Background: Never in mitosis gene A (NIMA)-related kinase 8 (NEK8) regulation is poorly understood.
Results: Hypoxia induced NEK8 expression in vitro and in vivo. NEK8-siRNA transfection blocked pVHL-knockdown-induced cilia disassemble.
Conclusion: pVHL down-regulates NEK8 via HIFs to maintain the primary cilia.
Significance: Identification of a new target for HIF. NEK8 was involved in pVHL modulating cilia.
Keywords: Cancer Biology, Cell Signaling, Cilia, Hypoxia, Hypoxia-inducible Factor (HIF), NEK8, pVHL, Renal Carcinoma
Abstract
NEK8 (never in mitosis gene A (NIMA)-related kinase 8) is involved in cytoskeleton, cilia, and DNA damage response/repair. Abnormal expression and/or dysfunction of NEK8 are related to cancer development and progression. However, the mechanisms that regulate NEK8 are not well declared. We demonstrated here that pVHL may be involved in regulating NEK8. We found that CAK-I cells with wild-type vhl expressed a lower level of NEK8 than the cells loss of vhl, such as 786-O, 769-P, and A-498 cells. Moreover, pVHL overexpression down-regulated the NEK8 protein in 786-O cells, whereas pVHL knockdown up-regulated NEK8 in CAK-I cells. In addition, we found that the positive hypoxia response elements (HREs) are located in the promoter of the nek8 sequence and hypoxia could induce nek8 expression in different cell types. Consistent with this, down-regulation of hypoxia-inducible factors α (HIF-1α or HIF-2α) by isoform-specific siRNA reduced the ability of hypoxia inducing nek8 expression. In vivo, NEK8 and HIF-1α expression were increased in kidneys of rats subjected to an experimental hypoxia model of ischemia and reperfusion. Furthermore, NEK8 siRNA transfection significantly blocked pVHL-knockdown-induced cilia disassembling, through impairing the pVHL-knockdown-up-regulated NEK8 expression. These results support that nek8 may be a novel hypoxia-inducible gene. In conclusion, our findings show that nek8 may be a new HIF target gene and pVHL can down-regulate NEK8 via HIFs to maintain the primary cilia structure in human renal cancer cells.
Introduction
Never in mitosis gene A (NIMA)3-related kinase 8 (NEK8) is a member of the serine/threonine-specific protein kinase family related to NIMA of Aspergillus nidulans that has been shown to have a role in the progression of mitosis. The family of human NEK kinases currently contains 11 members, named NEK1 to NEK11 (1–4). NEK2 is the closest NIMA homolog and required for G2/M progression and centrosome maturation during mitosis; NEK6 and NEK7 are components of a mitotic kinase cascade; NEK9 plays a role in chromosome alignment and segregation during mitosis; NEK1 and NEK8 are critical in cilia, cell cycle, and linked to activation of DNA damage response (1, 4, 5).
NEK8 is a new number of the NEK kinases family. The open reading frame of human nek8 encodes a 692-amino acid protein with a calculated molecular mass of 75 kDa. This protein has an N-terminal catalytic domain, a typical character of serine/threonine kinases, and a C-terminal domain, which bears homology to the seven-bladed β-propeller of the renal cell carcinoma 1 GTPase exchange factor. Unlike other NEK family numbers mostly being involved in cell cycle regulation, NEK8 was starting to be linked to cell cycle independent on microtubule dynamics (6). The well known function of NEK8 is its role in cilia, the microtubule-based structures that are nucleated from basal bodies (7–11)., NEK8 has been recently identified as a new effector of the ATR-mediated replication stress response, a critical component of the DNA damage response that links replication stress with cystic kidney disorders (5, 12). NEK8 dysfunction has been linked with polycystic kidney disease and some cancer (7, 8, 10, 11, 13–17). However, the mechanism underlying regulation of the NEK8 is poorly understood.
Von Hippel-Lindau syndrome (VHL) is a dominantly inherited familial cancer syndrome predisposing to a variety of malignant and benign tumors (18). It is well known that VHL protein (pVHL, the product of the vhl tumor suppressor gene) functions as the substrate recognition component of an E3-ubiquitin ligase complex that targets hypoxia-inducible factor α (HIF-α) for ubiquitination and degradation (19, 20). In the presence of oxygen, HIF-α subunits are hydroxylated by the HIF prolyl hydroxylases, and thus generates a binding site for pVHL. Under hypoxia or when pVHL is inactivated, stabilized HIF translocates to the nucleus, binds to HIF-β, and induces many genes (18, 20, 21). pVHL interacts with many other proteins in addition to HIF-α and has multiple functions, including microtubule dynamics, cell proliferation, neuronal apoptosis, extracellular matrix deposition, DNA damage response, and primary cilia maintenance (22, 23). pVHL can regulate primary cilia through both HIF-dependent and HIF-independent mechanisms (24–26). In the current report, we first show that NEK8 may be a new target gene of HIFs and pVHL can down-regulate NEK8 via HIFs to maintain the primary cilia structure in human renal cancer cells, which contributes to improve our knowledge of NEK8 regulation.
