Background: The properties of voltage-gated Ca2+ channels are regulated by auxiliary β and α2δ subunits.
Results: Retinal β2 and α2δ4 subunits interact with Cav1.4 and differentially modulate channel properties compared with other Cav subunits.
Conclusion: β2 and α2δ subunits are major determinants of Cav1.4 function.
Significance: Cav1.4 channels in retinal photoreceptors are composed of auxiliary subunits with distinct properties compared with other Cav1 channels.
Keywords: Calcium Channel, Calcium-binding Protein, Electrophysiology, Immunohistochemistry, Photoreceptor, Retina
Abstract
In photoreceptor synaptic terminals, voltage-gated Cav1.4 channels mediate Ca2+ signals required for transmission of visual stimuli. Like other high voltage-activated Cav channels, Cav1.4 channels are composed of a main pore-forming Cav1.4 α1 subunit and auxiliary β and α2δ subunits. Of the four distinct classes of β and α2δ, β2 and α2δ4 are thought to co-assemble with Cav1.4 α1 subunits in photoreceptors. However, an understanding of the functional properties of this combination of Cav subunits is lacking. Here, we provide evidence that Cav1.4 α1, β2, and α2δ4 contribute to Cav1.4 channel complexes in the retina and describe their properties in electrophysiological recordings. In addition, we identified a variant of β2, named here β2X13, which, along with β2a, is present in photoreceptor terminals. Cav1.4 α1, β2, and α2δ4 were coimmunoprecipitated from lysates of transfected HEK293 cells and mouse retina and were found to interact in the outer plexiform layer of the retina containing the photoreceptor synaptic terminals, by proximity ligation assays. In whole-cell patch clamp recordings of transfected HEK293T cells, channels (Cav1.4 α1 + β2X13) containing α2δ4 exhibited weaker voltage-dependent activation than those with α2δ1. Moreover, compared with channels (Cav1.4 α1 + α2δ4) with β2a, β2X13-containing channels exhibited greater voltage-dependent inactivation. The latter effect was specific to Cav1.4 because it was not seen for Cav1.2 channels. Our results provide the first detailed functional analysis of the Cav1.4 subunits that form native photoreceptor Cav1.4 channels and indicate potential heterogeneity in these channels conferred by β2a and β2X13 variants.
Introduction
Ca2+ ions are important for many cellular functions, including neurotransmitter release, muscle contraction, and gene transcription. In neurons, depolarizing stimuli initiate Ca2+ signaling mainly through activation of voltage-gated (Cav) Ca2+ channels. Neuronal Cav channels are multiprotein complexes composed of a major pore-forming α1 subunit, and two auxiliary subunits, β and α2δ, which not only regulate the functional properties of the α1 subunit but also are important for the proper trafficking of the α1 subunit to the plasma membrane and protection of the α1 subunit from proteosomal degradation (reviewed in Refs. 1–4). Ten different genes encode the Cav α1 subunits, whereas the β and α2δ subunits are encoded by four genes each (5, 6). Alternative splicing further adds to the molecular and functional diversity of α1 and β subunits (1, 7).
In photoreceptor terminals, Cav1 L-type channels are concentrated near the synaptic “ribbon,” a structure specialized for high throughput and tonic exocytosis (8). At the depolarized photoreceptor membrane potential in darkness, Cav1-mediated Ca2+ influx triggers the release of glutamate, which is required for transmission of visual stimuli to second-order neurons (9, 10). Of the different classes of Cav1 channels (Cav1.1–Cav1.4), multiple lines of evidence indicate that Cav1.4 is the major Cav1 channel in rod and cone photoreceptors. Antibodies against Cav1.4 label both rod and cone terminals (11–14). In mice, inactivation of CACNA1F, the gene encoding Cav1.4 (Cav1.4 KO), disrupts photoreceptor synaptic transmission and presynaptic calcium signaling (15) and prevents the maturation of photoreceptor synapses (13, 16). In addition, human mutations in CACNA1F cause vision disorders, including incomplete congenital stationary night blindness 2, which is characterized by impaired rod photoreceptor transmission and low visual acuity in darkness (17–20). Antibody labeling for the Cav1.3 α1 subunit has also been detected in the cones from tree shrew (21, 22) and chick (23). However, in mice lacking Cav1.3, morphological changes in photoreceptor synapses are observed, but visual function is largely normal (24). The auxiliary Cav1.4 subunits in photoreceptors are most likely β2 and α2δ4, because mice lacking functional β2 or α2δ4 subunits exhibit similar morphological defects in the retina and vision impairment as Cav1.4 KO mice (25, 26).
However, an understanding of the functional properties of this particular combination of Cav1.4 channel is lacking. Previous electrophysiological analyses of Cav1.4 channels in heterologous expression systems have employed alternate β and α2δ subunits (27–32) and so may not reflect the properties of native photoreceptor Cav channels. Therefore, the goal of this study was to investigate the association of Cav1.4 α1 with β2 and α2δ4 subunits cloned from human retina and to characterize the electrophysiological properties of the corresponding channels in transfected HEK293T cells. In the course of this work, we identified a splice variant of β2 in the retina, which is distinct from the brain β2a subunit and which differentially modulates the functional properties of Cav1.4.
EXPERIMENTAL PROCEDURES
Animals
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Iowa and the University of Washington. These procedures were in accordance with National Institutes of Health guidelines. CaBP4 KO (28) and Cav1.4 KO (33) mice were characterized previously. Cav1.4 KO mice (B6.Cg-Cacna1ftm1.1Sdie/J) were obtained from the Jackson Laboratory. Adult mice (WT, 2–4 months old) used in this study were maintained on a 12-h light/dark cycle.
Antibodies
Commercially available antibodies were alkaline phosphatase-conjugated anti-rat, anti-rabbit, and anti-mouse (Promega Corp., Madison, WI), mouse anti-FLAG (Sigma-Aldrich), Alexa Fluor 555 goat anti-mouse, Alexa Fluor 488 goat anti-rabbit, and Alexa Fluor 555 goat anti-rat (Invitrogen). The development of the anti-Cav1.4 antibody and demonstration of its specificity was described previously (13).
