Background: NCX1 regulates intracellular Ca2+ and Na+ homeostasis in neurons.
Results: The overexpression of NCX1 induced neuronal differentiation through Akt as well as NGF exposure. NCX1 knockdown prevented NGF-induced neurite outgrowth.
Conclusion: NCX1 participates in neuronal differentiation by ionic regulation and Akt phosphorylation.
Significance: Learning how NCX1 participates in neurite outgrowth will improve the knowledge of neuronal differentiation.
Keywords: Akt, Calcium Imaging, Calcium Transport, Neurite Outgrowth, PI3K
Abstract
NGF induces neuronal differentiation by modulating [Ca2+]i. However, the role of the three isoforms of the main Ca2+-extruding system, the Na+/Ca2+ exchanger (NCX), in NGF-induced differentiation remains unexplored. We investigated whether NCX1, NCX2, and NCX3 isoforms could play a relevant role in neuronal differentiation through the modulation of [Ca2+]i and the Akt pathway. NGF caused progressive neurite elongation; a significant increase of the well known marker of growth cones, GAP-43; and an enhancement of endoplasmic reticulum (ER) Ca2+ content and of Akt phosphorylation through an early activation of ERK1/2. Interestingly, during NGF-induced differentiation, the NCX1 protein level increased, NCX3 decreased, and NCX2 remained unaffected. At the same time, NCX total activity increased. Moreover, NCX1 colocalized and coimmunoprecipitated with GAP-43, and NCX1 silencing prevented NGF-induced effects on GAP-43 expression, Akt phosphorylation, and neurite outgrowth. On the other hand, the overexpression of its neuronal splicing isoform, NCX1.4, even in the absence of NGF, induced an increase in Akt phosphorylation and GAP-43 protein expression. Interestingly, tetrodotoxin-sensitive Na+ currents and 1,3-benzenedicarboxylic acid, 4,4′-[1,4,10-trioxa-7,13-diazacyclopentadecane-7,13-diylbis(5-methoxy-6,12-benzofurandiyl)]bis-, tetrakis[(acetyloxy)methyl] ester-detected [Na+]i significantly increased in cells overexpressing NCX1.4 as well as ER Ca2+ content. This latter effect was prevented by tetrodotoxin. Furthermore, either the [Ca2+]i chelator(1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) (BAPTA-AM) or the PI3K inhibitor LY 294002 prevented Akt phosphorylation and GAP-43 protein expression rise in NCX1.4 overexpressing cells. Moreover, in primary cortical neurons, NCX1 silencing prevented Akt phosphorylation, GAP-43 and MAP2 overexpression, and neurite elongation. Collectively, these data show that NCX1 participates in neuronal differentiation through the modulation of ER Ca2+ content and PI3K signaling.
Introduction
Neurite outgrowth is an important process in the development of the nervous system and in neuronal regeneration after brain injury (1). This process is mainly regulated by neurotrophins, such as NGF, that, by activating the tyrosine-kinase receptor TrkA, promote neuronal survival and neurite outgrowth (2). When activated, TrkA triggers several signaling cascades, including the ERK/MAPK and the PI3K/Akt pathways (3, 4). The role of these transductional cascades in neurite outgrowth has been studied extensively. Particularly the MAPK pathway is required for growth factor-induced differentiation of PC12 cells, although it is not sufficient for neurite outgrowth (5). In fact, MAPK activation appears to be a permissive signal for neurite extension in response to growth factor stimuli and calcium signaling (6). Furthermore, activation of PI3K/Akt signaling has been shown to mediate a number of processes, including NGF-induced neurite outgrowth in PC12 cells (7). Conversely, inhibition of the MEK/ERK/Akt pathway suppresses neurite outgrowth (8). Furthermore, varying [Ca2+]i alters neurite outgrowth through changes in the NGF-dependent transductional pathways (6, 9). In fact, the Ca2+ ion is considered an important key second messenger in growth cones because, depending on its concentration level, it modulates the rate, motility, and final collapse of growth cones. However, the [Ca2+]i modulators involved in the regulation of NGF-dependent pathways remain unknown.
Complex patterns regulate the specificity of Ca2+ signaling through the activity of channels and transporters. Among these is the Na+/Ca2+ exchanger (NCX),3 a bidirectional high-capacity and low-affinity ionic transporter that, by exchanging three Na+ ions for one Ca2+ ion, plays a relevant role in maintaining [Ca2+]i homeostasis (10, 11). Three different gene products of NCX have been cloned (12, 13, 14). Among these isoforms, NCX1, which is involved in the regulation of neuronal [Ca2+]i homeostasis, is modulated by NGF (15). In fact, we have demonstrated previously that, after an early exposure, NGF modulates NCX1 expression through a specific pathway involving ERK1/2 and p38 signaling (15). These kinases, in turn, determine an increase of ncx1 transcription through CREB1 (15, 16). Furthermore, NGF exposure determines a translocation of SP1 into the nucleus where it binds to a specific region of the ncx1 promoter between 200 and 79 bp upstream of the transcription start site (15, 17). Collectively, NGF induces up-regulation of NCX1 through MEK1/p38/cAMP response element-binding protein/SP1 signaling.
Although NCXs are specifically involved in many cell functions, their role in neurite outgrowth, together with the transductional pathway involved, remains unknown. In this work, we explored whether NCX isoforms, by regulating [Ca2+]i, could trigger neurite outgrowth during differentiation through the regulation of PI3K/Akt signaling.
EXPERIMENTAL PROCEDURES
Cell Cultures
PC12 cells were grown on plastic dishes in RPMI medium composed of 10% horse serum, 5% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Neuronal differentiation was induced by exposing PC12 cells to NGF (50 ng/ml) for 7 days. Cells were cultured in a humidified 5% CO2 atmosphere. The culture medium was changed every 2 days. For microfluorimetric, electrophysiological, and morphological studies, cells were seeded on glass coverslips (Fisher, Springfield, NJ) coated with poly-l-lysine (5 μg/ml) (Sigma) and used at least 12 h after seeding.
Primary Cortical Neuron Preparation
Postnatal Neurons
Mixed cultures of cortical neurons from Wistar rat pups, 2–4 days old, were prepared. The tissue was minced, trypsinized (0.1% for 15 min at 37 °C), triturated, and plated on poly-d-lysine-coated coverslips. Finally, it was cultured in Neurobasal medium (Invitrogen) supplemented with B-27 (Invitrogen) and 2 mm l-glutamine. Cells were plated at 1.8 × 106 on 25-mm glass coverslips precoated with poly-d-lysine (10 μg/ml). Cultures were kept at 37 °C in a humidified atmosphere of 5% CO2 and 95% air and fed once a week. The neurons were used after 7 days.
Embryonic Neurons
Cortical pure neurons were prepared from brains of 16-day-old Wistar rat embryos. Briefly, the rats were first anesthetized and then decapitated to minimize pain and distress. Dissection and dissociation were performed in Ca2+/Mg2+-free PBS containing glucose (30 mm). Tissues were incubated with papain for 10 min at 37 °C and dissociated by trituration in Earle's Balanced Salt Solution containing DNase, BSA, and ovomucoid. Cells were plated at 15 × 106 in 100-mm plastic Petri dishes precoated with poly-d-lysine (20 μg/ml) in minimum Eagle's medium/F12 (Invitrogen) containing glucose, 5% deactivated FCS, 5% horse serum (Invitrogen), glutamine, and antibiotics. Ara-C (10 μm) was added within 48 h of plating to prevent non-neuronal cell growth. Neurons were cultured at 37 °C in a humidified 5% CO2 atmosphere and used after 7 days of culture. All experiments on primary cortical neurons were performed according to the procedures described in experimental protocols approved by the ethical committee of the Federico II University of Naples, Italy.
Small Interfering RNA and NCX1 Overexpression
The mammalian expression vector pSUPER.retro.puro (OligoEngine, Seattle, WA) was used to express siRNA against NCX1 and its mismatch sequences in PC12 cells. These vectors were prepared as reported previously (16, 18). After 12 h of plating, PC12 cells were first transfected with pSUPER-NCX1 and pSUPER-mismatch sequences by means of the Ca2+ phosphate transfection standard method and then treated with NGF 48 h later. To obtain NCX1.4 overexpression, cells were transfected with 1–2 μg of pCEFL plasmid containing the cDNA of the neuronal splicing form of murine NCX1, NCX1.4, using Lipofectamine 2000 reagent (Invitrogen).