MATERIALS AND METHODS
Cell Culture and Reagents
CAK-I, 786-O, 769-P, and A-498 human renal cancer cells obtained from ATCC were cultured in RPIM-1640 medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (Hangzhou Sijiqing Biological Engineering Materials Co., Ltd., Hangzhou, China), l-glutamine (2 mm), penicillin (100 IU/ml), streptomycin (100 μg/ml), and HEPES (10 mm, pH 7.4). Cells were incubated in a humidified atmosphere of 95% air plus 5% CO2 at 37 °C. CoCl2 was obtained from Zhiyuan Chemical Reagent Co., Ltd. (Tianjin, China). Dimethyloxaloylglycine (DMOG) was obtained from MCE (Shanghai, China).
Immunoblotting
Immunoblotting was conducted with standard procedures (27), using antibodies against NEK8, HIF-1α, HIF-2α, and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA), PC1, and VHL (Cell Signaling Technology, Beverly, MA).
Real-time Quantitative PCR
Total RNA was extracted with TRIzol according to the manufacturer's instructions and transcribed using Prime ScriptTM RT reagent Kit (TaKaRa, Dalian, China). The cDNA template was amplified by real-time PCR using SYBR-PremixExTaqTM Kit (TaKaRa, Dalian, China). The primer sequences were as follows: 5′-CTCCAAGATCCTTAGCAGCAAGA-3′ (forward), 5′-GGCTTGCCCTCACACAGCT-3′ (reverse) for NEK8, 5′-GCACCGTCAAGGCTGAGAAC-3′ (forward), 5′-GCCTTCTCCATGGTGGTGAA-3′ (reverse) for GAPDH. Thermal cycling was programmed as follows: 95 °C for 30 s followed by 40 cycles of 95 °C for 5 s, 60 °C for 20 s, and 72 °C for 15 s, and then 72 °C for 10 min. Gene expression was assessed by ΔCt method and mRNA levels of nek8 were normalized to those of the GAPDH internal standard.
Transfection of siRNA
Synthetic siRNA were purchased from Shanghai GenePharma Co., Ltd. with sequences as follows: hif-1α, 5′-TACGTTGTGAGTGGTATTATT; 5′-CUGAUGACCAGCAACUUGATT; hif-2α, 5′-CCCGGATAGACTTATTGCCAA; 5′-GCGACAGCTGGAGTATGAA; nek8, 5′-UCGUCAAGAUCGGUGAUUUTT-3′; 5′-CUGGAAGACAAAGCCCUUATT-3′; 5′-GUGGUAUCGAUUCCUCCAUTT-3′; and vhl, 5′-CCAAUGGAUUCAUGGAGUA-3′; 5′-GGAGCGCAUUGCACAUCAA-3′; 5′-CCACCCAAAUGUGCAGAAA-3′. NC-siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-AATTCTCCGAACGTGTCACGT, was used as a negative control. The siRNA were transfected into cells using siRNA-Mate (Shanghai GenePharma Co., Ltd, Shanghai, China) according to the manufacturer's instructions.
Visualizing and Measuring Renal Cilia
Cultures were fixed in 4% paraformaldehyde in PBS for 30 min, treated with 0.2% Triton X-100 in PBS for 15 min, blocked in 2.5% BSA, and stained with a primary antibody against acetylated tubulin (Sigma) and anti-mouse Texas Red AffiniPure (Molecular Probes) to visualize the cilia and DAPI (49,6-diamidino-2-phenylindole) to visualize nuclei. Cultures were mounted in ProLong Gold antifade reagent (Invitrogen) and examined on a Provis fluorescence microscope (Olympus). Images of randomly chosen fields (×40) were collected, and AnalySIS version 5.0 software (Olympus) was used to trace the cilia in captured images.
Renal Ischemia/Reperfusion (I/R) Injury
Adult male Sprague-Dawley rats (250 g, n = 5 for each group) were anesthetized and maintained at body temperature of 37 °C. Both kidneys were exposed by a flank incision, and both renal arteries were occluded with a non-traumatic vascular clamp for 30 min. After 30 min of clamping, clamps were removed, renal blood flow was re-established, both incisions were sutured, and rats were allowed to recover in a warm room. Rats were euthanized at 24, 48, and 72 h after reperfusion; both kidneys were removed and processed for Western blotting. A group of sham animals were included; the kidneys of these animals were exposed by a flank incision, but they did not receive renal artery occlusion.
All animals used in the study were housed and cared for in accordance with the Chinese Pharmacological Society Guidelines for Animal Use. The work was approved by the Committee on the Ethics of Animal Experiments of the Taizhou University.
