay RE, Luscher TF, Gay S, Ruschitzka F. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106:2184–2187. doi: 10.1161/01.cir.0000037521.71373.44. [DOI] [PubMed] [Google Scholar]
- 21.Bergholm R, Leirisalo-Repo M, Vehkavaara S, Makimattila S, Taskinen MR, Yki-Jarvinen H. Impaired responsiveness to NO in newly diagnosed patients with rheumatoid arthritis. Arterioscler Thromb Vasc Biol. 2002;22:1637–1641. doi: 10.1161/01.atv.0000033516.73864.4e. [DOI] [PubMed] [Google Scholar]
- 22.Maki-Petaja KM, Hall FC, Booth AD, Wallace SM, Yasmin, Bearcroft PW, Harish S, Furlong A, McEniery CM, Brown J, Wilkinson IB. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation. 2006;114:1185–1192. doi: 10.1161/CIRCULATIONAHA.105.601641. [DOI] [PubMed] [Google Scholar]
- 23.Giles JT, Post WS, Blumenthal RS, Polak J, Petri M, Gelber AC, Szklo M, Bathon JM. Longitudinal predictors of progression of carotid atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2011;63:3216–3225. doi: 10.1002/art.30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Juanatey C, Llorca J, Gonzalez-Gay MA. Correlation between endothelial function and carotid atherosclerosis in rheumatoid arthritis patients with long-standing disease. Arthritis Res Ther. 2011;13:R101. doi: 10.1186/ar3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radovits BJ, Fransen J, Al Shamma S, Eijsbouts AM, van Riel PL, Laan RF. Excess mortality emerges after 10 years in an inception cohort of early rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62:362–370. doi: 10.1002/acr.20105. [DOI] [PubMed] [Google Scholar]
- 26.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524–1529. doi: 10.1136/annrheumdis-2011-200726. [DOI] [PubMed] [Google Scholar]
- 27.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 28.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, Gabriel SE. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52:402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 29.Aubry MC, Maradit-Kremers H, Reinalda MS, Crowson CS, Edwards WD, Gabriel SE. Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. J Rheumatol. 2007;34:937–942. [PubMed] [Google Scholar]
- 30.Semb AG, Rollefstad S, Provan SA, Kvien TK, Stranden E, Olsen IC, Hisdal J. Carotid plaque characteristics and disease activity in rheumatoid arthritis. J Rheumatol. 2013;40:359–368. doi: 10.3899/jrheum.120621. [DOI] [PubMed] [Google Scholar]
- 31.Maki-Petaja KM, Elkhawad M, Cheriyan J, Joshi FR, Ostor AJ, Hall FC, Rudd JH, Wilkinson IB. Anti-tumor necrosis factor-alpha therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126:2473–2480. doi: 10.1161/CIRCULATIONAHA.112.120410. [DOI] [PubMed] [Google Scholar]
- 32.Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60:221–225. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 33.Nossent J, Cikes N, Kiss E, Marchesoni A, Nassonova V, Mosca M, Olesinska M, Pokorny G, Rozman B, Schneider M, Vlachoyiannopoulos PG, Swaak A. Current causes of death in systemic lupus erythematosus in Europe, 2000–2004: relation to disease activity and damage accrual. Lupus. 2007;16:309–317. doi: 10.1177/0961203307077987. [DOI] [PubMed] [Google Scholar]
- 34.Kahlenberg JM, Kaplan MJ. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu Rev Med. 2013;64:249–263. doi: 10.1146/annurev-med-060911-090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters MJ, Nurmohamed MT. Cardiovascular risk management in rheumatoid arthritis: are we still waiting for the first step? Arthritis Res Ther. 2013;15:111. doi: 10.1186/ar4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Magadmi M, Bodill H, Ahmad Y, Durrington PN, Mackness M, Walker M, Bernstein RM, Bruce IN. Systemic lupus erythematosus: an independent risk factor for endothelial dysfunction in women. Circulation. 2004;110:399–404. doi: 10.1161/01.CIR.0000136807.78534.50. [DOI] [PubMed] [Google Scholar]
- 37.Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30:1837–1843. doi: 10.1093/eurheartj/ehp205. [DOI] [PubMed] [Google Scholar]