Nucleus-directed Akt Negative Mutant
A wild-type form of rat Akt1 (Akt WT) cDNA lacking the stop codon was cloned in the pEGFP-N1 vector (Clontech, Mountain View, CA) and provided with a nuclear localization signal (NLS) sequence at the C terminus (pEGFP-N1-NLS). The kinase-negative mutant form of Akt (Akt D−) was obtained with the substitution of lysine 179 with methionine by means of site-directed mutagenesis (Agilent Life Science, Milan, Italy) and cloned in the pEGFP-N1-NLS expressing vector. Amino acid sequence of EGFP-Akt-NLS (D−) mutant was as follows (the NLS is underlined): MNDVAIVKEGWLHKRGEYIKTWRPRYFLLKNDGTFIGYKERPQDVDQRESPLNNFSVAQCQLMKTERPRPNTFIIRCLQWTTVIERTFHVETPEEREEWATAIQTVADGLKRQEEETMDFRSGSPSDNSGAEEMEVSLAKPKHRVTMNEFEYLKLLGKGTFGKVILVKEKATGRYYAMKILKKEVIVAKDEVAHTLTENRVLQNSRHPFLTALKYSFQTHDRLCFVMEYANGGELFFHLSRERVFSEDRARFYGAEIVSALDYLHSEKNVVYRDLKLENLMLDKDGHIKITDFGLCKEGIKDGATMKTFCGTPEYLAPEVLEDNDYGRAVDWWGLGVVMYEMMCGRLPFYNQDHEKLFELILMEEIRFPRTLGPEAKSLLSGLLKKDPTQRLGGGSEDAKEIMQHRFFANIVWQDVYEKKLSPPFKPQVTSETDTRYFDEEFTAQMITITPPDQDDSMECVDSERRPHFPQFSYSASGTAWDPPVATMVSKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICTTGKLPVPWPTLVTTLTYGVQCFSRYPDHMKQHDFFKSAMPEGYVQERTIFFKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHNVYIMADKQKNGIKVNFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNHYLSTQSALSKDPNEKRDHMVLLEFVTAAGITLGMDELYKGSPKKKRKVEFGRD. Nuclear localization of EGFP-tagged Akt was observed under a Zeiss LSM510 META/laser-scanning confocal microscope.
Western Blot Analysis
Protein extraction and Western blotting were performed according to standard protocols. Briefly, PC12 cells were washed in phosphate-buffered saline and collected by gentle scraping in ice-cold lysis buffer containing 20 mm HEPES (pH 7.4), 1 mm sodium azide, 0.2 mm sodium orthovanadate, 0.05 mm sodium fluoride, 1% mm Triton X-100, and a protease inhibitor mixture (0.1% aprotinin, 0.7 mg/ml pepstatin, 1 μg/ml leupeptin) (Roche Diagnostic). After centrifugation (13,400 rpm, 4 °C, 20 min), the supernatants were collected, and 100 μg of proteins was loaded and separated on 8% SDS-polyacrylamide gels. Proteins were transferred onto Hybond-ECL nitrocellulose membranes (GE Healthcare). The filters were blocked for 1 h at ∼20 °C with 5% nonfat dry milk (Bio-Rad) in Tris-buffered saline-Tween 20 (2 mm Tris-HCl, 50 mm NaCl (pH 7.5) plus 0.1% Tween 20) and incubated overnight with the appropriate primary antibody in 5% nonfat dry milk. The final dilution of primary antibodies was 1:1000, except for the antibodies against NCX3, which were used at a final dilution of 1:1500. Filters were incubated with the following antibodies: anti-NCX1 (monoclonal mouse antibody, Swant, Bellinzona, Switzerland), anti-NCX2 (polyclonal rabbit antibody, Alpha Diagnostic), anti-NCX3 (a gift from Dr. K. Philipson, University of California, Los Angeles, CA), anti-phosphoAkt (monoclonal mouse antibody, Santa Cruz Biotechnology, catalog no. sc-81433), anti-Akt (polyclonal rabbit antibody, Santa Cruz Biotechnology), anti-ERK1/2 (polyclonal, catalog no. sc-153, Santa Cruz Biotechnology), anti-phosphoERK (polyclonal, catalog no. sc-7383, Santa Cruz Biotechnology), anti-EGFP (monoclonal mouse antibody, Clontech), and anti-GAP-43 (monoclonal mouse antibody, Calbiochem). Primary cortical neurons were washed in phosphate-buffered saline and lysed. Samples were loaded, separated on 10% SDS-PAGE gel, and then transferred to a nitrocellulose membrane. Immunoblot analysis was performed using anti NCX1 (1:1000 polyclonal mouse antibody, Swant), anti GAP-43 (1: 500 monoclonal mouse antibody, Calbiochem), anti phospho-Akt (1:1000 monoclonal mouse antibody, Santa Cruz Biotechnology, catalog no. sc-81433), anti-Akt (1:1000 polyclonal rabbit antibody, Santa Cruz Biotechnology), and anti-MAP2 (1:1000 monoclonal mouse antibody, Sigma). Then all membranes were washed with TBS-T (500 mm Tris, 60 mmm KCl, 2.8 mm NaCl, and 1.0% Tween 20) and incubated with the appropriate secondary antibodies (1:2000, GE Healthcare) for 1 h at ∼20 °C. Immunoreactive bands were detected using ECL reagent kits (GE Healthcare). The optical density of the bands was determined by a Chemi-Doc imaging system (Bio-Rad).
Immunoprecipitation and Immunoblot Analyses
Cells were homogenized in lysis buffer containing 50 mm HEPES, 100 mm NaCl, 1.5 mm MgCl2, 1 mm PMSF, 0.2% Nonidet P-40, 5 μg/ml aprotinin, 10 μg/ml leupeptin and 2 μg/ml pepstatin. The lysates were cleared by centrifugation (12,000 rpm, 10 min). 1 mg of cell lysate was immunoprecipitated with anti-NCX1 rabbit antibody (1:100), anti-GAP-43 antibody (1:50), or non-immune IgG antibody. Then the immunoprecipitates were resolved by SDS-PAGE gel and transferred to a nitrocellulose membrane. Immunoblot analysis was performed using anti-GAP-43 or anti-NCX1, respectively.
Immunocytochemistry and Confocal Microscopy
Immunolocalization of the NCX1 isoform was performed by mouse monoclonal anti-NCX1 (R3F1) purchased from Swant. PC12 cells were rinsed twice in cold 0.01 m PBS (pH 7.4) and fixed in 4% (w/v) paraformaldehyde (Sigma) for 20 min at room temperature. After three washes in PBS, cells were blocked with 3% (w/v) bovine serum albumin and 0.05% Triton X-100 (Bio-Rad) for 1 h at room temperature. The coverslips were then incubated with a primary antibody, anti-NCX1 (1:100 dilution), and, after three washes in PBS, incubated under dark conditions with a biotinylated secondary antibody. After incubation, the peroxidase reaction was developed with 3,3′-diaminobenzidine/4-HCl as the chromogen. For double labeling immunofluorescent analysis, anti-NCX1 (rabbit polyclonal antibody (Swant) was used together with anti-GAP-43 (monoclonal mouse antibody, Chemicon). PC12 cells were rinsed twice in cold 0.01 m PBS (pH 7.4) and fixed in 4% (w/v) paraformaldehyde (Sigma) for 20 min at room temperature. Following three washes in PBS, cells were blocked with 3% (w/v) bovine serum albumin and 0.05% Triton X-100 (Bio-Rad, Milan, Italy) for 1 h at room temperature. The coverslips were then incubated overnight with the primary antibody, anti-NCX1 (1:100 dilution), and anti-GAP-43 (1:100). Primary cortical neurons were fixed as described above and incubated with NeuN (1:1000 polyclonal rabbit antibody, Millipore) and MAP2 (1:1000 monoclonal mouse antibody, Sigma). Following three washes in PBS, the coverslips were incubated under dark conditions with two secondary antibodies: Cy3 anti-mouse IgG and Cy2 anti-rabbit IgG (1:200, Jackson ImmunoResearch Laboratories) for 1 h at room temperature. Nuclei were stained at the end of the experiment with Hoechst 33258 (1 μg/ml) for 5 min at room temperature. Phalloidin staining in PC12 cells and cortical neurons was performed after Hoechst 33258 staining using Phalloidin-Atto Rho6G (1:50, Sigma) for 15 min at room temperature. After the final wash, coverslips were mounted with Vectashield (Vector Labs, Burlingame, CA), and images were observed using a Zeiss LSM510 META/laser-scanning confocal microscope. Single images were taken with an optical thickness of 0.7 μm and a resolution of 1024 × 1024.