RESULTS
VHL-defective Renal Cancer Cells Expressed High Level of NEK8
We first investigated the NEK8 expression patterns in human renal carcinoma cells. As shown in Fig. 1A, 786-O, 769-P, and A-498 cells expressed high levels of NEK8, whereas CAK-I cells expressed very low levels of NEK8. A similar pattern was observed at the mRNA level of nek8 in these cells (Fig. 1B). Interestingly, it appeared that those cells expressing high levels of NEK8 were exactly the VHL-defective renal carcinoma cells, which indicated that pVHL was involved in down-regulation of NEK8.
FIGURE 1.
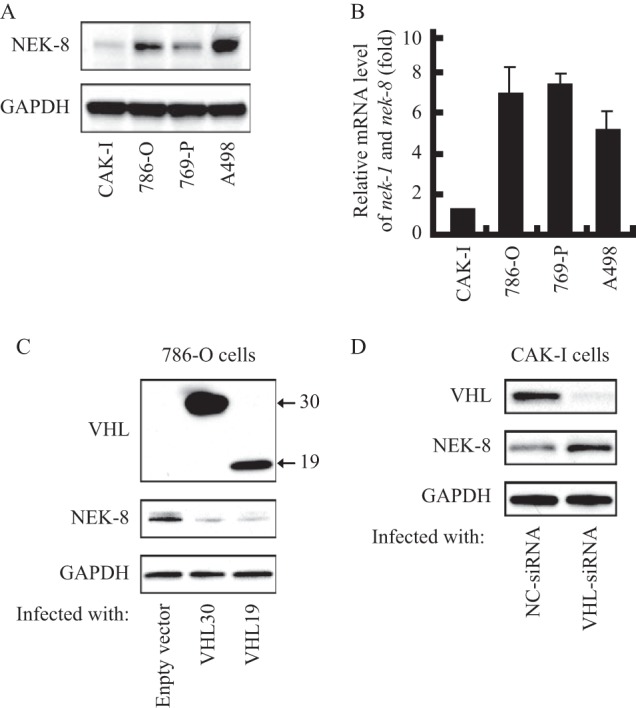
pVHL down-regulated NEK8. A, NEK8 protein expression profiles in renal cancer cells. GAPDH was employed as a loading control. B, nek8 mRNA levels in renal cancer cells. Basal levels of nek8 were measured by real-time PCR (with normalization relative to GAPDH levels). Data shown are fold-change relative to the nek8 levels in CAK-I cells. C, overexpression of pVHL leads to NEK8 degradation. 786-O cells were transfected with pcDNA3.0-VHL30, pcDNA3.0-VHL19, or empty vector to collect whole cell lysate 48 h later for immunoblot analysis of pVHL and NEK8, GAPDH was employed as a loading control. D, pVHL knockdown un-regulated NEK8. CAK-I cells were transfected with VHL-siRNA or negative control siRNA to collect whole cell lysate 48 h later for immunoblot analysis of pVHL and NEK8, GAPDH was employed as a loading control.
To determine whether there was a possible regulation of NEK8 by pVHL, we next examined the effect of pVHL overexpression on NEK8 in 786-O cells. As shown in Fig. 1C, pVHL was obviously increased in 786-O cells transfected with pcDNA3.0-VHL30 for 48 h. Compared with empty vector, the transfection of pVHL30 led to a noticeable decrease of NEK8 (Fig. 1C). As we know, the VHL protein is produced in two forms, 19- and 30-kDa proteins. Both isoforms appear to retain similar tumor suppressor activity (28). We next detected whether the short form pVHL could down-regulate the NEK8 protein. As shown in Fig. 1C, the protein level of NEK8 was remarkably decreased in cells transfected with pVHL19.
To further verify the obtained results that pVHL could down-regulate NEK8, we next studied whether pVHL knockdown would affect NEK8. As shown in Fig. 1D, the transfection of vhl-specific siRNA in CAK-I cells significantly reduced the protein level of pVHL. Accordingly, knockdown of pVHL markedly induced NEK8 expression in CAK-I cells. Based on the data, we can see that pVHL is related to the regulation of NEK8.
pVHL Down-regulated NEK8 via Hypoxia Inducible Factors
pVHL employs multiple functions that have relationships to tumor suppression, among which the best recognized and closely related to the development of renal cell carcinoma is the role in targeting hypoxia-inducible factors (HIFs) for ubiquitin-mediated degradation (18, 21). Therefore, we suppose that NEK8 may be a target of HIFs. We first analyzed the promoter of nek8 and found some possible hypoxia reaction elements (HRE). Among seven putative HREs in the nek8 promoter, results of the chromatin immunoprecipitation (ChIP) assay showed a specific binding of HIF-1α to region 2 (−643 to −639) upstream of the transcription initiation (Fig. 2A), indicating that nek8 may be a hypoxia-induced gene.
FIGURE 2.