- 38.Bruce IN, Burns RJ, Gladman DD, Urowitz MB. Single photon emission computed tomography dual isotope myocardial perfusion imaging in women with systemic lupus erythematosus. I. Prevalence and distribution of abnormalities . J Rheumatol. 2000;27:2372–2377. [PubMed] [Google Scholar]
- 39.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, Crow MK, Schwartz JE, Paget SA, Devereux RB, Salmon JE. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 40.Salmon JE, Roman MJ. Subclinical atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Am J Med. 2008;121:S3–S8. doi: 10.1016/j.amjmed.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magder LS, Petri M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am J Epidemiol. 2012;176:708–719. doi: 10.1093/aje/kws130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, Cote R, Grover SA, Fortin PR, Clarke AE, Senecal JL. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 43.Hak AE, Karlson EW, Feskanich D, Stampfer MJ, Costenbader KH. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses’ health study. Arthritis Rheum. 2009;61:1396–1402. doi: 10.1002/art.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, D'Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 45.Mok CC, Ho LY, To CH. Annual incidence and standardized incidence ratio of cerebrovascular accidents in patients with systemic lupus erythematosus. Scand J Rheumatol. 2009;38:362–368. doi: 10.1080/03009740902776927. [DOI] [PubMed] [Google Scholar]
- 46.Fischer LM, Schlienger RG, Matter C, Jick H, Meier CR. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first-time acute myocardial infarction. Am J Cardiol. 2004;93:198–200. doi: 10.1016/j.amjcard.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 47.Skaggs BJ, Hahn BH, McMahon M. Accelerated atherosclerosis in patients with SLE – mechanisms and management. Nat Rev Rheumatol. 2012;8:214–223. doi: 10.1038/nrrheum.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vuilleumier N, Bas S, Pagano S, Montecucco F, Guerne PA, Finckh A, Lovis C, Mach F, Hochstrasser D, Roux-Lombard P, Gabay C. Anti-apolipoprotein A-1 IgG predicts major cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheum. 2010;62:2640–2650. doi: 10.1002/art.27546. [DOI] [PubMed] [Google Scholar]
- 49.Montecucco F, Mach F. Common inflammatory mediators orchestrate pathophysiological processes in rheumatoid arthritis and atherosclerosis. Rheumatology (Oxford) 2009;48:11–22. doi: 10.1093/rheumatology/ken395. [DOI] [PubMed] [Google Scholar]
- 50.Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10:356–364. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 51.Loyer X, Vion AC, Tedgui A, Boulanger CM. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res. 2014;114:345–353. doi: 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]
- 52.Sandoo A, Kitas GD, Carroll D, Veldhuijzen van Zanten JJ. The role of inflammation and cardiovascular disease risk on microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: a cross-sectional and longitudinal study. Arthritis Res Ther. 2012;14:R117. doi: 10.1186/ar3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 54.Robertson J, Peters MJ, McInnes IB, Sattar N. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat Rev Rheumatol. 2013;9:513–523. doi: 10.1038/nrrheum.2013.91. [DOI] [PubMed] [Google Scholar]
- 55.McMahon M, Grossman J, FitzGerald J, Dahlin-Lee E, Wallace DJ, Thong BY, Badsha H, Kalunian K, Charles C, Navab M, Fogelman AM, Hahn BH. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54:2541–2549. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- 56.Parker B, Urowitz MB, Gladman DD, Lunt M, Bae SC, Sanchez-Guerrero J, Romero-Diaz J, Gordon C, Wallace DJ, Clarke AE, Bernatsky S, Ginzler EM, Isenberg DA, Rahman A, Merrill JT, Alarcon GS, Fessler BJ, Fortin PR, Hanly JG, Petri M, Steinsson K, Dooley MA, Manzi S, Khamashta MA, Ramsey-Goldman R, Zoma AA, Sturfelt GK, Nived O, Aranow C, Mackay M, Ramos-Casals M, van Vollenhoven RF, Kalunian KC, Ruiz-Irastorza G, Lim S, Kamen DL, Peschken CA, Inanc M, Bruce IN. Clinical associations of the metabolic syndrome in systemic lupus erythematosus: data from an international inception cohort. Ann Rheum Dis. 2012;72:1308–1314. doi: 10.1136/annrheumdis-2012-202106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mankad R, Gabriel SE. Rheumatoid arthritis: Treating cardiovascular risk in RA requires multidisciplinary care. Nat Rev Rheumatol. 2014;10:202–204. doi: 10.1038/nrrheum.2014.37. [DOI] [PubMed] [Google Scholar]