Cloning of Cav1.4 α1, β2, and α2δ4 Subunits and Partial β1b and α2δ1
Human Cav1.4 α1 Subunit
The Cav1.4 coding sequence was isolated and cloned from a human retina cDNA library. Five fragments were amplified by PCR with Platinum Pfx DNA polymerase (Invitrogen): nucleotides 1–399 (F1, ATG initiation codon-SnaBI), 393–1306 (F2, SnaBI-SfiI), 1294–3292 (F3, SfiI-ClaI), 3286–3918 (F4, ClaI-HindIII), and 3913–5934 (F5, HindIII-TGA stop codon). A FLAG epitope was added to the first fragment covering the N terminus of Cav1.4 (F1′, 1–399) by PCR with primers FH736 (5′-CTAGACCATGGATTACAAGGATGACGACGATAAGTCGGAATCTGAAGGCGGAAAG-3′) and FH 720 (5′-CCAGGAATACGTACTCCACCTGC-3′). All PCR fragments were subcloned into the pCR-blunt II vector (Invitrogen) and sequenced. Fragments F1′ and F2 were first assembled by subcloning into the XbaI and KpnI sites of the pBluescript vector using restriction sites provided by the pCR-blunt II vector for the Cav1.4 fragments. The F3 fragment was then added by cloning into the SfiI and ClaI sites of pBluescript-F1-F2. The human Cav1.4 full coding sequence was then cloned between the XbaI and NotI sites of the pcDNA3.1 mammalian expression vector by ligating fragments XbaI-ClaI (F1-F3) with ClaI-HindIII (F4) and HindIII-NotI (F5).
β2 Subunits
The β2X13 coding sequence was cloned from human retina cDNA library using PCR with primers FH1168 (5′-CACCATGCAGTGCTGCGGGCTGGT-3′) and FH 1169 (5′-TCATTGGCGGATGTAAACATCCCTG-3′) that hybridizes on the initiation and stop codons, respectively, of β2a and β2X13. After subcloning into the pCR-blunt II vector and confirmation of correct sequence, β2X13 was transferred into the XbaI and BamHI sites of pcDNA3.1 vector using the XbaI and BamHI restriction sites provided by the pCR-blunt II vector. The β2a subunit was cloned from a human retina cDNA library using PCR in two fragments: F1 with primers FH1168 and FH1291 (5′-GGTTTAGGGGACCGGTGGTTTGC-3′) that inserts a silent mutation (underlined) at nucleotide 567, creating an AgeI site, and F2 with primers FH1290 (5′-GCAAACCACCGGTCCCCTAAACC-3′) and FH1169. The human β2a full coding sequence was then cloned between the XbaI and BamHI sites of the pcDNA3.1 vector by ligating fragments F1 Xba-AgeI (ATG-563) and F2 AgeI-BamHI (564-Stop codon). For bacterial expression of partial β2 protein fused to a His6 tag, a fragment covering from nucleotide 1225 to the stop codon of human β2 was excised with BclI and HindIII from the pcDNA3.1-β2X13 plasmid and cloned into the BamHI and HindIII sites of the pET30B vector (EMD Millipore, Billerica, MA).
The full-length mouse β2X13 coding sequence was cloned from mouse retina cDNA using PCR with primers FH1168 (5′- CACCATGCAGTGCTGCGGGCTGGT-3′) and FH1278 (5′-TCATTGGCGGATGTATACATCCCTG-3′).
Human α2δ4 Subunit
The α2δ4 coding sequence was cloned from a human retina cDNA library using PCR in two fragments covering nucleotides 1–988 (F1, ATG-BamHI) using primers FH1227 (5′-CTCATGGTCTGTGGCTGCTCTGCCCTC-3′) and FH1173 (5′-CTGGACGAGGATCCCTTTAAAACAAG-3′) and nucleotides 982–3363 (F2, BamHI-Stop codon) using primers FH1274 (5′-CTTGTTTTAAAGGGATCCTCGTCCAG-3′) and FH1272 (5′-TCACCGCAGGAGTTGGGGCAGTAG-3′). After subcloning in pCR-blunt II and sequencing, the F1 fragment was then first subcloned into the XbaI and BamHI sites of the pcDNA3.1 vector. Fragment F2 was then added between the BamHI and KpnI sites of pcDNA3.1-F1 in two fragments BamHI-XhoI (nucleotides 983–2778) and XhoI-KpnI (2778-Stop codon). For bacterial expression of a partial α2δ4 protein fused to a His6 tag, a fragment covering nucleotides 1–1591 of human α2δ4 cDNA was excised with NcoI and HindIII from the pcDNA3.1-α2δ4 plasmid and cloned into the pET-SUMO vector (Invitrogen).
β1b and α2δ1 Fragments
For bacterial expression, a fragment of β1b corresponding to the sequence used to generate anti-β2 antibodies was fused to a His6 tag. A PCR fragment was amplified with primers 5′-GACCGGGCCACTGGGGAGCAT-3′ and 5′-TCAGCGGATGTAGACGCCTTGTC-3′ and then cloned into the pdest17 vector (Invitrogen). Similarly, a partial fragment of α2δ1 corresponding to the sequence used to generate the anti-α2δ4 antibody was amplified by PCR using primers (5′-ATGGCTGCTGGCTGCCTGCTG-3′) and (5′-TCATGTAAAACGTGGTGTCAATCT-3′) and cloned into the pET-SUMO vector.
Quantitative RT-PCR
Human retinas were obtained from donors without known eye diseases from the Oregon Lions eye bank as allowed by the Institutional Human Subjects Division of the University of Washington. Total RNAs were isolated from human retina using the UltraSpec RNA isolation system (Biotecx, Houston, TX). A two-step quantitative PCR was then carried out to determine the relative expression of splice variants. Total RNA (1 μg) was subjected to first strand cDNA synthesis using Superscript III reverse transcriptase and oligo(dT) in a volume of 20 μl according to the manufacturer's protocol (Invitrogen). For the human β2X13 quantitative PCR (qPCR),2 primers were designed on exon 7B (FH1280, 5′-GCTAAGCAGAAGCAGAAATCGAC-3′) and ∼290 bp downstream of FH1280 on the exon 9-exon 10 junction (FH1284, 5′-TTCACTCTGAACTTCCGCTAAG-3′). For human β2a, primers were designed on exon 7A (FH1279, 5′-GCTATAGACATAGATGCTACTGGC-3′) and ∼400 bp downstream of FH1279 on the exon 9-exon 10 junction (FH1284, 5′-TTCACTCTGAACTTCCGCTAAG-3′). For normalization of the qPCR products, primers FH812 (5′-TCAACGGATTTGGTCGTATTGGGC-3′) and FH813 (5′-AGTGATGGCATGGACTGTGGTCAT-3′) were used to amplify human glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Triplicate quantitative PCRs were carried out using 0.5 μl of cDNA, a 400 nm concentration of each primer, and 10 μl of QuantiTect SYBR Green PCR mix (Qiagen) in a 20-μl total reaction volume. After an initial incubation at 95 °C for 15 min, the qPCR was carried out for 40 cycles of denaturation at 95 °C for 15 s, annealing at 68 °C for 30 s, and extension at 72 °C for 1 min on a real-time PCR system (ABI PRISM 7000, Applied Biosystems). Single bands of the predicted size were verified post-PCR by agarose gel electrophoresis. Threshold cycle (Ct) was determined using the ABI Prism 7000 software. Data were analyzed by comparing Ct values. All cDNAs were normalized relative to the Ct values of the internal control, GAPDH.