[Ca2+]i and [Na+]i Measurement
[Ca2+]i was measured by single cell computer-assisted video imaging (19). Briefly, PC12 cells grown on glass coverslips were loaded with 10 μm Fura-2/AM for 1 h at room temperature in normal Krebs solution containing 5.5 mm KCl, 160 mm NaCl, 1.2 mm MgCl2, 1.5 mm CaCl2, 10 mm glucose, and 10 mm HEPES-NaOH (pH 7.4). At the end of the Fura-2/AM loading period, the coverslips were placed into a perfusion chamber (Medical System Co., Greenvale, NY) mounted onto a Zeiss Axiovert 200 microscope (Carl Zeiss, Germany) equipped with a FLUAR ×40 oil objective lens. The experiments were carried out with a digital imaging system composed of MicroMax 512BFT cooled charge-coupled device camera (Princeton Instruments, Trenton, NJ), a Lambda 10-2 filter wheeler (Sutter Instruments, Novato, CA, and Meta-Morph/MetaFluor imaging system software (Universal Imaging, West Chester, PA). After loading, cells were alternatively illuminated at wavelengths of 340 and 380 nm by a xenon lamp. The emitted light was passed through a 512-nm barrier filter. The fluorescence intensity of Fura-2/AM was measured every 3 s. Because the FURA-2/AM Kd was assumed to be 224 nm, the equation in Grynkiewicz et al. (20), whose parameters were determined for individual cells as described previously (21), was used for calibration. All results are presented as cytosolic Ca2+ concentration. [Na+]i measurement was performed by 1,3-benzenedicarboxylic acid, 4,4′-[1,4,10-trioxa-7,13-diazacyclopentadecane-7,13-diylbis(5-methoxy-6,12-benzofurandiyl)]bis-, tetrakis[(acetyloxy)methyl] ester incubated at 10 μm in the presence of pluronic acid (0.02%) for 1 h at 37 °C (22).
Measurement of NCX Activity Evaluated as Na+-dependent 45Ca2+ Uptake
Na+-dependent 45Ca2+ uptake into cells was measured as described previously (18). Briefly, PC12 cells were plated on 6-well plates (∼500,000 cells/well). After 48 h, cells were incubated at 37 °C for 10 min in normal Krebs solution (5.5 mm KCl, 145 mm NaCl, 1.2 mm MgCl2, 1.5 mm CaCl2, 10 mm glucose, and 10 mm HEPES-NaOH (pH 7.4)) containing 1 mm ouabain and 10 μm monensin. Then 45Ca2+ uptake was initiated by switching the normal Krebs medium to Na+-free N-methyl-d-glucamine (5.5 mm KCl, 147 mm N-methyl glucamine, 1.2 mm MgCl2, 1.5 mm CaCl2, 10 mm glucose, and 10 mm HEPES-NaOH (pH 7.4)) containing 10 μm 45Ca2+ (74 kBq/ml) and 1 mm ouabain. After 30 s of incubation, cells were washed with an ice-cold solution containing 2 mm La3+ to stop 45Ca2+ uptake. Cells were subsequently solubilized with 0.1 N NaOH, and aliquots were taken to determine radioactivity and protein content by the Bradford method (23).
Electrophysiological Recording of NCX and Voltage-gated Sodium Channel Activity by Patch Clamp
INCX was recorded from differentiated PC12 cells with the whole-cell patch clamp technique (22). Currents were filtered at 5 kHz and digitized with a Digidata 1322A interface (Molecular Devices). Data were acquired and analyzed with pClamp software (version 9.0, Molecular Devices). INCX was recorded starting from a holding potential of −60 mV up to a short-step depolarization at +60 mV (60 ms) (24). Then a descending voltage ramp from +60 to −120 mV was applied. The current recorded in the descending portion of the ramp (from +60 to −120 mV) was used to plot the current voltage (I-V) relation curve. The magnitude of INCX was measured at the end of +60 mV (reverse mode) and at the end of −120 mV (forward mode). The Ni2+-insensitive components were subtracted from total currents to isolate INCX. INCX was normalized for membrane capacitance as reported previously (25, 26). For tetrodotoxin (TTX)-sensitive Na+ channel recordings, PC12 cells were perfused with an extracellular Ringer's solution (25) containing 20 mm tetraethylammonium (TEA) and 5 μm nimodipine. The pipettes were filled with 110 mm CsCl, 10 mm TEA, 2 mm MgCl2, 10 mm EGTA, 8 mm glucose, 2 mm Mg-ATP, 0.25 mm cAMP, and 10 mm HEPES (pH 7.3). TTX-sensitive Na+ currents were recorded by applying, from a holding potential of −70 mV, depolarizing voltage steps of 50-ms duration in 10 mV from −100 to +50 mV elicited at 0.066-Hz frequency (1 pulse every 15 s), as reported previously (25).
Statistical Analysis
Data are expressed as mean ± S.E. Statistical comparisons between controls and treated experimental groups were performed using one-way analysis of variance followed by Newman Keul's test. p < 0.05 was considered statistically significant.
RESULTS
Effect of NGF on Neurite Elongation, Akt Activation, and GAP-43 Protein Expression in PC12 Cells
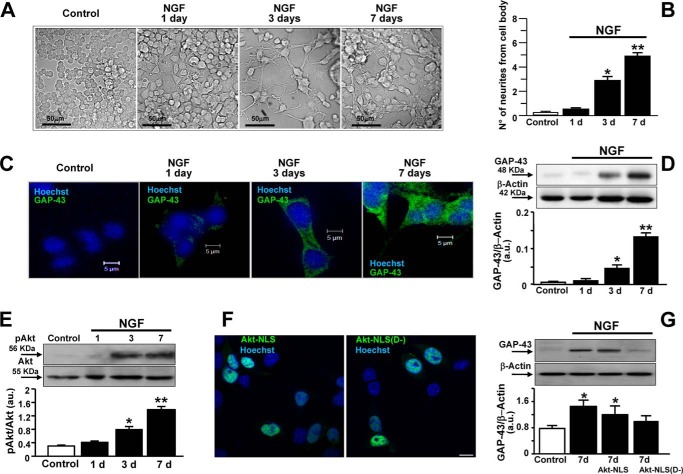
To induce neuronal differentiation, PC12 cells were exposed to NGF (50 ng/ml). As reported already, neurite elongation increased progressively after 3 and 7 days of exposure to NGF (Fig. 1, A and B). In fact, the number of neurites from the cell body of PC12 cells increased in a time-dependent manner (Fig. 1B). Accordingly, Western blot analysis and immunocytochemistry showed that GAP-43 protein expression appeared after only 3 days of exposure, peaking 7 days after treatment (Fig. 1, C and D). Because the activation of the serine/threonine protein kinase Akt has been shown already to play a key role in neuronal differentiation (27), Akt phosphorylation was studied under the experimental conditions described above. Western blot analysis revealed that Akt phosphorylation increased in a time-dependent manner in PC12 cells when exposed to NGF for 3 and 7 days (Fig. 1E). To verify whether the effect of the phosphorylated form of Akt on neurite outgrowth was exerted at the nuclear level per se or through such a mediator, a dominant negative form of Akt (Akt D−) lacking kinase activity was linked to the EGFP protein and to the NLS (Akt-NLS(D−)) that favors its translocation into the nucleus. Confocal microscopy images showed that the fluorescent mutant chimera was localized in the nucleus as well as the wild-type mutant Akt-NLS (see Fig. 1F). More importantly, Akt-NLS(D−) transfection completely prevented GAP-43 expression in PC12 cells exposed to NGF for 7 days compared with NGF-untreated cells and cells overexpressing the wild-type mutant Akt containing an NLS sequence (Akt-NLS) (Fig. 1G). The amino acid sequence of the Akt-NLS(D−) protein conjugated to EGFP and with the K179M substitution in the Akt sequence is reported under “Experimental Procedures.”
FIGURE 1.
Effect of NGF on neurite elongation, Akt activation, and GAP-43 protein expression in PC12 cells. A, representative image sequence depicting PC12 cells during differentiation with NGF (50 ng/ml). B, quantification of neurite number from each cell body. Data are mean ± S.E. from three independent experimental sessions. *, p < 0.05 versus control; **, p < 0.05 versus all. C, GAP-43 immunosignal in PC12 cells exposed to NGF for 1, 3, and 7 days. D, representative Western blot and relative quantification of GAP-43 expression in PC12 cells exposed to NGF for 1, 3, and 7 days. *, p < 0.05 versus control and 1 day; **, p < 0.05 versus all. a.u., arbitrary units. E, representative Western blots and relative quantifications of Akt phosphorylation under control conditions and after the exposure to NGF for 1, 3, and 7 days. Data are mean ± S.E. from three independent experimental sessions. *, p < 0.05 versus control and 1 day; **, p < 0.05 versus all. F, representative fluorescent image of Hoechst-positive nuclei (blue) overexpressing EGFP-tagged Akt-NLS and EGFP-tagged Akt-NLS(D−) (green). Scale bar, 10 μm. G, representative Western blot and relative quantification of GAP-43 expression in PC12 cells overexpressing Akt-NLS or Akt-NLS(D−) exposed to NGF for 7 days. Data are mean ± S.E. from three independent experimental sessions. *, p < 0.05 versus control.