- 58.Van Doornum S, Brand C, Sundararajan V, Ajani AE, Wicks IP. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther. 2010;12:R183. doi: 10.1186/ar3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis JM, III, Roger VL, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603–2611. doi: 10.1002/art.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fleet JC, Clinton SK, Salomon RN, Loppnow H, Libby P. Atherogenic diets enhance endotoxin-stimulated interleukin-1 and tumor necrosis factor gene expression in rabbit aortae. J Nutr. 1992;122:294–305. doi: 10.1093/jn/122.2.294. [DOI] [PubMed] [Google Scholar]
- 62.Shah MA, Shah AM, Krishnan E. Poor outcomes after acute myocardial infarction in systemic lupus erythematosus. J Rheumatol. 2009;36:570–575. doi: 10.3899/jrheum.080373. [DOI] [PubMed] [Google Scholar]
- 63.Maksimowicz-McKinnon K, Selzer F, Manzi S, Kip KE, Mulukutla SR, Marroquin OC, Smitherman TC, Kuller LH, Williams DO, Wasko MC. Poor 1-year outcomes after percutaneous coronary interventions in systemic lupus erythematosus: report from the National Heart, Lung, and Blood Institute Dynamic Registry. Circ Cardiovasc Interv. 2008;1:201–208. doi: 10.1161/CIRCINTERVENTIONS.108.788745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gasparyan AY, Ayvazyan L, Cocco G, Kitas GD. Adverse cardiovascular effects of antirheumatic drugs: implications for clinical practice and research. Curr Pharm Des. 2012;18:1543–1555. doi: 10.2174/138161212799504759. [DOI] [PubMed] [Google Scholar]
- 65.Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol. 2011;7:399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 66.Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernan MA, Ridker PM, Mozaffarian D. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362–1370. doi: 10.1016/j.amjcard.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 68.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 69.Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ. Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL, editors. Task Force M, Guidelines ESCCfP. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 70.Arts EE, Popa C, Den Broeder AA, Semb AG, Toms T, Kitas GD, van Riel PL, Fransen J. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204024. E-pub Jan] [DOI] [PubMed] [Google Scholar]
- 71.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, McInnes IB, Haentzschel H, Gonzalez-Gay MA, Provan S, Semb A, Sidiropoulos P, Kitas G, Smulders YM, Soubrier M, Szekanecz Z, Sattar N, Nurmohamed MT. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–331. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 72.Corrales A, Gonzalez-Juanatey C, Peiro ME, Blanco R, Llorca J, Gonzalez-Gay MA. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: results of a population-based study. Ann Rheum Dis. 2014;73:722–727. doi: 10.1136/annrheumdis-2012-203101. [DOI] [PubMed] [Google Scholar]
- 73.Maki-Petaja KM, Wilkinson IB. Arterial Stiffness and inflammation – a potential target for drug therapy. Artery Res. 2010;4:99–107. [Google Scholar]
- 74.Bisoendial RJ, Stroes ES, Kastelein JJ, Tak PP. Targeting cardiovascular risk in rheumatoid arthritis: a dual role for statins. Nat Rev Rheumatol. 2010;6:157–164. doi: 10.1038/nrrheum.2009.277. [DOI] [PubMed] [Google Scholar]
- 75.Stojan G, Petri M. Atherosclerosis in systemic lupus erythematosus. J Cardiovasc Pharmacol. 2013;62:255–262. doi: 10.1097/FJC.0b013e31829dd857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petri MA, Kiani AN, Post W, Christopher-Stine L, Magder LS. Lupus atherosclerosis prevention study (LAPS) Ann Rheum Dis. 2011;70:760–765. doi: 10.1136/ard.2010.136762. [DOI] [PubMed] [Google Scholar]