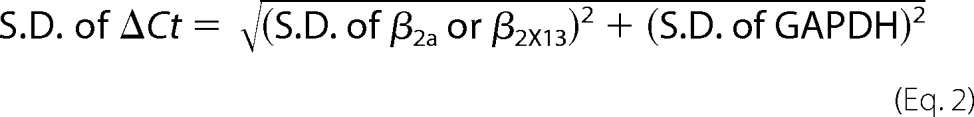 |
The normalized Ct values of β2X13 and β2a were then compared by determining ΔΔCt = ΔCt of β2X13 − ΔCt of β2a. -Fold difference was calculated as 2(−ΔΔCt) (34).
For mouse RT-PCR, total RNA was isolated from mouse brain or retina using the RNeasy kit (Qiagen, Valencia, CA). After synthesis of cDNA using superscript reverse transcriptase, mouse β2a was analyzed by PCR using primers FH1279 and FH1284, whereas mouse β2X13 was amplified with primers FH1338 (5′-GCAAAGCAGAAGCAGAAGTCGAC-3′) and FH1284. Primers GAPDH-F (5′-GAAGGGCTAATGACCACAGTCCAT-3′) and GAPDH-R (5′-TAGCCATATTCGTTGTCGTACCAGG-3′) were used to amplify mouse GAPDH.
RNA-seq
A retinal RNA-seq experiment using samples from donor eyes (35) was used to determine the expression of human retinal β2 splice variants. These sequences were aligned to the human genome (release GRCh 37) using the Tuxedo pipeline (36). The resulting alignments were visually evaluated using IGV (37) to determine the specificity of transcriptional inclusion for exons 7A, 7B, and 7C of the CACNB2 gene.
Generation of Anti-β2, Anti-α2 δ4, and Anti-CaBP4 Antibodies
Partial β2X13 (amino acids 409–569) and α2δ4 (amino acids 1–532) were expressed fused to a His6 tag and purified from bacteria. Anti-β2 and anti-α2δ4 polyclonal antibodies were raised in rats by subcutaneous immunization with purified β2 or α2δ4 recombinant proteins mixed with Freund's adjuvant (Cocalico Biologicals, Inc., Reamstown, PA). Mouse anti-CaBP4 was produced similarly by immunization with purified mouse full-length CaBP4 (28) (Cocalico Biologicals). For affinity purification of rat antibodies, purified β2 or α2δ4 was coupled to CNBr-activated Sepharose (GE Healthcare) according to the manufacturer's protocol. After loading of a 10-fold dilution of the sera in PBS, the columns were washed with 20 volumes of PBS. The bound antibodies were eluted with 0.1 m glycine buffer, pH 2.5, and dialyzed overnight against PBS. To test the specificity of the purified anti-β2 by Western blot, the antibody (1 μg/ml) was preadsorbed by preincubation with His-β2a fusion protein (2 μg/ml) or His-tagged β1b (2 μg/ml) for 1 h at room temperature before incubation with the membranes. Similarly, to test the specificity of purified anti-α2δ4, the antibody (1 μg/ml) was preadsorbed by preincubation with His-tagged α2δ4 (2 μg/ml) or α2δ1 (2 μg/ml).
Expression of Cav1.4 α1, β2, α2δ4, and CaBP4 in HEK293 Cells
HEK293 cells were maintained in DMEM + 10% FBS and 100 units/ml penicillin/streptomycin. For coimmunoprecipitation assays, cells were cotransfected with Cav1.4 α1, β2X13, α2δ4, and CaBP4 plasmids using the Ca2+-phosphate method. The transfection medium was replaced 6 h after transfection, and the transfected cells were collected 3 days after transfection. For the analysis of anti-β2 antibody specificity, cells were cotransfected with Cav1.4 α1, α2δ4, and β2X13 or β1b (GenBankTM number NM017346), β3 (GenBankTM number NM012838), or β4 (GenBankTM number L02315) (38). For the analysis of anti-α2δ4 antibody specificity, cells were cotransfected with Cav1.4 α1, β2X13, and α2δ4, α2δ1, α2δ2, or α2δ3 (plasmids encoding the latter three were a generous gift from Dr. Annette Dolphin (39)).
For electrophysiological experiments, HEK293T cells were grown to 70–80% confluence and cotransfected with human Cav1.4 or Cav1.2 α1, β2X13 or β2a, and α2δ4 or α2δ1 cDNAs cloned in pcDNA3.1 and pEGFP cDNA as a transfection marker. Fugene transfection reagent (Promega) was used according to the manufacturer's protocol. After transfection, cells were maintained at 30 °C. Cells were dissociated 18–24 h after transfection and plated at low density for electrophysiological recording of single cells.