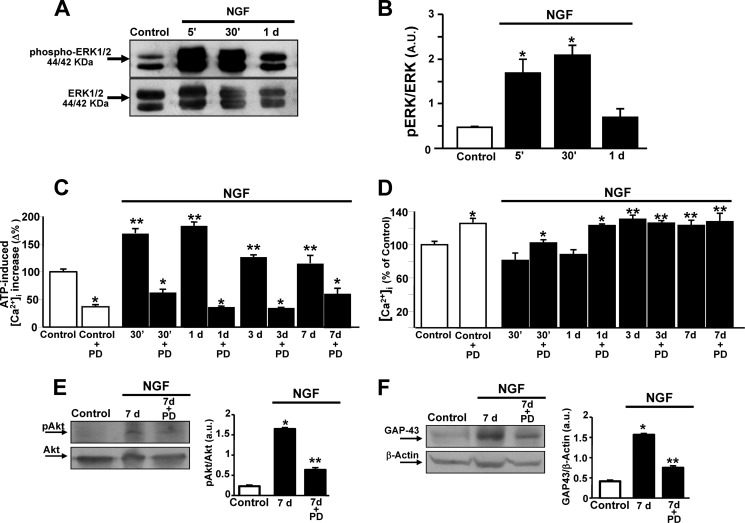
Effect of ERK1/2 Modulation on Intracellular Ca2+ Release from the ER, Akt Activation, and GAP-43 Protein Expression in NGF-induced Neuronal Differentiation
To study an upstream regulator of Akt, MAPKs were investigated early after NGF exposure. Western blot analysis revealed that ERK1/2 phosphorylation increased in PC12 cells when exposed to NGF for 5 and 30 min, decreasing thereafter at 1 day (Fig. 2, A and B). In accordance with the acquisition of the neuronal phenotype, Ca2+ release from the ER, induced by both the purinergic receptor agonist ATP and the irreversible sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitor thapsigargin (Tg), peaked 30 min after NGF exposure. This effect was also maintained at 1 day but decreased in cells exposed to NGF for 3 and 7 days, although it remained higher than that of the control (Fig. 2C). Interestingly, the MAPK inhibitor PD 098059 (20 μm) prevented an ATP- and Tg-induced [Ca2+]i peak in cells exposed to NGF for 30 min, 1 day, 3 days, and 7 days as well as under control conditions (Fig. 2C). Furthermore, [Ca2+]i progressively increased upon NGF administration (Fig. 2D). Interestingly, PD 098059 further enhanced this increase under control conditions and in cells exposed to NGF for 30 min and 1 day when compared with the respective controls (Fig. 2D).
FIGURE 2.
Effect of ERK1/2 modulation on [Ca2+]i homeostasis, Akt phosphorylation, and GAP-43 protein expression in NGF-induced PC12 differentiation. A and B, representative Western blot and relative quantification of ERK1/2 in PC12 cells exposed to NGF for 5 min (5′), 30 min (30′), and 1 day. Data are mean ± S.E. from three independent experimental sessions. *, p < 0.05 versus untreated cells (control). a.u., arbitrary units. C and D, quantification of the effect of NGF on ATP-induced (100 μm) and Tg-induced (1 μm) [Ca2+]i increase and [Ca2+]i, respectively, in PC12 cells treated with the growth factor for 30 min, 1 day, 3 days, and 7 days in the presence or absence of the pharmacological inhibitor of ERK1/2, PD 098059 (PD, 20 μm). ATP and Tg were administered in a Ca2+-free solution containing EGTA (1 mm). Data are mean ± S.E. from three independent experimental sessions. *, p < 0.05 versus respective internal control; **, p < 0.05 versus untreated cells. E and F, representative Western blot and relative quantification of Akt phosphorylation and GAP-43 protein expression after 7 days of exposure to NGF in the presence or absence of PD 098059 (20 μm). Data are mean ± S.E. from three independent experimental sessions. *, p < 0.05 versus control; **, p < 0.05 versus 7 days of exposure to NGF.
Finally, the effect of ERK1/2 inhibition on the activation of Akt and GAP-43 protein expression was also investigated. PD 098059 effectively prevented NGF-induced Akt phosphorylation detected in PC12 cells after 7 days of exposure (Fig. 2E). The same effect was also observed after 3 days (data not shown). Similarly, PD 098059 prevented GAP-43 overexpression in PC12 cells exposed to NGF for 7 days (Fig. 2F), therefore suggesting a prominent role of ERK1/2 in the complex Ca2+-dependent regulation of neuronal differentiation by NGF.
Effect of NGF on the Expression and Activity of the Three NCX Isoforms in PC12 Cells
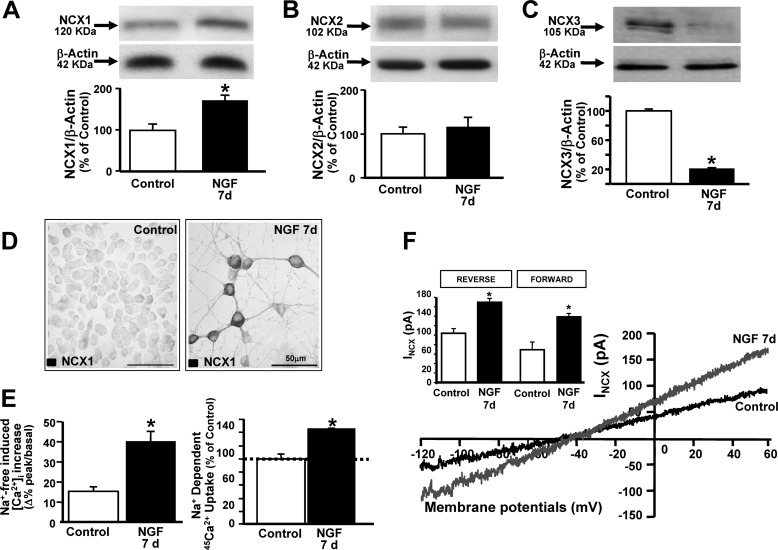
Because, among the proteins regulated by MAPKs and involved in [Ca2+]i handling, NCX represents a potential player in the Ca2+-dependent regulation of neuronal differentiation, the expression and function of NCX isoforms upon NGF administration were investigated. When PC12 cells were exposed to NGF for 7 days, NCX1 protein expression increased significantly, NCX3 decreased, and NCX2 remained unaffected (Fig. 3, A–C). Indeed, the diffused NCX1 immunosignal significantly increased after 7 days of exposure to NGF (Fig. 3D). NCX activity was then recorded by single-cell Fura-2/AM microfluorimetry, radioactive 45Ca2+ uptake assays, and patch clamp electrophysiology (Fig. 3, E and F). In particular, NCX activity, measured in reverse mode of operation as [Ca2+]i increase and as 45Ca2+ uptake, both elicited by the addition of a Na+-deficient NMDG+ medium, increased significantly after 7 days of exposure to NGF, as opposed to controls (Fig. 3E). Accordingly, patch clamp experiments revealed that the magnitude of INCX, measured as reverse mode at the end of +60 mV and as forward mode at the end of −120 mV, increased significantly after 7 days of exposure to NGF compared with controls (Fig. 3F). Interestingly, in PC12 exposed to NGF for 3 and 7 days, the NCX1 immunosignal increased progressively and colocalized significantly with GAP-43 (data not shown), therefore suggesting the involvement of this isoform of exchanger in the NGF-induced differentiation of PC12 cells.
FIGURE 3.
Effect of NGF on the expression and activity of the three NCX isoforms in neuronal PC12 cells. A–C, representative Western blots and relative quantifications of NCX1, NCX2, and NCX3 protein expression in PC12 cells under control conditions and after 7 days of exposure to NGF. *, p < 0.05 versus control. D, immunocytochemical images of NCX1 expression in control and differentiated PC12 (NGF 7 d). E, NCX activity measured in the reverse mode of operation as Na+-free-induced [Ca2+]i increase and 45Ca2+ uptake under control conditions and after 7 days of exposure to NGF. *, p < 0.05 versus control. F, representative superimposed traces of INCX recorded from control and differentiated PC12 cells (NGF 7 d). Inset, quantification of INCX recorded in reverse and forward modes of operation under the above described conditions. *, p < 0.05 versus control.