- 77.Schanberg LE, Sandborg C, Barnhart HX, Ardoin SP, Yow E, Evans GW, Mieszkalski KL, Ilowite NT, Eberhard A, Imundo LF, Kimura Y, von Scheven E, Silverman E, Bowyer SL, Punaro M, Singer NG, Sherry DD, McCurdy D, Klein-Gitelman M, Wallace C, Silver R, Wagner-Weiner L, Higgins GC, Brunner HI, Jung L, Soep JB, Reed AM, Provenzale J, Thompson SD. Use of atorvastatin in systemic lupus erythematosus in children and adolescents. Arthritis Rheum. 2012;64:285–296. doi: 10.1002/art.30645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Landewe RB, van den Borne BE, Breedveld FC, Dijkmans BA. Methotrexate effects in patients with rheumatoid arthritis with cardiovascular comorbidity. Lancet. 2000;355:1616–1617. doi: 10.1016/S0140-6736(00)02222-4. [DOI] [PubMed] [Google Scholar]
- 79.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–1177. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 80.van Halm VP, Nurmohamed MT, Twisk JW, Dijkmans BA, Voskuyl AE. Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther. 2006;8:R151. doi: 10.1186/ar2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E, Ostor AJ, Edwards CJ. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2010;49:295–307. doi: 10.1093/rheumatology/kep366. [DOI] [PubMed] [Google Scholar]
- 82.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207 e115. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Juni P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goodson NJ, Brookhart AM, Symmons DP, Silman AJ, Solomon DH. Non-steroidal anti-inflammatory drug use does not appear to be associated with increased cardiovascular mortality in patients with inflammatory polyarthritis: results from a primary care based inception cohort of patients. Ann Rheum Dis. 2009;68:367–372. doi: 10.1136/ard.2007.076760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lindhardsen J, Gislason GH, Jacobsen S, Ahlehoff O, Olsen AM, Madsen OR, Torp-Pedersen C, Hansen PR. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: a nationwide cohort study. Ann Rheum Dis. 2014;73:1515–1521. doi: 10.1136/annrheumdis-2012-203137. [DOI] [PubMed] [Google Scholar]
- 86.Becker MC, Wang TH, Wisniewski L, Wolski K, Libby P, Luscher TF, Borer JS, Mascette AM, Husni ME, Solomon DH, Graham DY, Yeomans ND, Krum H, Ruschitzka F, Lincoff AM, Nissen SE. Rationale, design, and governance of Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen Or Naproxen (PRECISION), a cardiovascular end point trial of nonsteroidal antiinflammatory agents in patients with arthritis. Am Heart J. 2009;157:606–612. doi: 10.1016/j.ahj.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 87.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–732. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 88.Angel K, Provan SA, Fagerhol MK, Mowinckel P, Kvien TK, Atar D. Effect of 1-year anti-TNF-alpha therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. Am J Hypertens. 2012;25:644–650. doi: 10.1038/ajh.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Doornum S, McColl G, Wicks IP. Tumour necrosis factor antagonists improve disease activity but not arterial stiffness in rheumatoid arthritis. Rheumatology (Oxford) 2005;44:1428–1432. doi: 10.1093/rheumatology/kei033. [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez-Juanatey C, Testa A, Garcia-Castelo A, Garcia-Porrua C, Llorca J, Gonzalez-Gay MA. Active but transient improvement of endothelial function in rheumatoid arthritis patients undergoing long-term treatment with anti-tumor necrosis factor alpha antibody. Arthritis Rheum. 2004;51:447–450. doi: 10.1002/art.20407. [DOI] [PubMed] [Google Scholar]
- 91.Ljung L, Simard JF, Jacobsson L, Rantapaa-Dahlqvist S, Askling J. Treatment with tumor necrosis factor inhibitors and the risk of acute coronary syndromes in early rheumatoid arthritis. Arthritis Rheum. 2012;64:42–52. doi: 10.1002/art.30654. [DOI] [PubMed] [Google Scholar]