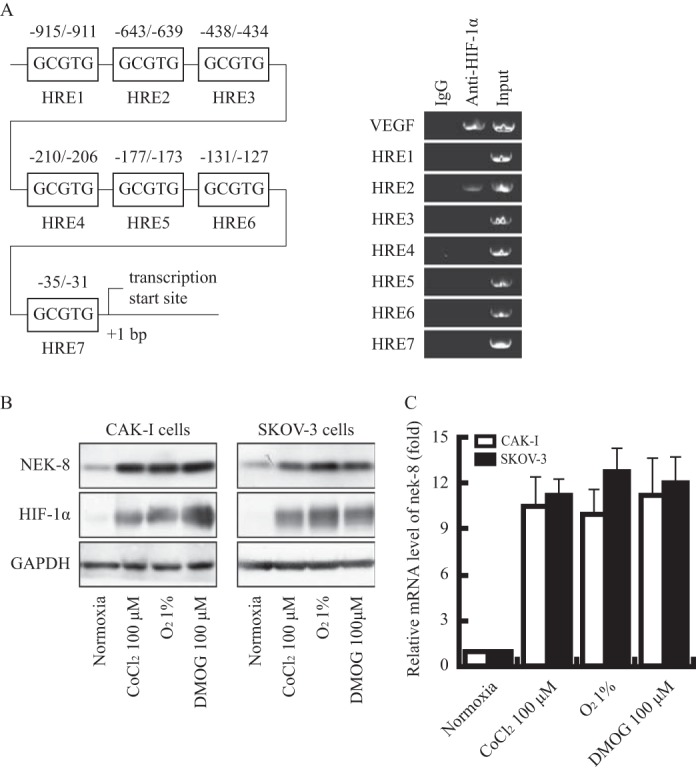
nek8 is a new target gene of HIFs. A, left: schematic representation of putative hypoxia responsive element sites in the proximal nek8 promoter. Right, semi-quantitative PCR with ChIP samples obtained using HIF-1α or control antibodies. B, hypoxia induced the expression of NEK8. Cells were treated with CoCl2 (100 μm) or DMOG (100 μm) for 6 h or cultured for 12 h in hypoxia (1.0% oxygen). HIF-1α and NEK8 were analyzed by immunoblotting, GAPDH was employed as a loading control. C, values are the fold-increase in mRNA in cells treated with CoCl2 (100 μm) or DMOG (100 μm) for 6 h or cultured for 12 h in hypoxia (1.0% oxygen) relative to parallel cultures in normoxia (21% oxygen).
To verify this, we exposed two cell lines to hypoxia and detected the expression at both protein and mRNA levels by immunoblotting and real-time quantitative PCR. As shown in Fig. 2B, HIF-1α protein expression was obviously up-regulated in CAK-I and SKOV-3 cells after incubation with CoCl2 (100 μm) or deprivation from oxygen (1% O2), suggesting that the hypoxia models worked well. Expression of the NEK8 protein was consistently up-regulated in all cell lines in response to hypoxia (Fig. 2, B and C). At the same time, we measured nek8 mRNA using the same treatment of cells. Similar up-regulation patterns were observed (Fig. 2, B and C).
To further explore the dependence of NEK8 induction on the HIFs system, we also used DMOG, an inhibitor of prolyl hydroxylase and the asparaginyl hydroxylase factor to inhibit HIF. The results were shown in the Fig. 2, B and C. DMOG (100 μm) treatment could also significantly induce nek8 expression in both CAKI and SKOV3 cells.
HIF is the key transcription factor that regulates cellular responses to hypoxia. To further determine whether HIF regulates NEK8, we examined the hypoxia-induced NEK8 expression after down-regulation of HIF-1α or HIF-2α by transient transfection of siRNA targeting HIF-1α or HIF-2α. As shown in Fig. 3, A and B, transfection of isoform-specific siRNA in CAK-I cells apparently reduced the protein level of HIF-1α or HIF-2α. Knockdown of both HIF-1α and HIF-2α with two different independent hairpins remarkably impaired hypoxia-induced NEK8 expression. Altogether, these results show that NEK8 is regulated by both HIF-α subunits in response to hypoxia.
FIGURE 3.