Immunohistochemistry
C57Bl/6J mouse eyecups were fixed in 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4 (PB) for 30 min to 1 h. After fixation, tissues were incubated with increasing concentration of sucrose to 20% sucrose in PB and then embedded in 33% OCT compound (Miles, Elkhart, NY) diluted with 20% sucrose in PB. Eye tissues were cut in 12-μm sections. To block nonspecific labeling, retinal sections were incubated with 3% normal goat serum in PBST buffer (10 mm sodium phosphate, 150 mm NaCl, 0.1% Triton X-100, pH 7.4) for 20 min at room temperature. Sections were incubated overnight at 4 °C in a mix of diluted primary antibodies: mouse anti-CaBP4 (1:100) plus rabbit anti-Cav1.4 (1:1000), rat anti-β2 (1:25) plus rabbit anti-Cav1.4 (1:1000), or rat anti-α2δ4 (1:25) plus rabbit anti-Cav1.4 (1:1000). A mix of Alexa Fluor 555-conjugated goat anti-mouse IgG or Alexa Fluor 555-conjugated goat anti-rat IgG and Alexa 488-conjugated goat anti-rabbit IgG was reacted with the sections for 1 h at room temperature. Then the sections were rinsed in PBST and mounted with Prolong antifade reagent (Molecular Probes, Inc., Eugene, OR). Sections were analyzed under a confocal microscope (Zeiss LSM710). Immunofluorescent images were obtained with a Plan-Neofluar ×40/1.3 numerical aperture (Zeiss) objective lens. For immunohistochemistry experiments with rat anti-β2 and rat anti-α2δ4, antigen retrieval was performed by incubating the sections at 80 °C for 20 min in 10 mm sodium citrate, pH 6.0, 0.05% Tween 20 and washing with PBS before blocking and incubation with the antibodies. Quantification of colocalization was performed using the JACoP plugin for ImageJ (National Institutes of Health), which uses correlation analysis based on Pearson's coefficient (40). A Pearson's coefficient near 1 suggests perfect correlation, whereas a number near 0 indicates no correlation.
Coimmunoprecipitation
Three days after transfection, whole cell lysates were prepared by incubation of transfected cells at 4 °C for 1 h in 20 mm Tris, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and inhibitors of proteases (Sigma-Aldrich) with or without 0.1 mm MgCl2 and 0.1 mm CaCl2. Lysates were centrifuged at 22,000 × g for 30 min and incubated with mouse IgG or anti-FLAG (5 μg). After a 1-h incubation at 4 °C, 20 μl of protein G-magnetic beads (Invitrogen) were added, and the incubation proceeded for 3 h at 4 °C. After four washes with lysis buffer, proteins were eluted with SDS-sample buffer and analyzed by Western blotting with specific antibodies.
For coimmunoprecipitations from retinas, 12 retinas from WT or Cav1.4 KO mice were lysed in 20 mm Hepes, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% n-dodecyl-β-d-maltoside and a mix of protease inhibitors (Sigma-Aldrich) at 4 °C for 30 min. Lysates were centrifuged at 10,000 × g for 10 min, and 500 μg of supernatant was incubated with 5 μg of anti-α2δ4 or anti-β2 at 4 °C. After 1 h of incubation, 30 μl of protein A-Sepharose bead slurry was added to the mix and incubated for 1 h at 4 °C. Beads were washed three times with lysis buffer without detergent, and the proteins were eluted with SDS-sample buffer plus 10 mm DTT before analysis by Western blotting with Cav1.4 antibodies.
Proximity Ligation Assay
The proximity ligation assay was performed using the Duolink kit (Sigma-Aldrich). Mouse retina sections were prepared as described above for immunohistochemistry and incubated overnight with rabbit anti-Cav1.4 and mouse anti-CaBP4, rat anti-β2, or rat anti-α2δ4. For the assay using rat anti-β2 and rat anti-α2δ4, the sections were then incubated for 1 h at room temperature with mouse anti-rat antibody. Anti-rabbit PLUS probe and anti-mouse MINUS probe were then added to the sections for 1 h at 37 °C. Ligation, amplification, and detection of the probes were carried out according to the manufacturer's protocol. The sections were mounted with antifade reagent and analyzed under a confocal microscope as described above. To quantify proximity ligation assay (PLA) signals, an arbitrary area of identical size (x = 50 μm × y = 10 μm) was selected in the outer plexiform layer (OPL) of single z plane images as well as in the outer nuclear layer (ONL) to be able to normalize the measurements. Indeed, because there is variability in the background signals from one section to the other, we subtracted the number of PLA signals in the ONL from the number of PLA signals in the OPL. Quantification of PLA spots was performed using the “analyze particles” tools in ImageJ version 1.48.
Electrophysiological Recordings
Whole-cell patch clamp recordings of cells transfected with Cav1 channel subunits were performed 36–48 h after transfection. Data were acquired with an EPC-9 patch clamp amplifier driven by Pulse software (HEKA Elektronik, Lambrecht/Pfalz, Germany) and analyzed with Igor Pro software (Wavemetrics, Lake Oswego, OR). Extracellular recording solutions contained 140 mm Tris, 1 mm MgCl2, and 20 mm BaCl2. Intracellular solutions consisted of 140 mm N-methyl-d-glucamine, 10 mm HEPES, 2 mm MgCl2, 2 mm Mg-ATP, and 5 mm EGTA. The pH of intracellular and extracellular recording solutions was adjusted to 7.3 with methanesulfonic acid. Electrode resistances were typically 1–2 megaohms, and series resistance compensated up to 80%. All averaged data are presented as the mean ± S.E. Statistical significance of differences between groups was determined by Student's t test as indicated (SigmaPlot, SPSS Science, Chicago, IL). Normalized tail currents were fit with the Boltzmann equation: I/(1 + exp(−(V − Vh)/k) + b where I is the maximal current, V is the test voltage, Vh is the voltage of half-activation, k is the slope factor, and b is the baseline).