- 92.Solomon DH, Curtis JR, Saag KG, Lii J, Chen L, Harrold LR, Herrinton LJ, Graham DJ, Kowal MK, Kuriya B, Liu L, Griffin MR, Lewis JD, Rassen JA. Cardiovascular risk in rheumatoid arthritis: comparing TNF-alpha blockade with nonbiologic DMARDs. Am J Med. 2013;126:730 e739–730 e717. doi: 10.1016/j.amjmed.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dixon WG, Watson KD, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2007;56:2905–2912. doi: 10.1002/art.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63:522–529. doi: 10.1002/acr.20371. [DOI] [PubMed] [Google Scholar]
- 95.Ljung L, Askling J, Rantapaa-Dahlqvist S, Jacobsson L. The risk of acute coronary syndrome in rheumatoid arthritis in relation to tumour necrosis factor inhibitors and the risk in the general population: a national cohort study. Arthritis Res Ther. 2014;16:R127. doi: 10.1186/ar4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol. 2007;293:F616–F623. doi: 10.1152/ajprenal.00507.2006. [DOI] [PubMed] [Google Scholar]
- 97.van Leuven SI, Mendez-Fernandez YV, Wilhelm AJ, Wade NS, Gabriel CL, Kastelein JJ, Stroes ES, Tak PP, Major AS. Mycophenolate mofetil but not atorvastatin attenuates atherosclerosis in lupus-prone LDLr(-/-) mice. Ann Rheum Dis. 2012;71:408–414. doi: 10.1136/annrheumdis-2011-200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Leuven SI, van Wijk DF, Volger OL, de Vries JP, van der Loos CM, de Kleijn DV, Horrevoets AJ, Tak PP, van der Wal AC, de Boer OJ, Pasterkamp G, Hayden MR, Kastelein JJ, Stroes ES. Mycophenolate mofetil attenuates plaque inflammation in patients with symptomatic carotid artery stenosis. Atherosclerosis. 2010;211:231–236. doi: 10.1016/j.atherosclerosis.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 99.Kiani AN, Magder LS, Petri M. Mycophenolate mofetil (MMF) does not slow the progression of subclinical atherosclerosis in SLE over 2 years. Rheumatol Int. 2012;32:2701–2705. doi: 10.1007/s00296-011-2048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 101.Libby P. Pathophysiology of vasculitis. In: Creager MA, Beckman JA, Loscalzo J, editors. Vascular Medicine: A companion to Braunwald's Heart Disease. 2nd ed. 2012. pp. 126–132. [Google Scholar]
- 102.Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, Baslund B, Brenchley P, Bruchfeld A, Chaudhry AN, Cohen Tervaert JW, Deloukas P, Feighery C, Gross WL, Guillevin L, Gunnarsson I, Harper L, Hruskova Z, Little MA, Martorana D, Neumann T, Ohlsson S, Padmanabhan S, Pusey CD, Salama AD, Sanders JS, Savage CO, Segelmark M, Stegeman CA, Tesar V, Vaglio A, Wieczorek S, Wilde B, Zwerina J, Rees AJ, Clayton DG, Smith KG. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–223. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jennette JC, Falk RJ, Hu P, Xiao H. Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Annu Rev Pathol. 2013;8:139–160. doi: 10.1146/annurev-pathol-011811-132453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Booth AD, Jayne DRW, Kharbanda RK, McEniery CM, Mackenzie IS, Brown J, Wilkinson IB. Infliximab improves endothelial dysfunction in systemic vasculitis: a model of vascular inflammation. Circulation. 2004;109:1718–1723. doi: 10.1161/01.CIR.0000124720.18538.DD. [DOI] [PubMed] [Google Scholar]
- 105.Booth AD, Wallace S, McEniery CM, Yasmin, Brown J, Jayne DRW, Wilkinson IB. Inflammation and arterial stiffness in systemic vasculitis. A model of inflammation. Arthritis Rheum. 2004;50:581–588. doi: 10.1002/art.20002. [DOI] [PubMed] [Google Scholar]
- 106.Raza K, Thambyrajah J, Townend JN, Exley AR, Hortas C, Filer A, Carruthers DM, Bacon PA. Suppression of inflammation in primary systemic vasculitis restores vascular endothelial function: lessons for atherosclerotic disease? Circulation. 2000;102:1470–1472. doi: 10.1161/01.cir.102.13.1470. [DOI] [PubMed] [Google Scholar]
- 107.Faurschou M, Mellemkjaer L, Sorensen IJ, Svalgaard Thomsen B, Dreyer L, Baslund B. Increased morbidity from ischemic heart disease in patients with Wegener's granulomatosis. Arthritis Rheum. 2009;60:1187–1192. doi: 10.1002/art.24386. [DOI] [PubMed] [Google Scholar]