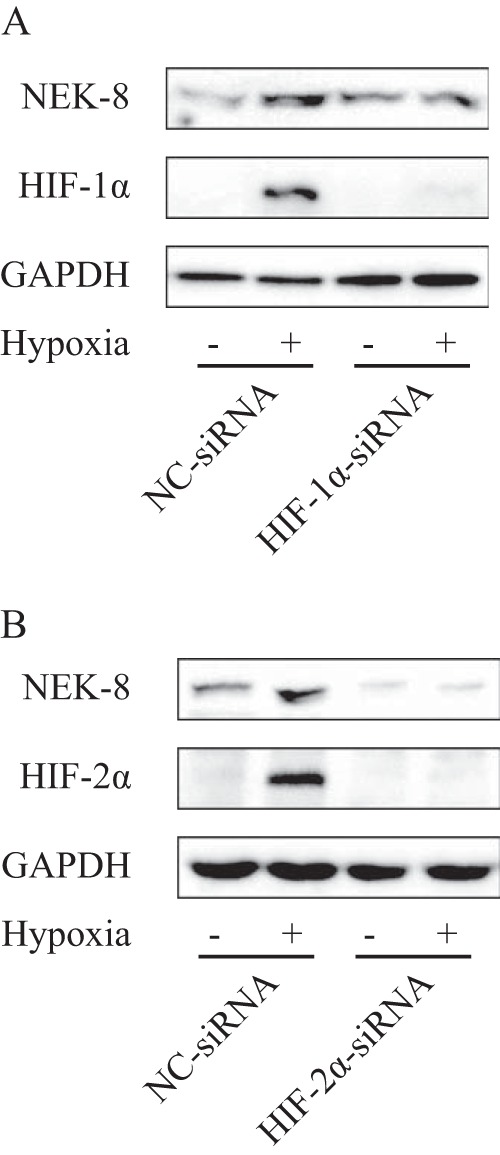
Down-regulation of the HIF subunit by transient transfection of siRNA targeting HIF-1α or HIF-2α blocked NEK8 up-regulation induced by hypoxia. After transfection with the respective siRNA for 24 h, CAK-I cells were cultured in hypoxia for 12 h. NEK8 and HIF-1α (A) or HIF-2α (B) were analyzed by immunoblotting, GAPDH was employed as a loading control.
NEK8 Expression in Kidney Exposed to Experimental I/R
Up-regulation of the NEK8 expression profile was further evaluated in animal hypoxia model. NEK8 expression was examined in kidneys from sham and experimental I/R animals by Western blot. From Fig. 4, we can see that after 48 h of reperfusion, the abundance of NEK8 protein was significantly increased in kidneys from experimental I/R animals, compared with that from sham animals. By contrast, 24 h after reperfusion, kidneys of experimental I/R animals did express a much higher level of HIF-1α protein than that of sham animals. These outcomes indicated that hypoxia was a positive regulator of NEK8 in vivo and HIF induction was earlier than NEK8.
FIGURE 4.
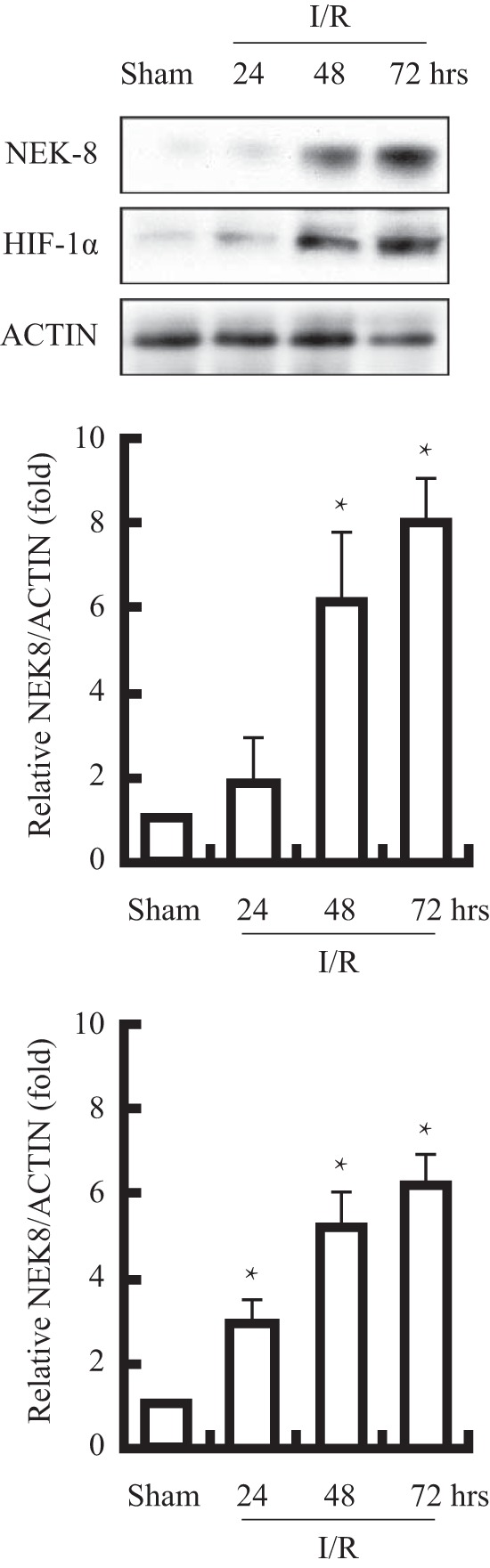
NEK8 protein abundance in kidneys of rats exposed to experimental I/R was determined by immunoblotting, GAPDH was employed a loading control. A representative picture is shown in the upper section. Bar graph represents mean ± S.D. * indicates p < 0.05; n = 5 (* versus sham).