RESULTS
Molecular Identification of Cav1.4 Subunits in Human Retina
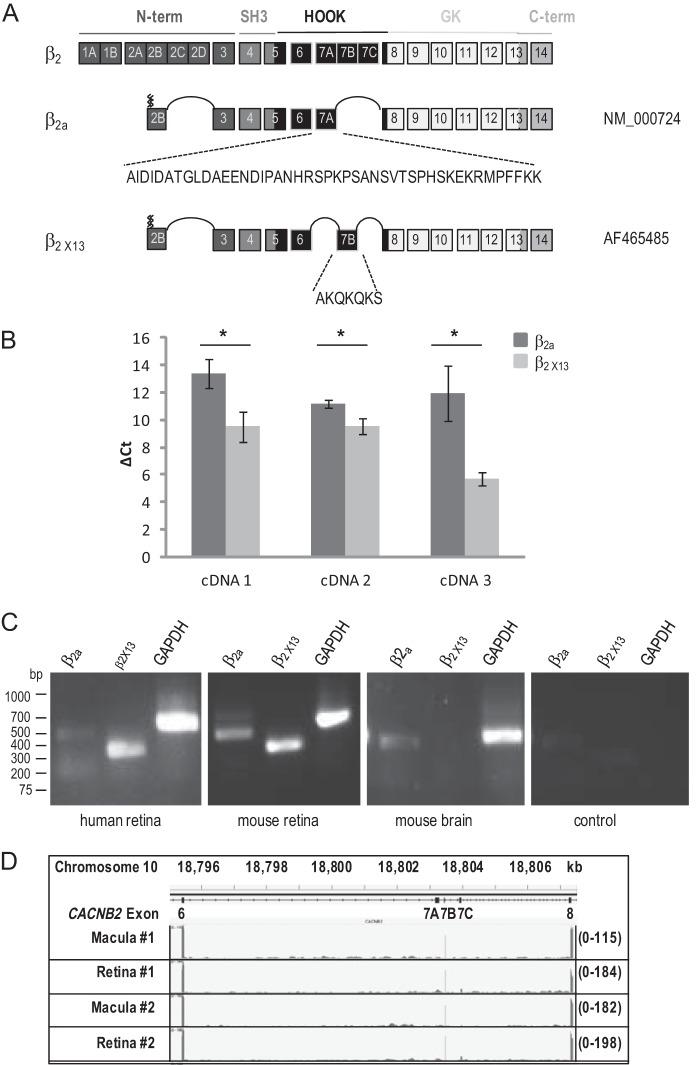
To characterize the molecular constituents of Cav1.4 complexes in the retina, we performed RT-PCR from human retinas using primers specific for Cav1.4 α1, β2, and α2δ4. We isolated full-length wild-type Cav1.4 α1 and α2δ4 cDNAs that were identical to those described previously (GenBankTM accession numbers AF201304 and NM_172364, respectively). The large majority (≥90%; Fig. 1) of PCR products for β2 corresponded to an alternative splice variant that includes a different exon 7. This retinal β2 variant includes palmitoylation sites known so far to be specific to β2a and includes exon 7B instead of the exon 7A that is incorporated in β2a (Fig. 1A, β2a GenBankTM number NM_000724; exon numbering according to Buraei et al. (1)). Exon 7B is analogous to the exon 7B included in β1b and β1c mRNAs (1). This new retinal variant has here been named β2X13 (Fig. 2). A β2 variant with sequence identical to the retinal β2X13 was isolated from human jejunum (GenBankTM number AF465485) (41). Although β2X13 is expressed at higher levels than β2a in the retina, we were also able to detect the expression of β2a transcripts that include exon 7A (Fig. 1, B and C). By quantitative RT-PCR, the relative expression of β2a and β2X13 was variable between human retinas, with β2X13 being 9–77 times more abundant than β2a (Fig. 1B and Table 1). β2a was undetectable in one human retina (data not shown). By RT-PCR, we also detected β2X13 expression in mouse retina (Fig. 1C), where its expression was also greater than that of β2a (data not shown). β2X13 was not detected in mouse brain and is thus probably tissue-specific (Fig. 1C). Further evidence for the predominance of β2X13 in the human retina was obtained in a retinal RNA-seq data set (35), which was specifically evaluated for the inclusion rates of exons 7A, 7B, and 7C. A β2 variant including the 7B exon and concomitantly excluding the 7A and 7C exons was observed to be the dominant retinal isoform in both the macular and peripheral retina (Fig. 1D). In general, exon 7B was found in greater than 95% of sampled transcripts. The mouse β2X13 sequence was submitted to GenBankTM and assigned accession number KJ789960 (Fig. 2).
FIGURE 1.
Cloning and expression of retinal β2X13 variant. A, schematic of the human CACNB2 gene with exon numbering according to Buraei and Yang (1). Alternatively spliced exons 1, 2, and 7 are linked and denoted by letters. The different domains are indicated: N-terminal (N-term), Src homology 3 (SH3), HOOK, guanylyl kinase (GK), and C-terminal (C-term). GenBankTM accession numbers are indicated on the right. The distinct peptide sequence encoded by exon 7A in β2a and exon 7B in β2X13 is indicated. The zig zag lines indicate the palmitoylation sites at the N terminus. B, qPCR analysis of β2a and β2X13 expression in human retina. Values represent mean ΔCt values (Ct values for β2a and β2X13 normalized to the Ct values for the internal standard GAPDH) for all cDNA preparations (1–3). Error bars, S.D. (*, p ≤ 0.015, unpaired t test, n = 3). C, RT-PCR analysis of β2a and β2X13 expression in human and mouse tissue. For human cDNA, primers were the same as those used in B and Table 1. For mouse cDNA, primers were designed on exon 7A and exon 10 (for β2a) or on exon 7B and exon 10 (for β2X13). D, profile of RNA-seq reads from human macula or peripheral retina mapped to exon 6 to exon 8 of the CACNB2 gene. The numbers between parentheses on the right indicate the scale. The location of exon 6 to exon 8 from 18,795.363 to 18,807.311 kb on chromosome 11 is shown at the top.
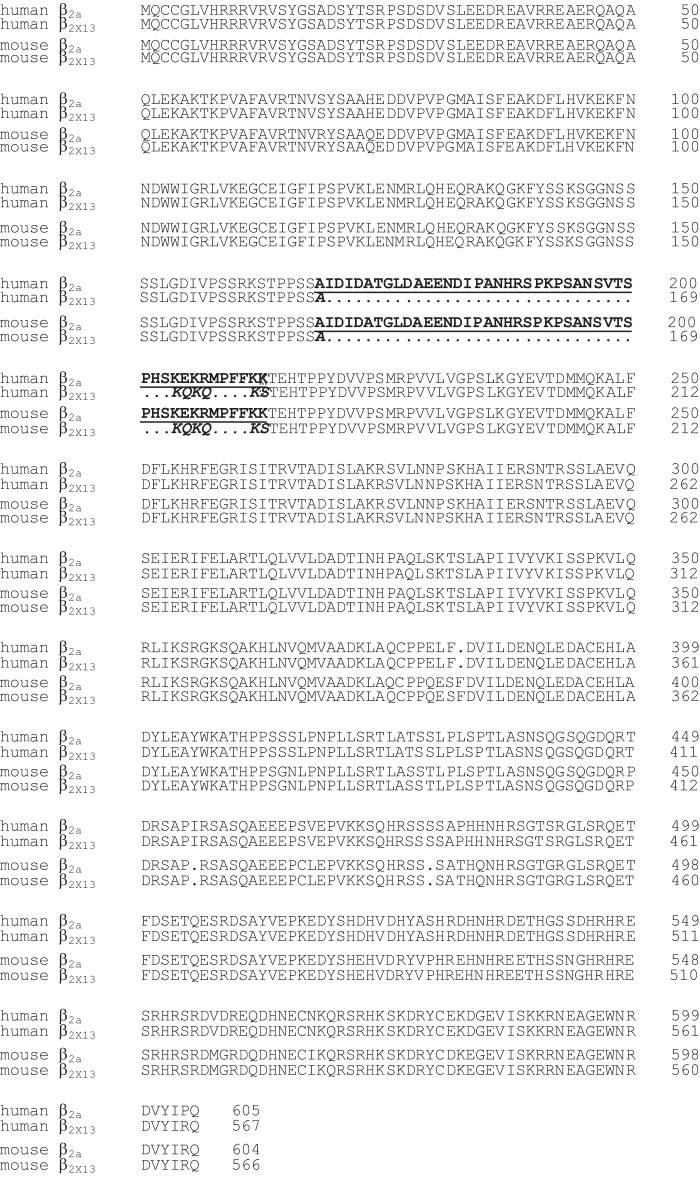
FIGURE 2.