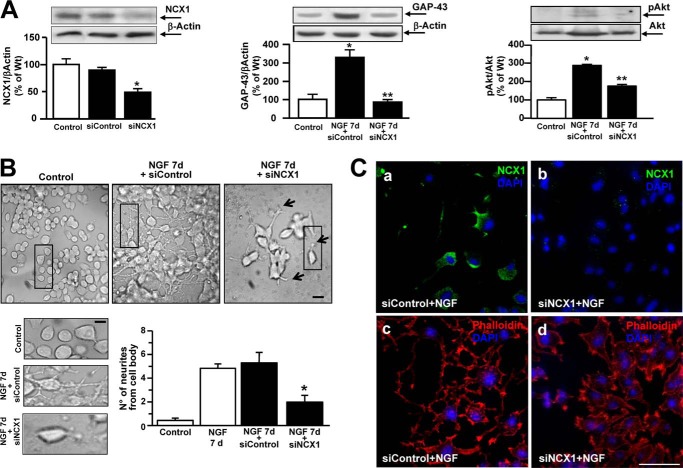
Effect of NCX1 Silencing on GAP-43 Protein Expression and Neurite Outgrowth in PC12 Cells
The role of NCX1 in neuronal differentiation was explored by knocking down its expression with specific siRNA. Western blot analysis revealed that NCX1 silencing, by reducing NCX1 protein expression by almost 60% (Fig. 4A, left panel), prevented the increase in GAP-43 protein expression after 7 days of exposure to NGF (Fig. 4A, center panel). The mismatch sequence failed to modify GAP-43 expression (Fig. 4A, center panel). Interestingly, NCX1 silencing prevented NGF-induced Akt phosphorylation (Fig. 4A, right panel). Under these conditions, the number of processes from the cell body was measured in PC12 exposed to NGF (Fig. 4B). siRNA against NCX1 significantly reduced the number of neurites after 7 days of exposure to NGF compared with control conditions (Fig. 4B). Furthermore, silencing of NCX1 induced a dysregulation of cytoskeleton organization in PC12 cells exposed to NGF for 3 days, as revealed by phalloidin-rhodamine staining (Fig. 4C, a–d).
FIGURE 4.
Effect of siNCX1 on neurite elongation, GAP-43 protein expression, and Akt phosphorylation in neuronal PC12 cells. A, left panel, representative Western blot and quantification of NCX1 protein expression in control cells and in PC12 cells exposed to siNCX1 for 48 h or to siControl. *, p < 0.05 versus control. Center panel, representative Western blot and quantification of GAP-43 expression in control and in PC12 cells exposed to NGF for 7 days in the presence of siControl or siNCX1. *, p < 0.05 versus control; **, p < 0.05 NGF 7 d + siControl. Right panel, representative Western blot and quantification of Akt phosphorylation in control and PC12 cells exposed to NGF for 7 days in the presence of siControl or siNCX1. *, p < 0.05 versus control; **, p < 0.05 NGF 7 d + siControl. B, top panel, representative image sequence depicting PC12 cells under control conditions after 7 days of exposure to NGF + siControl and after 7 days of exposure to NGF + siNCX1. Scale bars = 10 μm (5 μm for the images at higher magnification). Bottom panels, quantification of neurite number from each cell body in PC12 cells under the conditions B. Data are mean ± S.E. from three independent experimental sessions. *, p < 0.05 versus NGF 7 d and NGF 7 d + siControl. C, immunocytochemical images depicting NCX1 expression (a and b) and phalloidin-rhodamine staining (c and d) in PC12 cells exposed to NGF after treatment with siControl or siNCX1 (see “Experimental Procedures”). Nuclei were stained with DAPI. Scale bar = 50 μm.
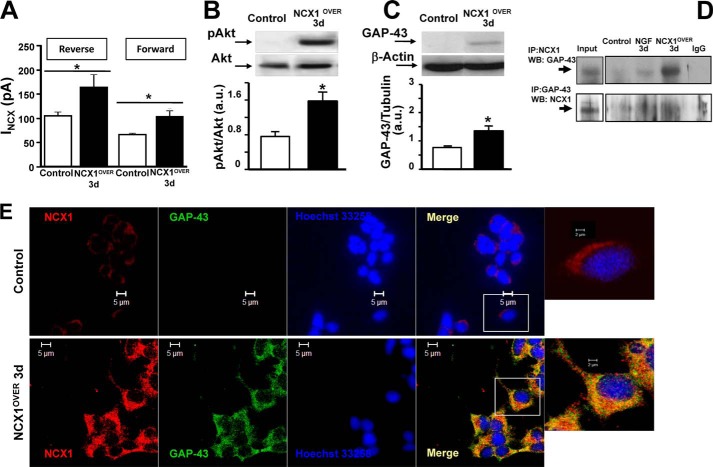
Effect of NCX1 Overexpression on GAP-43 Protein Expression, ER Ca2+ Content, and Akt Phosphorylation in PC12 Cells
The role of the neuronal isoform of NCX1 (NCX1.4) in neuronal differentiation was tested further by overexpressing this isoform in PC12 cells. After 3 days, NCX1.4 overexpression produced an increase in INCX detected by patch clamp in both reverse and forward modes of operation (Fig. 5A). Furthermore, NCX1.4 overexpression induced a neuronal phenotype in PC12 cells even in the absence of NGF. In fact, under these experimental conditions, the activation of Akt and a significant increase in GAP-43 protein expression occurred in PC12 cells (Fig. 5, B and C). Interestingly, under the same conditions, NCX1 significantly colocalized and coimmunoprecipitated with GAP-43 after 3 days in culture (see Fig. 5, D and E).
FIGURE 5.
Effect of NCX1 overexpression on GAP-43 protein expression and Akt phosphorylation in neuronal PC12 cells. A, quantification of INCX in the reverse and forward modes of operation in PC12 cells transfected with the empty expression vector pCEFL (control) and PC12 cells transfected for 3 days with pCEFL expressing NCX1.4 (NCX1OVER). *, p < 0.05 versus its respective control. B, representative Western blot and relative quantification of Akt phosphorylation in control cells and in NCX1OVER. *, p < 0.05 versus control. C, representative Western blot and relative quantification of GAP-43 expression under the conditions of A. *, p < 0.05 versus control. a.u., arbitrary units. D, lysates from control and PC12 cells exposed to NGF for 3 days and PC12 NCX1OVER cells for 3 days were subjected to immunoprecipitation (IP) using anti-NCX1 (top row) or anti-GAP-43 (bottom row). The presence of GAP-43 (top row) or NCX1 (bottom row) was analyzed by immunoblotting. WB, Western blot. E, NCX1 (red) and GAP-43 (green) immunosignal in control cells and in NCX1OVER (Pearson's correlation factor, −0.08 ± 0.008 in control cells and 0.39 ± 0.09 in NCX1OVER cells). p < 0.05 versus control.
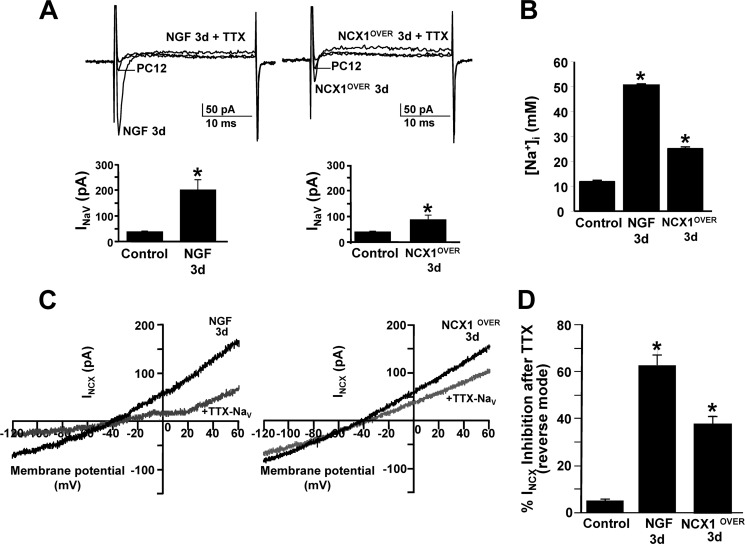
In accordance with the acquisition of the neuronal phenotype, TTX-sensitive Na+ currents increased significantly in PC12 cells exposed to NGF for 3 days and in cells overexpressing NCX1.4 for 3 days compared with controls (Fig. 6A). Accordingly, 1,3-benzenedicarboxylic acid, 4,4′-[1,4,10-trioxa-7,13-diazacyclopentadecane-7,13-diylbis(5-methoxy-6,12-benzofurandiyl)]bis-, tetrakis[(acetyloxy)methyl] ester-detected [Na+]i increased significantly in PC12 cells exposed to NGF for 3 days and in cells overexpressing NCX1.4 for 3 days (Fig. 6B). Interestingly, TTX-induced blockade of voltage-gated sodium currents decreased INCX in PC12 cells exposed to NGF for 3 days and in cells overexpressing NCX1.4 for 3 days (Fig. 6, C and D). Furthermore, the overexpression of NCX1.4 profoundly modulated [Ca2+]i homeostasis. In fact, ATP plus Tg, inducing ER Ca2+ release and preventing its reuptake, produced in NCX1-overexpressing cells a significantly higher increase of [Ca2+]i than in controls, as detected by single-cell microfluorimetry (Fig. 7, A and B).
FIGURE 6.