NEK8 Was Involved in pVHL Maintaining the Primary Cilia Structure
A novel function of pVHL is its influence on the development and function of the primary cilium, the singular and antenna-like structures that appear on the surface of most differentiated vertebrate cells. pVHL can regulate primary cilia through both HIF-dependent and HIF-independent mechanisms (23). We also detected primary cilia structure in human renal cancer cell CAK-I with wild-type vhl. Whereas, there was no cilium in 786-O cells with loss of vhl, the observation was consistent with previous reports that loss of pVHL results in aberrant orientation of newly formed microtubules and prevents ciliogenesis (29). Considering the fact that both pVHL and NEK8 play a role in ciliary regulation and cyst formation, and our above results that cells with wild-type vhl express higher levels of NEK8 than that of cells with loss of vhl, we postulated that NEK8 may be linked to pVHL in maintaining cilia.
To further distinguish the role of NEK8 in pVHL-regulated cilia, we examined the effect of pVHL and NEK8 knockdown on cilia in cells with wild-type vhl. As shown in the Fig. 5A, pVHL was significantly decreased in CAK-I cells transfected with VHL-siRNA for 48 h, by contrast, the NEK8 protein was obviously up-regulated. When the cell was co-transfected with VHL-siRNA and NEK8-siRNA, both pVHL and NEK8 protein were significantly down-regulated, indicating that NEK8-siRNA transfection blocked the VHL-knockdown-induced NEK8 up-regulation (Fig. 5A). Compared with NC-siRNA, the transfection of VHL-siRNA apparently decreased the number of cilia, whereas, co-transfection of NEK8-siRNA impaired VHL-knockdown-induced cilia disassemble (Fig. 5B). The results showed that pVHL may maintain the cell cilia structure via down-regulating the NEK8 protein.
FIGURE 5.
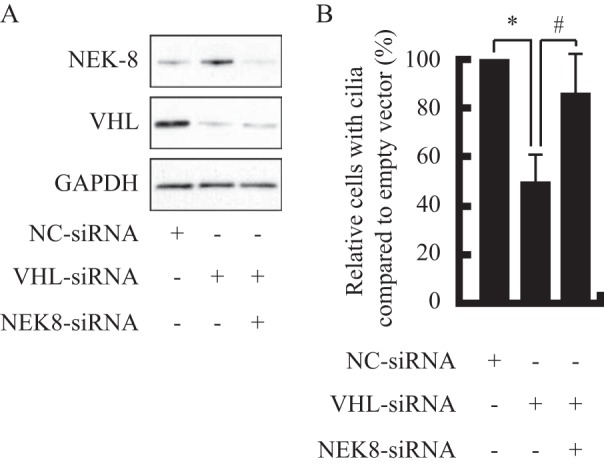
pVHL maintain cilia structure via down-regulating NEK8. A, CAK-I cells transfected with VHL-siRNA or co-transfected with VHL-siRNA and NEK8-siRNA for 48 h were analyzed by immunoblotting, GAPDH was employed a loading control. B, effects of pVHL knockdown or co-transfection of siRNA targeting VHL and NEK8 on the cilia numbers were determined by immunofluorescence. Quantitative data (mean ± S.D. of three independent experiments) were shown. * and #, p < 0.05, difference with NC-siRNA or VHL-siRNA group.
DISCUSSION
In the present study, we showed that vhl-defective renal cancer cells 786-O, 769-P, and A-498 expressed higher levels of NEK8, compared with CAK-I cells with wild-type vhl. Re-expression of pVHL reduced NEK8 in vhl-defective 786-O cells, whereas down-regulation of pVHL by VHL-siRNA induced the expression of NEK8 in CAK-I cells with wild-type vhl. pVHL appeared to be involved in regulating NEK8. As has been reported, pVHL targets are HIFs and there are positive HREs in the promoter of nek8 sequence, suggesting that nek8 may be a novel hypoxia-inducible gene. The fact that hypoxia-induced nek8 expression and HIFs knockdown reduced the ability of hypoxia-inducing NEK8 expression confirmed the above hypothesis. We also found that NEK8 overexpression or pVHL knockdown in cells with wild-type vhl obviously decreased the number of cilia. In a word, these results indicate that nek8 may be a new HIF target gene and pVHL can down-regulate NEK8 via HIFs to maintain the primary cilia structure in human renal cancer cells.