Alignment of human β2a and β2X13 amino acid sequences with mouse β2a and mouse β2X13. Cloning of the β2X13 cDNAs is described in the “Experimental Procedures.” The aligned amino acid sequences are derived from GenBankTM accession number AF423189.1 for human β2A, AF465485_1 for human β2X13, XM_006497320.1 for mouse β2a, and KJ789960 for mouse β2X13. Similarly to the human β2X13 retinal subunit, the mouse retinal β2X13 also includes the shorter exon 7B shown in boldface italic letters instead of exon 7A indicated in boldface underlined letters.
TABLE 1.
-Fold difference in transcript levels of β2X13 and β2a in human retina
qPCR data in Fig. 1B were analyzed using the 2−ΔΔCt as described by Livak et al. (34). -Fold differences in Ct values between β2X13 and β2a were calculated after adjustment to GAPDH control and are presented as mean ± S.D. (n = 3).
| cDNA | β2a ΔCt (mean β2a Ct − mean GAPDH Ct) | β2×13 ΔCt (mean β2×13 Ct − mean GAPDH Ct) | ΔΔCt (mean β2×13 ΔCt − mean β2a ΔCt) | Normalized β2×13 RNA amount relative to β2a RNA or 2−ΔΔCt |
|---|---|---|---|---|
| cDNA1 | 13.36 ± 1.04 | 9.51 ± 1.09 | −3.85 ± 1.09 | 14.45 ± 10.5 |
| cDNA2 | 11.15 ± 0.28 | 9.55 ± 0.55 | −3.15 ± 0.55 | 8.9 ± 3.4 |
| cDNA3 | 11.96 ± 2.00 | 5.69 ± 0.47 | −6.27 ± 0.47 | 77.5 ± 25.1 |
Co-localization of Cav1.4 Subunits in Mouse Retina
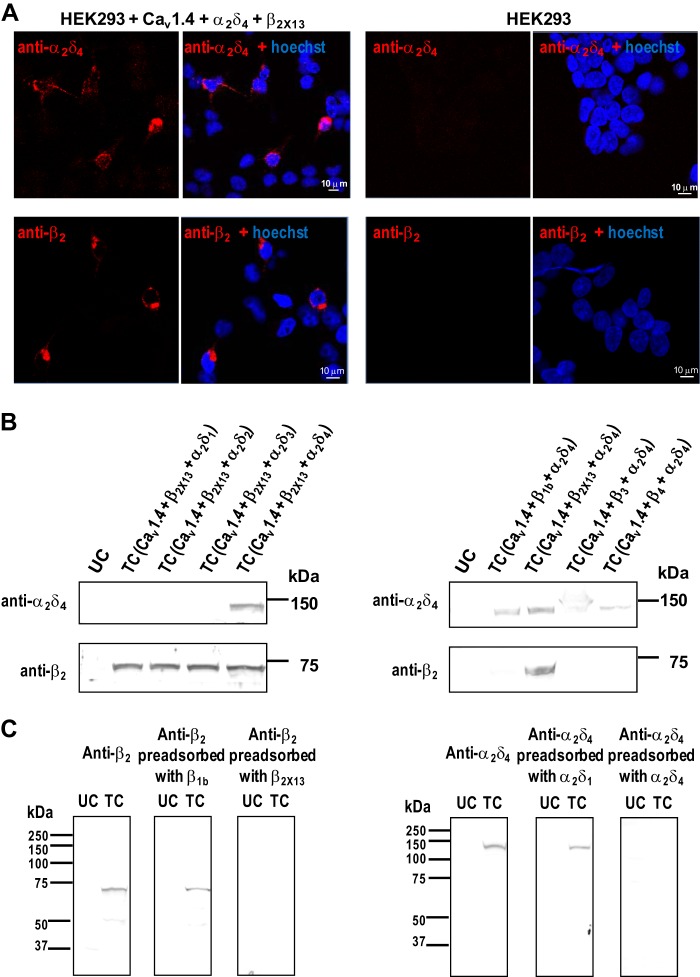
We next tested whether Cav1.4 α1 colocalized with β2 and α2δ4 in the retina using rabbit polyclonal anti-Cav1.4 α1 subunit antibodies that we characterized previously (13). For double labeling, we generated rat polyclonal antibodies against the β2 and α2δ4 subunits and tested their specificity by immunohistochemistry and Western blot. Immunolabeling with anti-β2 and anti-α2δ4 was observed for cells transfected with Cav1.4 α1, β2X13, and α2δ4 but not for untransfected cells (Fig. 3A). In Western blot analysis, the anti-α2δ4 detected a protein of ∼135 kDa that corresponds to the predicted molecular mass of α2δ4 in lysates of cells transfected with Cav1.4 α1, β2X13, and α2δ4 but not in cells expressing α2δ1, α2δ2, or α2δ3 (Fig. 3B, left. A protein of ∼70 kDa, close to the calculated molecular mass of β2X13, was detected by the anti-β2 antibody in cells transfected with β2X13, but no signal was observed in cells transfected with β1b, β3, or β4 (Fig. 3B, right). In addition, preadsorption of anti-β2 with β2a but not β1b abolished detection of β2a (Fig. 3C, left). As well, α2δ4 detection was blocked by preadsorption of anti-α2δ4 with α2δ4 but not α2δ1 (Fig. 3C, right). These results validate the specificity of these antibodies for detection of β2X13 and α2δ4 by Western blot or immunohistochemistry.