Role of TTX-sensitive voltage-gated sodium currents and [Na+]i on INCX in neuronal PC12 cells. A, top panel, representative superimposed traces of voltage-gated sodium currents (INaV) recorded from PC12 cells under control conditions (n = 6) and after exposure to NGF for 3 days (n = 10) and from PC12 cells overexpressing NCX1.4 (NCX1OVER) for 3 days (n = 6) in the presence and in absence of TTX (50 nm). Bottom panel, quantification of voltage-gated sodium currents under the conditions described above. *, p < 0.05 versus control. B, quantification of 1,3-benzenedicarboxylic acid, 4,4′-[1,4,10-trioxa-7,13-diazacyclopentadecane-7,13-diylbis(5-methoxy-6,12-benzofurandiyl)]bis-, tetrakis[(acetyloxy)methyl] ester-detected [Na+]i under the same conditions as in A. Data are mean ± S.E. from three independent experimental sessions (n = 60 cells). *, p < 0.05 versus control. C, representative superimposed traces of INCX recorded in reverse and forward modes of operation from PC12 cells exposed to NGF for 3 d and from NCX1OVER for 3 d in the presence (gray traces) and in absence (black traces) of TTX (50 nm). D, quantification of INCX inhibition under the conditions described above. *, p < 0.05 versus control.
FIGURE 7.
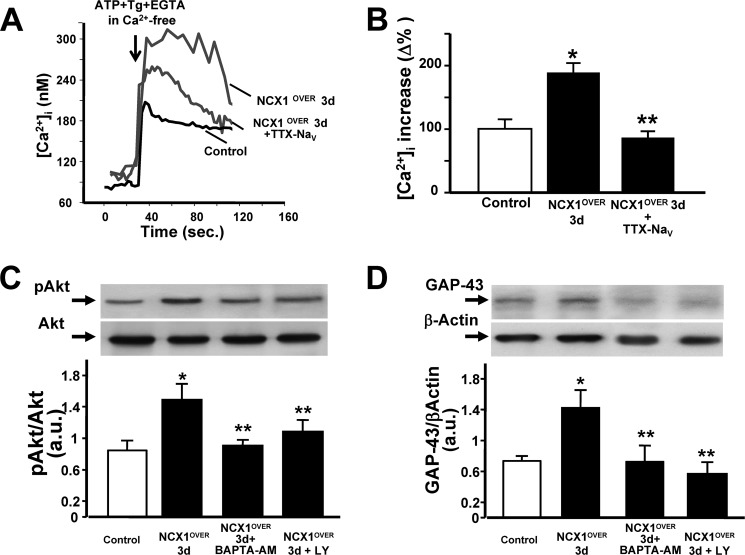
Effect of NCX1 overexpression on ER Ca2+ content and effect of the Ca2+ chelator BAPTA-AM and the PI3K inhibitor LY 294002 on NCX1-induced differentiation in neuronal PC12 cells. A, superimposed single cells representative of the effect on [Ca2+]i of ATP (100 μm) and Tg (1 μm) in Ca2+-free solution containing EGTA (1 mm) in control cells, in cells overexpressing NCX1 for 3 days in vitro (NCX1OVER 3 d), and in NCX1OVER 3 d exposed to TTX (50 nm). B, quantification of A. Data are mean ± S.E. from three independent experimental sessions. *, p < 0.05 versus control; **, p < 0.05 versus NCX1OVER 3 d. C, representative Western blot and relative quantification of Akt phosphorylation in PC12 cells after NCX1OVER alone, after NCX1OVER in the presence of BAPTA-AM, and after NCX1OVER in the presence of LY 294002. All treatments lasted 3 days. *, p < 0.05 versus control; **, p < 0.05 versus NCX1OVER 3 d. D, representative Western blot and relative quantification of GAP-43 expression in PC12 cells after NCX1OVER alone, after NCX1OVER in the presence of BAPTA-AM, and after NCX1OVER in the presence of LY 294002. a.u., arbitrary units. *, p < 0.05 versus control; **, p < 0.05 versus NCX1OVER 3 d.
This increased ER Ca2+ content, induced by NCX1.4 overexpression, was prevented by TTX (50 nm), therefore suggesting a relationship between the increased INav and ER Ca2+ refilling. Concomitantly, the activation of Akt occurred in PC12 cells after NCX1.4 overexpression, even in the absence of NGF (Fig. 7C). In particular, the overexpression of the neuronal isoform NCX1.4 induced Akt activation as early as 1 day after culture in vitro (data not shown). Furthermore, the intracellular Ca2+ chelator BAPTA-AM prevented both Akt phosphorylation and GAP-43 protein expression induced by NCX1.4 overexpression (Fig. 7, C and D). Similarly, pharmacological inhibition of PI3K LY 294002 prevented both Akt phosphorylation and GAP-43 protein expression induced by NCX1.4 overexpression (Fig. 7, C and D).
Effect of NCX1 Silencing on GAP-43 and MAP2 Protein Expression, Akt Phosphorylation, and Neurite Outgrowth in Primary Cortical Neurons
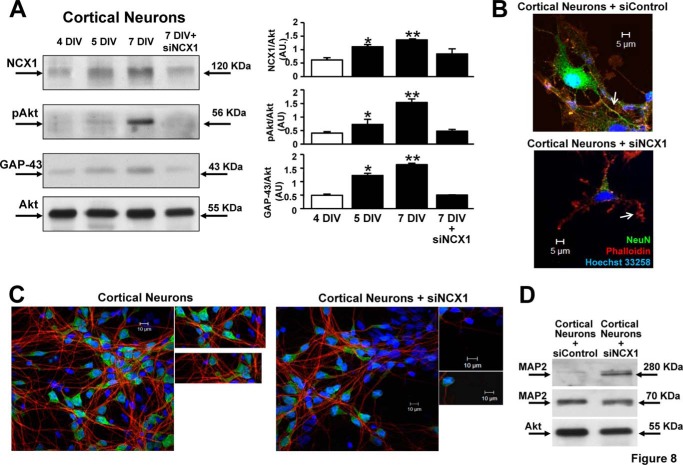
Both NCX1 and GAP-43 protein expression, as well as Akt phosphorylation, increased progressively in cortical neurons during differentiation, reaching a peak at 7 DIV (Fig. 8A). NCX1 silencing (siNCX1) prevented the activation of Akt and GAP-43 up-regulation during in vitro differentiation. Furthermore, siNCX1 counteracted both the increase of the 70-kDa band and the reduction of 280-kDa band of the microtubule-associated protein MAP2 during in vitro differentiation (Fig. 8D). Accordingly, siNCX1 prevented neurite outgrowth of cortical neurons (7 DIV), as detected by phalloidin-rhodamine staining (Fig. 8B), and reduced NeuN-positive neurons (Fig. 8C).
FIGURE 8.
Effect of siNCX1 on in vitro differentiation of rat cortical neurons. A, representative Western blots and relative quantifications of NCX1, GAP-43 protein expression, and Akt phosphorylation in cortical neurons at 4, 5, and 7 DIV and in neurons at 7 DIV plus siRNA against NCX1 (siNCX1). This treatment was performed in cortical neurons at 1 DIV. Akt protein expression was used as an internal control. B, immunocytochemical images depicting NeuN and phalloidin-rhodamine staining in a representative cortical neuron at 7 DIV and in a cortical neuron treated with siNCX1. Nuclei, Hoechst (blue)). The arrows indicate neurites. C, immunocytochemical images depicting NeuN and MAP2 staining in cortical neurons at 7 DIV and cortical neurons treated with siNCX1. Nuclei, Hoechst (blue)). siNCX1 treatment was performed in cortical neurons at 1 DIV. D, representative Western blots of MAP2 forms at 280 and 70 kDa and of Akt protein expression, used as internal control, in cortical neurons at 7 DIV + siControl and in cortical neurons treated with siNCX1.
DISCUSSION
This study demonstrates that, among the three isoforms of the Na+/Ca2+ exchanger, the neuronal splicing form of NCX1, NCX1.4, plays a relevant role in triggering neurite outgrowth and neuronal differentiation in PC12 cells and in cortical neurons. The expression and activity of NCX1 increased progressively during differentiation, reaching a peak after 7 days of NGF treatment in PC12 cells and at 7 DIV in cortical neurons. In addition, the NCX1 isoform colocalized and coimmunoprecipitated significantly with GAP-43 during differentiation with NGF. Furthermore, siRNA against NCX1 in PC12 cells significantly reduced the number of neurites and the increase in GAP-43 after NGF exposure. Our data are in accordance with Oda et al. (28), showing that the pharmacological blockade of NCX reduces neurite outgrowth in PC12 cells. Accordingly, in cortical neurons, silencing of NCX1 prevented the activation of Akt and protein expression of GAP-43 during in vitro differentiation. Furthermore, silencing of NCX1 reduced MAP2 and prevented neurite outgrowth of cortical neurons (7 DIV), as detected by phalloidin-rhodamine staining and reduced NeuN-positive neurons. On the other hand, in PC12 cells exposed to NGF, NCX3 expression was reduced significantly, whereas NCX2 remained unchanged. Furthermore, NCX1.4 overexpression induced GAP-43 protein expression, neurite outgrowth, and Akt phosphorylation, even in the absence of NGF. Interestingly, neuronal differentiation of PC12 cells was mediated by the nuclear translocation of Akt. In fact, nuclear delivery of a dominant negative form of Akt prevented GAP-43 protein expression in NGF-treated cells. Furthermore, in differentiated PC12 cells, Akt phosphorylation was prevented by NCX1 knockdown and was also induced in a time-dependent manner when the neuronal form of NCX1 was overexpressed.