NEK8 belongs to the NIMA-related serine-threonine kinase family, which is involved in cilia dynamics, cell cycle progression, and DNA damage response (12). In 2002, Holland and colleagues (6) purified, cloned, and characterized the human NEK8 and its candidate substrate Bicd2. NEK8 is capable of autophosphorylation and oligomerization (6). Its activity is not cell cycle regulated, but like other NEK family members, NEK8 protein expression levels are consistently higher in G0-arrested cells (6). However, the mechanism underlying the regulation of NEK8 is unclear. In this study, we showed that the pVHL was involved in regulating NEK8. We further found that there are some possible HRE in the promoter of nek8 gene sequence. HIFα subunits heterodimerize with HIFβ subunits, and can bind to the same DNA sequence called HRE (5′-RCGTG-3′, r = A/G), then promote gene transcription. It indicates that nek8 may be a novel target of HIFs, which was verified by the following experiments, hypoxia-induced nek8 expression in the different cell types and HIFs knockdown reduced the ability of hypoxia inducing NEK8 expression.
The primary cilia structure can only be detected in human renal cancer cell CAK-I with wild-type vhl but not in 786-O cells with loss of vhl, which is consistent with the previous report that pVHL was utilized as an essential part for cilia assembling (23–25, 29). Moreover, the cells with wild-type vhl expressed higher levels of NEK8 than the cells with loss of vhl. Therefore, we suspected that NEK8 may be linked to pVHL in maintaining cilia. The transfection of NEK8-siRNA blocked the pVHL-knockdown-induced cilia disassemble in cells with wild-type vhl, supporting the notion that pVHL down-regulates NEK8 to maintain cilia.
Based on our data, we draw a conclusion that NEK8 may be a new target gene of HIFs and pVHL can down-regulate NEK8 via HIFs to maintain the primary cilia structure in human renal cancer cells. To the best of our knowledge, this is the first report declaring that NEK8 may be a new target gene of HIFs.
This work was supported by National Natural Science Foundation of China Grant 81201530, Zhejiang Provincial Natural Science Foundation of China Grants Y15H310005 and Y15H310006, Foundation of Zhejiang Educational Committee Grant Y201121896, and Key Disciplines of Applied Chemistry of Zhejiang Providence, Taizhou University.
- NIMA
- never in mitosis gene A
- NEK8
- NIMA-related kinase 8
- VHL
- Von Hippel-Lindau syndrome
- HIFα
- hypoxia inducible factor α
- DMOG
- dimethyloxaloylglycine
- I/R
- ischemia/reperfusion
- HRE
- hypoxia reaction element.
REFERENCES
- 1. Fry A. M., O'Regan L., Sabir S. R., Bayliss R. (2012) Cell cycle regulation by the NEK family of protein kinases. J. Cell Sci. 125, 4423–4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quarmby L. M., Mahjoub M. R. (2005) Caught Nek-ing: cilia and centrioles. J. Cell Sci. 118, 5161–5169 [DOI] [PubMed] [Google Scholar]
- 3. O'Regan L., Blot J., Fry A. M. (2007) Mitotic regulation by NIMA-related kinases. Cell Div. 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moniz L., Dutt P., Haider N., Stambolic V. (2011) Nek family of kinases in cell cycle, checkpoint control and cancer. Cell Div. 6, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi H. J., Lin J. R., Vannier J. B., Slaats G. G., Kile A. C., Paulsen R. D., Manning D. K., Beier D. R., Giles R. H., Boulton S. J., Cimprich K. A. (2013) NEK8 links the ATR-regulated replication stress response and S phase CDK activity to renal ciliopathies. Mol. Cell 51, 423–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holland P. M., Milne A., Garka K., Johnson R. S., Willis C., Sims J. E., Rauch C. T., Bird T. A., Virca G. D. (2002) Purification, cloning, and characterization of Nek8, a novel NIMA-related kinase, and its candidate substrate Bicd2. J. Biol. Chem. 277, 16229–16240 [DOI] [PubMed] [Google Scholar]
- 7. Zalli D., Bayliss R., Fry A. M. (2012) The Nek8 protein kinase, mutated in the human cystic kidney disease nephronophthisis, is both activated and degraded during ciliogenesis. Hum. Mol. Genet. 21, 1155–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Natoli T. A., Gareski T. C., Dackowski W. R., Smith L., Bukanov N. O., Russo R. J., Husson H., Matthews D., Piepenhagen P., Ibraghimov-Beskrovnaya O. (2008) Pkd1 and Nek8 mutations affect cell-cell adhesion and cilia in cysts formed in kidney organ cultures. Am. J. Physiol. Renal Physiol. 294, F73–F83 [DOI] [PubMed] [Google Scholar]