FIGURE 3.
Characterization of polyclonal antibodies against β2 and α2δ4. A, immunolabeling of HEK293 cells cotransfected with Cav1.4 α1, β2X13, and α2δ4 (left panels) or untransfected cells (right panels) with rat anti-β2 or rat anti-α2δ4. B, Western blot of lysates of HEK293 cells untransfected (UC) or cotransfected (TC) with Cav1.4 α1, β2X13 and α2δ1, α2δ2, α2δ3, or α2δ4 (left) or cotransfected with Cav1.4 α1, α2δ4, and β1b, β2X13, β3, or β4 (right). Blots were probed with anti-α2δ4 or anti-β2, respectively. C, Western blot of lysates of HEK293 cells untransfected (UC) or cotransfected (TC) with Cav1.4 α1, β2X13, and α2δ4 and probed with anti-β2, anti-β2 preadsorbed with specific β2X13 or nonspecific β1b (left), or anti-α2δ4, or anti-α2δ4 preadsorbed with specific α2δ4 or nonspecific α2δ1 (right). Immunoreactivity was blocked by preadsorption with specific antigen only.
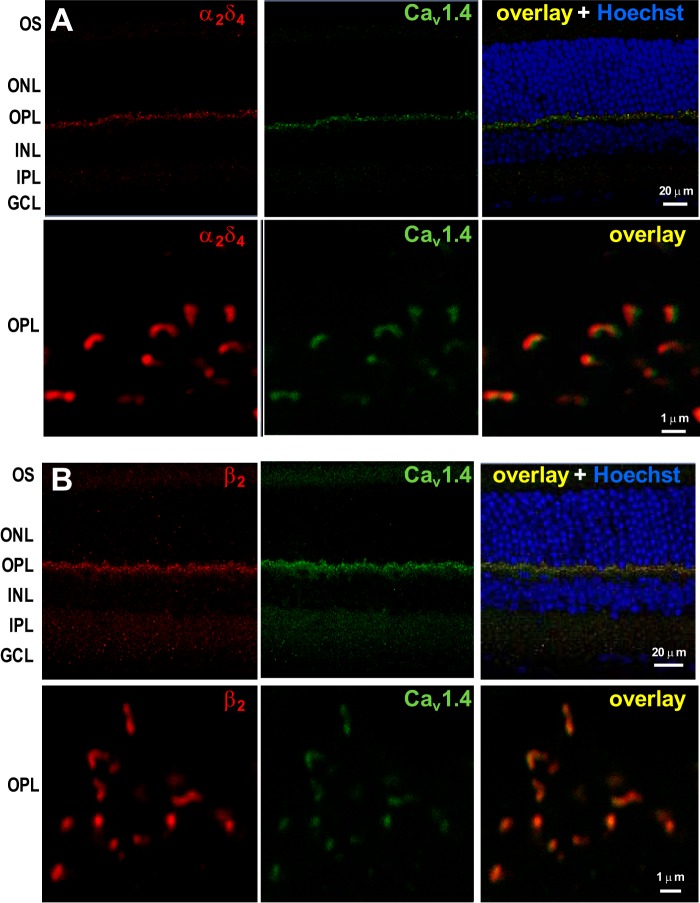
Using these antibodies for double-labeling of mouse retina, we observed selective labeling of the outer plexiform layer containing the photoreceptor terminals; no labeling was observed in other retinal layers (Fig. 4). High magnification images showed anti-Cav1.4 α1 labeling of elongated and horseshoe-shaped structures corresponding to the photoreceptor synaptic ribbon that arches around the postsynaptic terminals, as described previously (13, 33). The anti-β2 or anti-α2δ4 signals colocalized at these structures with anti-Cav1.4 α1 staining, as indicated by a Pearson's correlation coefficient of ≥0.710 or ≥0.608, respectively. These findings are consistent with the presence of Cav1.4 α1-β2-α2δ4 complexes that are concentrated near photoreceptor synaptic ribbons.
FIGURE 4.
Colocalization of Cav1.4 α1 with β2 or α2δ4 at the photoreceptor synaptic ribbon. Mouse retina sections were double-labeled with anti-Cav1.4 α1 (green) and anti-α2δ4 (red) (A) or anti-β2 (red) (B). The overlay is shown in the right panel together with Hoechst staining. Higher magnification images of the OPL are shown in the bottom panels. Cav1.4 shows a high degree of colocalization with β2 and α2δ4, as indicated by the calculated Pearson's correlation coefficient between the Alexa 488-stained Cav1.4 and the Alexa 555-stained β2 (r ≥ 0.710) or α2δ4 (r ≥ 0.608). OS, outer segment; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Cav1.4 α1, β2X13, and α2δ4 Subunits Interact in HEK293 Cells and in Mouse Retina
We next tested whether Cav1.4 α1 associates with β2X13 and α2δ4 in transfected HEK293 cells. The Cav1.4 α1 subunit was tagged with a FLAG epitope, and anti-FLAG antibodies were used to immunoprecipitate FLAG-Cav1.4 and associated proteins. In these experiments, we also tested for the co-immunoprecipitation of CaBP4, a known Cav1.4-interacting protein (28, 42). Because the association of CaBP4 with Cav1.4 can be affected by Ca2+, coimmunoprecipitation experiments were done in the presence and absence of Ca2+. Due to the limited sensitivity of FLAG antibodies, FLAG-Cav1.4 α1 was not detected in cell lysates by Western blot (Fig. 5, left panels). However, FLAG-Cav1.4 α1 was visualized after enrichment using immunoprecipitation (Fig. 5, right panels). Western blotting with the corresponding antibodies revealed the co-immunoprecipitation of β2, α2δ4, and CaBP4 with FLAG-Cav1.4 both with and without Ca2+ (Fig. 5, A and B). This result was not reproduced in cells transfected without FLAG-Cav1.4, which argued against nonspecific immunoprecipitation of β2, α2δ4, and CaBP4 by FLAG antibodies (Fig. 5A). No immunoprecipitated proteins were detected either when control mouse IgG was used instead of FLAG antibodies (Fig. 5, A and B). We also tested whether Cav1.4 α1 associates with β2 and α2δ4 in mouse retina using coimmunoprecipitation with anti-β2 and anti-α2δ4. Cav1.4 α1 coimmunoprecipitated with β2 and α2δ4 (Fig. 5C).