That Akt activation is involved in neuronal differentiation is a well known fact. However, the pathways involved in its activation remain elusive. In our study, we showed a strong correlation between NCX1 activation and Akt phosphorylation, therefore unraveling a new mechanism for Akt activation in neuronal differentiation. In particular, we showed that, in NCX1-overexpressing cells, a precocious activation of Akt occurred, demonstrating that, in PC12 cells, the exchanger isoform may guide NGF-induced differentiation through kinase phosphorylation. In particular, we showed that NCX1 increased the amount of Ca2+ in ER during PC12 differentiation. Accordingly, the Gq-coupled purinergic receptor agonist ATP plus the irreversible SERCA inhibitor thapsigargin induced a significantly higher increase of [Ca2+]i than in undifferentiated PC12 cells. This is consistent with the acquisition of the neuronal phenotype. Accordingly, Ciccolini et al. (29) reported that neurons become increasingly competent at Ca2+ signaling as differentiation proceeds and show an increased amount of Ca2+ in the ER in the late phase of the process (29). Furthermore, in BDNF-stimulated neuronal differentiation, Akt activation is abolished by the intracellular Ca2+ chelator BAPTA-AM but not by the elimination of extracellular Ca2+ ions with EGTA, therefore reinforcing the role played by stored Ca2+ release during differentiation (30). That NCX1 is involved in the refilling of Ca2+ ions into ER has already been reported as a neuroprotective mechanism to reduce ER stress under hypoxic conditions (31). Our results strongly demonstrated the involvement of the NCX1 reverse mode in mediating ER Ca2+ refilling during neuronal differentiation. Indeed, our data demonstrated that the activation of the reverse mode of NCX1 during neuronal differentiation is linked to the increase in the currents of the voltage-dependent Na+ channels. These currents, by increasing intracellular Na+ concentrations, may force NCX1.4 to work in the reverse mode of operation, as demonstrated previously (32, 33, 34). NCX1.4 working in the Ca2+-influx mode promoted ER Ca2+ refilling, as revealed by the relevant increase in [Ca2+]i observed following ER depletion.
Moreover, that intracellular Ca2+ is essential to gate Akt signaling in NCX1-dependent neuronal differentiation was demonstrated by our data showing that BAPTA-AM prevented both Akt phosphorylation and GAP-43 protein expression, both evoked by NCX1 overexpression. This further suggested a tight relationship between the neuronal isoform of NCX1 and Akt. It should be noted that, in a previous paper, we showed that the PI3K/Akt pathway is one of the main regulators of ncx1 gene transcription (16). Moreover, in this study, we show that NCX1 activated Akt to induce neuronal differentiation. Presumably, Akt could represent an amplification mechanism ensuring continuous ncx1 gene transcription and cell survival in PC12 cells (16).
Several mechanisms could regulate, in a Ca2+-dependent way, the phosphorylation of the Akt transcription factor at the level of the cytosol and, more directly, within the nucleus. Among these mechanisms, PKC-α and CaMK IV could play an important role (35, 36). Furthermore, in PC12 cells, the specific Akt downstream activator PI3K is localized in the nuclear matrix (37) or translocates into the nucleus after NGF exposure (38). We showed consistently that the pharmacological inhibition of PI3K by LY 294002 prevented neuronal differentiation induced by NCX1 overexpression. Therefore, in our model, the PI3K/Akt pathway may play a crucial role in modulating neuronal differentiation induced by NCX1 up-regulation.
Regarding the mechanisms involved in the activation of Akt pathway, our data demonstrated a relevant role played by ERK1/2 activation. This factor could be considered an early NGF mediator in triggering neuronal differentiation. In fact, ERK1/2 not only represents the upstream signal of Akt upon NGF exposure, but it seems to control ER Ca2+ refilling before the activation of NCX1, therefore representing an important regulator of [Ca2+]i homeostasis in neuronal differentiation. Moreover, upon NGF stimulation, ERK1/2 specifically up-regulates NCX1. Likewise, in NGF-untreated cells, NCX1 is the only isoform controlled by JNK (15). All of these findings suggest that ERK1/2 controls neuronal differentiation through a transductional cascade involving the fine regulation of NCX1 function.
However, concerning the mechanisms involved in the turning off of the Akt pathway during differentiation, it should be underlined that phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase (PTEN), a phosphatase that converts phosphatidylinositol 3,4,5-trisphosphate to phosphatidylinositol 3,4-bisphosphate, reduces the ability of PI3K to be recruited at the plasma membrane level for Akt activation. In addition, PTEN appears during NGF-induced elongation of nascent neurites (39) and increases progressively at the early stage of differentiation, possibly to prevent aberrant neurite extension. In conclusion, this study shows that, among NCX isoforms, the neuronal isoform NCX1.4 guides neuronal differentiation in PC12 cells and in primary cortical neurons by promoting ER Ca2+ refilling, PI3K signaling activation, and Akt phosphorylation.
Acknowledgments
We thank Dr. Paola Merolla for editorial revision and Vincenzo Grillo and Carmine Capitale for technical assistance.
This work was supported by Grant COFIN 2008, Ricerca-Sanitaria Grant RF-FSL352059, Ricerca Finalizzata (2006), Progetto-Strategico (2007), Progetto Ordinario (2007), Ricerca Finalizzata (2009), Ricerca-Sanitaria Progetto Ordinario (2008) from the Ministero della Salute (to L. A.) and by Progetto Giovani Ricercatori Grant GR-2010-2318138 from the Ministero della Salute (to A. S.), and Federazione Italiana Sclerosi Multipla progetto R/01 (to F. B.).
- NCX
- Na+/Ca2+ exchanger
- NLS
- nuclear localization signal
- EGFP
- enhanced GFP
- TTX
- tetrodotoxin
- Tg
- thapsigargin
- DIV
- day(s) in vitro
- BAPTA-AM
- (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid).