- 9. Sohara E., Luo Y., Zhang J., Manning D. K., Beier D. R., Zhou J. (2008) Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J. Am. Soc. Nephrol. 19, 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trapp M. L., Galtseva A., Manning D. K., Beier D. R., Rosenblum N. D., Quarmby L. M. (2008) Defects in ciliary localization of Nek8 is associated with cystogenesis. Pediatr. Nephrol. 23, 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahjoub M. R., Trapp M. L., Quarmby L. M. (2005) NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J. Am. Soc. Nephrol. 16, 3485–3489 [DOI] [PubMed] [Google Scholar]
- 12. Jackson P. K. (2013) Nek8 couples renal ciliopathies to DNA damage and checkpoint control. Mol. Cell 51, 407–408 [DOI] [PubMed] [Google Scholar]
- 13. Bowers A. J., Boylan J. F. (2004) Nek8, a NIMA family kinase member, is overexpressed in primary human breast tumors. Gene 328, 135–142 [DOI] [PubMed] [Google Scholar]
- 14. Manning D. K., Sergeev M., van Heesbeen R. G., Wong M. D., Oh J. H., Liu Y., Henkelman R. M., Drummond I., Shah J. V., Beier D. R. (2013) Loss of the ciliary kinase Nek8 causes left-right asymmetry defects. J. Am. Soc. Nephrol. 24, 100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cai Y., Somlo S. (2008) Too much of a good thing: does Nek8 link polycystic kidney disease and nephronophthisis? J. Am. Soc. Nephrol. 19, 418–420 [DOI] [PubMed] [Google Scholar]
- 16. Frank V., Habbig S., Bartram M. P., Eisenberger T., Veenstra-Knol H. E., Decker C., Boorsma R. A., Göbel H., Nürnberg G., Griessmann A., Franke M., Borgal L., Kohli P., Völker L. A., Dötsch J., Nürnberg P., Benzing T., Bolz H. J., Johnson C., Gerkes E. H., Schermer B., Bergmann C. (2013) Mutations in NEK8 link multiple organ dysplasia with altered Hippo signalling and increased c-MYC expression. Hum. Mol. Genet. 22, 2177–2185 [DOI] [PubMed] [Google Scholar]
- 17. Valkova N., Yunis R., Mak S. K., Kang K., Kültz D. (2005) Nek8 mutation causes overexpression of galectin-1, sorcin, and vimentin and accumulation of the major urinary protein in renal cysts of jck mice. Mol. Cell Proteomics 4, 1009–1018 [DOI] [PubMed] [Google Scholar]
- 18. Kaelin W. G., Jr. (2002) Molecular basis of the VHL hereditary cancer syndrome. Nat. Rev. Cancer 2, 673–682 [DOI] [PubMed] [Google Scholar]
- 19. Semenza G. L. (2007) Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE 2007, cm8. [DOI] [PubMed] [Google Scholar]
- 20. Kaelin W. G., Jr. (2008) The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat. Rev. Cancer 8, 865–873 [DOI] [PubMed] [Google Scholar]
- 21. Shen C., Kaelin W. G., Jr. (2013) The VHL/HIF axis in clear cell renal carcinoma. Semin. Cancer Biol. 23, 18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frew I. J., Krek W. (2007) Multitasking by pVHL in tumour suppression. Curr. Opin. Cell Biol. 19, 685–690 [DOI] [PubMed] [Google Scholar]
- 23. Li M., Kim W. Y. (2011) Two sides to every story: the HIF-dependent and HIF-independent functions of pVHL. J. Cell Mol. Med. 15, 187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schermer B., Ghenoiu C., Bartram M., Müller R. U., Kotsis F., Höhne M., Kühn W., Rapka M., Nitschke R., Zentgraf H., Fliegauf M., Omran H., Walz G., Benzing T. (2006) The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J. Cell Biol. 175, 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thoma C. R., Frew I. J., Hoerner C. R., Montani M., Moch H., Krek W. (2007) pVHL and GSK3β are components of a primary cilium-maintenance signalling network. Nat. Cell. Biol. 9, 588–595 [DOI] [PubMed] [Google Scholar]
- 26. Xu J., Li H., Wang B., Xu Y., Yang J., Zhang X., Harten S. K., Shukla D., Maxwell P. H., Pei D., Esteban M. A. (2010) VHL inactivation induces HEF1 and Aurora kinase A. J. Am. Soc. Nephrol. 21, 2041–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen G., Chen S. M., Wang X., Ding X. F., Ding J., Meng L. H. (2012) Inhibition of chemokine (CXC motif) ligand 12/chemokine (CXC motif) receptor 4 axis (CXCL12/CXCR4)-mediated cell migration by targeting mammalian target of rapamycin (mTOR) pathway in human gastric carcinoma cells. J. Biol. Chem. 287, 12132–12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim W. Y., Kaelin W. G. (2004) Role of VHL gene mutation in human cancer. J. Clin. Oncol. 22, 4991–5004 [DOI] [PubMed] [Google Scholar]
- 29. Kuehn E. W., Walz G., Benzing T. (2007) Von Hippel-Lindau: a tumor suppressor links microtubules to ciliogenesis and cancer development. Cancer Res. 67, 4537–4540 [DOI] [PubMed] [Google Scholar]