- 108.Morgan MD, Turnbull J, Selamet U, Kaur-Hayer M, Nightingale P, Ferro CJ, Savage CO, Harper L. Increased incidence of cardiovascular events in patients with antineutrophil cytoplasmic antibody-associated vasculitides: a matched-pair cohort study. Arthritis Rheum. 2009;60:3493–3500. doi: 10.1002/art.24957. [DOI] [PubMed] [Google Scholar]
- 109.Numano F, Okawara M, Inomata H, Kobayashi Y. Takayasu's arteritis. Lancet. 2000;356:1023–1025. doi: 10.1016/S0140-6736(00)02701-X. [DOI] [PubMed] [Google Scholar]
- 110.Seyahi E, Ugurlu S, Cumali R, Balci H, Seyahi N, Yurdakul S, Yazici H. Atherosclerosis in Takayasu arteritis. Ann Rheum Dis. 2006;65:1202–1207. doi: 10.1136/ard.2005.047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ng WF, Fantin F, Ng C, Dockery F, Schiff R, Davies KA, Rajkumar C, Mason JC. Takayasu's arteritis: a cause of prolonged arterial stiffness. Rheumatology (Oxford) 2006;45:741–745. doi: 10.1093/rheumatology/kei274. [DOI] [PubMed] [Google Scholar]
- 112.Keenan NG, Mason JC, Maceira A, Assomull R, O'Hanlon R, Chan C, Roughton M, Andrews J, Gatehouse PD, Firmin DN, Pennell DJ. Integrated cardiac and vascular assessment in Takayasu arteritis by cardiovascular magnetic resonance. Arthritis Rheum. 2009;60:3501–3509. doi: 10.1002/art.24911. [DOI] [PubMed] [Google Scholar]
- 113.Mason JC. Takayasu arteritis – advances in diagnosis and management. Nat Rev Rheumatol. 2010;6:406–415. doi: 10.1038/nrrheum.2010.82. [DOI] [PubMed] [Google Scholar]
- 114.Gonzalez-Gay MA, Blanco R, Abraira V, Garcia-Porrua C, Ibanez D, Garcia-Pais MJ, Rigueiro MT, Sanchez-Andrade A, Guerrero J, Casariego E. Giant cell arteritis in Lugo, Spain, is associated with low longterm mortality. J Rheumatol. 1997;24:2171–2176. [PubMed] [Google Scholar]
- 115.Matteson EL, Gold KN, Bloch DA, Hunder GG. Long-term survival of patients with giant cell arteritis in the American College of Rheumatology giant cell arteritis classification criteria cohort. Am J Med. 1996;100:193–196. doi: 10.1016/s0002-9343(97)89458-2. [DOI] [PubMed] [Google Scholar]
- 116.Tomasson G, Peloquin C, Mohammad A, Love TJ, Zhang Y, Choi HK, Merkel PA. Risk for cardiovascular disease early and late after a diagnosis of giant-cell arteritis: a cohort study. Ann Intern Med. 2014;160:73–80. doi: 10.7326/M12-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mohammad AJ, Nilsson JA, Jacobsson LT, Merkel PA, Turesson C. Incidence and mortality rates of biopsy-proven giant cell arteritis in southern Sweden. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204652. E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 118.Mackie SL, Dasgupta B. Vasculitis syndromes: dealing with increased vascular risk and mortality in GCA. Nat Rev Rheumatol. 2014;10:264–265. doi: 10.1038/nrrheum.2014.38. [DOI] [PubMed] [Google Scholar]
- 119.Abbott RD, Brand FN, Kannel WB, Castelli WP. Gout and coronary heart disease: the Framingham Study. J Clin Epidemiol. 1988;41:237–242. doi: 10.1016/0895-4356(88)90127-8. [DOI] [PubMed] [Google Scholar]
- 120.Stack AG, Hanley A, Casserly LF, Cronin CJ, Abdalla AA, Kiernan TJ, Murthy BV, Hegarty A, Hannigan A, Nguyen HT. Independent and conjoint associations of gout and hyperuricaemia with total and cardiovascular mortality. QJM. 2013;106:647–658. doi: 10.1093/qjmed/hct083. [DOI] [PubMed] [Google Scholar]
- 121.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116:894–900. doi: 10.1161/CIRCULATIONAHA.107.703389. [DOI] [PubMed] [Google Scholar]
- 122.Clarson L, Chandratre P, Hider S, Belcher J, Heneghan C, Roddy E, Mallen C. Increased cardiovascular mortality associated with gout: a systematic review and meta-analysis. Eur J Prev Cardiol. 2013 doi: 10.1177/2047487313514895. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lottmann K, Chen X, Schadlich PK. Association between gout and all-cause as well as cardiovascular mortality: a systematic review. Curr Rheumatol Rep. 2012;14:195–203. doi: 10.1007/s11926-011-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim SY, De Vera MA, Choi HK. Gout and mortality. Clin Exp Rheumatol. 2008;26:S115–S119. [PubMed] [Google Scholar]
- 126.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]