REFERENCES
- 1. Kolb B., Teskey C. G., Gibb R. (2010) Factors influencing cerebral plasticity in the normal and injured brain. Front. Hum. Neurosci. 4, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang E. J., Reichardt L. F. (2001) Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chao M. V., Hempstead B. L. (1995) p75 and Trk: a two-receptor system. Trends Neurosci. 18, 321–326 [PubMed] [Google Scholar]
- 4. Vaudry D., Stork P. J., Lazarovici P., Eiden L. E. (2002) Signaling pathways for PC12 cell differentiation: making the right connections. Science 296, 1648–1649 [DOI] [PubMed] [Google Scholar]
- 5. Stephens R. M., Loeb D. M., Copeland T. D., Pawson T., Greene L. A., Kaplan D. R. (1994) Trk receptors use redundant transduction pathways involving SHC and PLC-γ 1 to mediate NGF responses. Neuron 12, 691–705 [DOI] [PubMed] [Google Scholar]
- 6. Solem M., McMahon T., Messing R. O. (1995) Depolarization-induced neurite outgrowth in PC12 cells requires permissive, low level NGF receptor stimulation and activation of calcium/calmodulin-dependent protein kinase. J. Neurosci. 15, 5966–5975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shibata A., Laurent C. E., Smithgall T. E. (2003) The c-Fes protein-tyrosine kinase accelerates NGF-induced differentiation of PC12 through a PI3K-dependent mechanism. Cell Signal. 15, 279–288 [DOI] [PubMed] [Google Scholar]
- 8. Wang Z., Wang J., Li J., Wang X., Yao Y., Zhang X., Li C., Cheng Y., Ding G., Liu L., Ding Z. (2011) MEK/ERKs signaling is essential for lithium-induced neurite outgrowth inN2a cells. Int. J. Dev. Neurosci. 29, 415–422 [DOI] [PubMed] [Google Scholar]
- 9. Kater S. B., Mills L. R. (1991) Regulation of growth cone behavior by calcium. J. Neurosci. 11, 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blaustein M. P., Lederer W. J. (1999) Sodium/calcium exchange: its physiological implications. Physiol. Rev. 79, 763–854 [DOI] [PubMed] [Google Scholar]
- 11. Annunziato L., Pignataro G., Di Renzo G. F. (2004) Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol. Rev. 56, 633–654 [DOI] [PubMed] [Google Scholar]
- 12. Nicoll D. A., Longoni S., Philipson K. D. (1990) Molecular cloning and functional expression of the cardiac sarcolemmal Na+-Ca2+exchanger. Science 250, 562–565 [DOI] [PubMed] [Google Scholar]
- 13. Li Z., Matsuoka S., Hryshko L. V., Nicoll D. A., Bersohn M. M., Burke E. P., Lifton R. P., Philipson K. D. (1994) Cloning of the NCX2 isoform of the plasma membrane Na+-Ca2+ exchanger. J. Biol. Chem. 269, 17434–17439 [PubMed] [Google Scholar]
- 14. Nicoll D. A., Quednau B. D., Qui Z., Xia Y. R., Lusis A. J., Philipson K. D. (1996) Cloning of a third mammalian Na+-Ca2+ exchanger, NCX3. J. Biol. Chem. 271, 24914–24921 [DOI] [PubMed] [Google Scholar]
- 15. Sirabella R., Secondo A., Pannaccione A., Molinaro P., Formisano L., Guida N., Di Renzo G., Annunziato L., Cataldi M. (2012) ERK1/2, p38, and JNK regulate the expression and the activity of the three isoforms of the Na+ /Ca2+ exchanger, NCX1, NCX2, and NCX3, in neuronal PC12 cells. J. Neurochem. 122, 911–922 [DOI] [PubMed] [Google Scholar]
- 16. Formisano L., Saggese M., Secondo A., Sirabella R., Vito P., Valsecchi V., Molinaro P., Di Renzo G., Annunziato L. (2008) The two isoforms of the Na+/Ca2+ exchanger, NCX1 and NCX3, constitute novel additional targets for the prosurvival action of Akt/protein kinase B pathway. Mol. Pharmacol. 73, 727–737 [DOI] [PubMed] [Google Scholar]
- 17. Valsecchi V., Pignataro G., Del Prete A., Sirabella R., Matrone C., Boscia F., Scorziello A., Sisalli M. J., Esposito E., Zambrano N., Di Renzo G., Annunziato L. (2011) NCX1 is a novel target gene for hypoxia-inducible factor-1 in ischemic brain preconditioning. Stroke 42, 754–763 [DOI] [PubMed] [Google Scholar]
- 18. Secondo A., Staiano R. I., Scorziello A., Sirabella R., Boscia F., Adornetto A., Valsecchi V., Molinaro P., Canzoniero L. M., Di Renzo G., Annunziato L. (2007) BHK cells transfected with NCX3 are more resistant to hypoxia followed by reoxygenation than those transfected with NCX1 and NCX2: Possible relationship with mitochondrial membrane potential. Cell Calcium 42, 521–535 [DOI] [PubMed] [Google Scholar]
- 19. Secondo A., Molinaro P., Pannaccione A., Esposito A., Cantile M., Lippiello P., Sirabella R., Iwamoto T., Di Renzo G., Annunziato L. (2011) Nitric oxide stimulates NCX1 and NCX2 but inhibits NCX3 isoform by three distinct molecular determinants. Mol. Pharmacol. 79, 558–568 [DOI] [PubMed] [Google Scholar]
- 20. Grynkiewicz G., Poenie M., Tsien R. Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 21. Urbanczyk J., Chernysh O., Condrescu M., Reeves J. P. (2006) Sodium-calcium exchange does not require allosteric calcium activation at high cytosolic sodium concentrations. J. Physiol. 575, 693–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pannaccione A., Secondo A., Molinaro P., D'Avanzo C., Cantile M., Esposito A., Boscia F., Scorziello A., Sirabella R., Sokolow S., Herchuelz A., Di Renzo G., Annunziato L. (2012) A new concept: Aβ1–42 generates a hyperfunctional proteolytic NCX3 fragment that delays caspase-12 activation and neuronal death. J. Neurosci. 32, 10609–10617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bradford M. M. (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 24. He L. P., Cleemann L., Soldatov N. M., Morad M. (2003) Molecular determinants of cAMP-mediated regulation of the Na+-Ca2+ exchanger expressed in human cell lines. J. Physiol. 548, 677–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Secondo A., Pannaccione A., Molinaro P., Ambrosino P., Lippiello P., Esposito A., Cantile M., Khatri P. R., Melisi D., Di Renzo G., Annunziato L. (2009) Molecular pharmacology of the amiloride analog 3-amino-6-chloro-5-[(4-chloro-benzyl)amino]-n-[[(2,4-dimethylbenzyl)-amino]iminomethyl]-yrazinecarboxamide (CB-DMB) as a pan inhibitor of the Na+-Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in stably transfected cells. J. Pharmacol. Exp. Ther. 331, 212–221 [DOI] [PubMed] [Google Scholar]
- 26. Molinaro P., Cuomo O., Pignataro G., Boscia F., Sirabella R., Pannaccione A., Secondo A., Scorziello A., Adornetto A., Gala R., Viggiano D., Sokolow S., Herchuelz A., Schurmans S., Di Renzo G., Annunziato L. (2008) Targeted disruption of Na+/Ca2+ exchanger 3 (NCX3) gene leads to a worsening of ischemic brain damage. J. Neurosci. 28, 1179–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vojtek A. B., Taylor J., DeRuiter S. L., Yu J. Y., Figueroa C., Kwok R. P., Turner D. L. (2003) Akt regulates basic helix-loop-helix transcription factor-coactivator complex formation and activity during neuronal differentiation. Mol. Cell. Biol. 23, 4417–4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oda T., Kume T., Izumi Y., Ishihara K., Sugmimoto H., Akaike A. (2011) Na+/Ca2+ exchanger inhibitors inhibit neurite outgrowth in PC12 cells. J. Pharmacol. Sci. 116, 128–131 [DOI] [PubMed] [Google Scholar]
- 29. Ciccolini F., Collins T. J., Sudhoelter J., Lipp P., Berridge M. J., Bootman M. D. (2003) Local and global spontaneous calcium events regulate neurite outgrowth and onset of GABAergic phenotype during neural precursor differentiation. J. Neurosci. 23, 103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zheng F., Soellner D., Nunez J., Wang H. (2008) The basal level of intracellular calcium gates the activation of phosphoinositide 3-kinase-Akt signaling by brain-derived neurotrophic factor in cortical neurons. J. Neurochem. 106, 1259–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sirabella R., Secondo A., Pannaccione A., Scorziello A., Valsecchi V., Adornetto A., Bilo L., Di Renzo G., Annunziato L. (2009) Anoxia-induced NF-κB-dependent upregulation of NCX1 contributes to Ca2+ refilling into endoplasmic reticulum in cortical neurons. Stroke 40, 922–929 [DOI] [PubMed] [Google Scholar]
- 32. Bouron A., Becker C., Porzig H. (1999) Functional expression of voltage-gated Na+ and Ca2+ channels during neuronal differentiation of PC12 cells with nerve growth factor or forskolin. Naunyn Schmiedebergs Arch. Pharmacol. 359, 370–377 [DOI] [PubMed] [Google Scholar]
- 33. Drummond H. A., Furtado M. M., Myers S., Grifoni S., Parker K. A., Hoover A., Stec D. E. (2006) ENaC proteins are required for NGF-induced neurite growth. Am. J. Physiol. Cell. Physiol. 290, C404–C410 [DOI] [PubMed] [Google Scholar]
- 34. Larbig R., Torres N., Bridge J. H., Goldhaber J. I., Philipson K. D. (2010) Activation of reverse Na+-Ca2+ exchange by the Na+ current augments the cardiac Ca2+ transient: evidence from NCX knockout mice. J. Physiol. 588, 3267–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cárdenas C., Müller M., Jaimovich E., Pérez F., Buchuk D., Quest A. F., Carrasco M. A. (2004) Depolarization of skeletal muscle cells induces phosphorylation of cAMP response element binding protein via calcium and protein kinase Cα. J. Biol. Chem. 279, 39122–39131 [DOI] [PubMed] [Google Scholar]
- 36. Hardingham G. E., Cruzalegui F. H., Chawla S., Bading H. (1998) Mechanisms controlling gene expression by nuclear calcium signals. Cell Calcium 23, 131–134 [DOI] [PubMed] [Google Scholar]
- 37. Neri L. M., Borgatti P., Capitani S., Martelli A. M. (2002) The nuclear phosphoinositide 3-kinase/AKT pathway: a new second messenger system. Biochim. Biophys. Acta 1584, 73–80 [DOI] [PubMed] [Google Scholar]
- 38. Salinas M., López-Valdaliso R., Martín D., Alvarez A., Cuadrado A. (2000) Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide-activated protein phosphatase in PC12 cells. Mol. Cell. Neurosci. 15, 156–169 [DOI] [PubMed] [Google Scholar]
- 39. Musatov S., Roberts J., Brooks A. I., Pena J., Betchen S., Pfaff D. W., Kaplitt M. G. (2004) Inhibition of neuronal phenotype by PTEN in PC12 cells. Proc. Natl. Acad. Sci. U.S.A. 101, 3627–3631 [DOI] [PMC free article] [PubMed] [Google Scholar]