FIGURE 5.
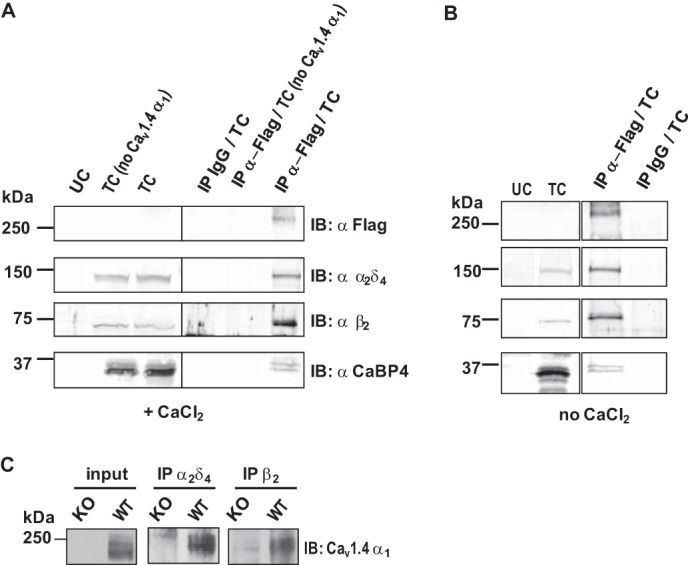
Isolation of Cav1.4 α11-β2-α2δ4 complexes from cotransfected HEK293 cells and mouse retina. A and B, Cav1.4 α1 interacts with β2X13, α2δ4, and CaBP4 expressed in HEK293 cells. Coimmunoprecipitations were performed with control mouse IgG or anti-FLAG antibody using lysates of HEK293 cells untransfected (UC) or cotransfected (TC) with β2X13, α2δ4, and CaBP4 with or without (no Cav1.4) FLAG-Cav1.4 α1. Experiments were performed in the presence (A) or in the absence of 0.1 mm Ca2+ (B). Western blotting (IB) was with anti-FLAG, anti-β2, anti-α2δ4, or anti-CaBP4. Left panels show input lysates. Right panels show coimmunoprecipitated (IP) proteins. Results shown are representative of three experiments. C, Cav1.4 α1 interacts with β2 and α2δ4 in mouse retina. Coimmunoprecipitations were performed using lysates from WT or KO Cav1.4 α1 mouse retina with anti-α2δ4 or anti-β2 antibodies. Anti-Cav1.4 α1 antibody was used for Western blotting. 8% of lysate was used for input.
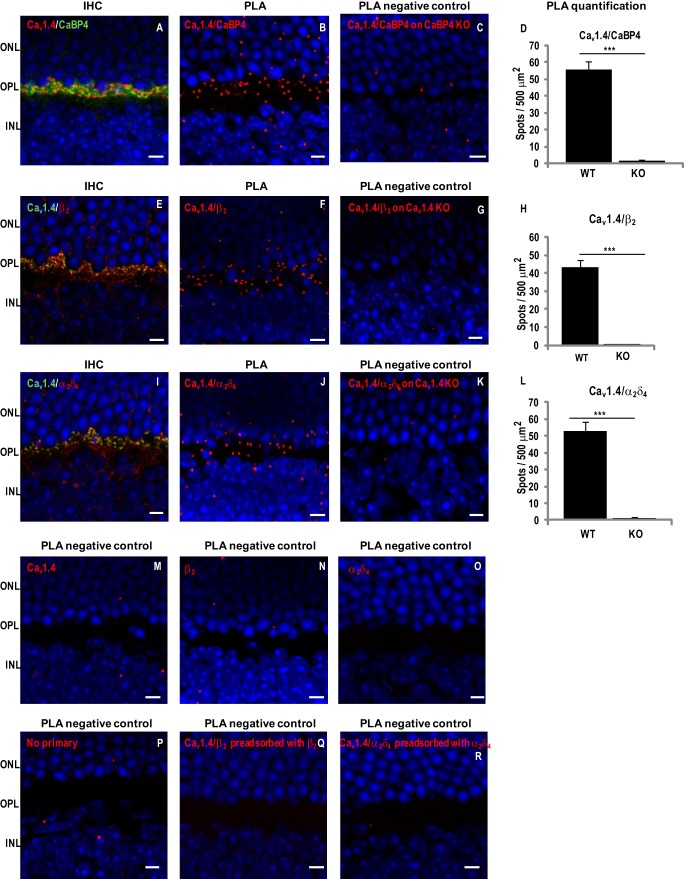
To test whether Cav1.4 subunits interact in photoreceptor synapses, we performed in situ PLAs, a technique that allows visualization of protein interactions in fixed tissue. With this method, antibodies against two potentially interacting proteins are coupled to oligonucleotides. If the two proteins interact, the antibodies that recognize these proteins in fixed tissue should be in close proximity (<40 nm) such that their coupled oligonucleotides can be ligated and further visualized with fluorescent probes. As proof of principle, we first tested the interaction of Cav1.4 α1 and CaBP4, which is known to colocalize with Cav1.4 at the photoreceptor terminals in the OPL (Fig. 6A) (28). PLA performed with anti-CaBP4 and anti-Cav1.4 α1 produced strong signals in the OPL, where both proteins are colocalized (Fig. 6, A and B). These signals were specific for the presence of CaBP4 because they were strongly reduced in the retina of mice lacking CaBP4 (CaBP4 KO; Fig. 6C). We have shown previously that immunolabeling for Cav1.4 channels is still present in the OPL of CaBP4 KO retina (13), so the absence of PLA signal is probably due to the lack of CaBP4/Cav1.4 interactions. A few fluorescent puncta were observed in the outer and inner nuclear layers of WT retina, but these were probably nonspecific because they were also seen in CaBP4 KO retina (Fig. 6C). With anti-Cav1.4 α1 and either anti-β2 or anti-α2δ4 antibodies, PLA generated strong signals in the OPL of WT but not Cav1.4 KO mice (Fig. 6, E–L). Again, only a few puncta were observed in the outer and inner nuclear layers of both WT and Cav1.4 KO retina, so they were probably nonspecific (Fig. 6, G and K). Quantification showed significantly greater density of PLA signals in WT compared with Cav1.4 KO retina using antibodies for Cav1.4-CaBP4, Cav1.4-β2, or Cav1.4-α2δ4 complexes (Fig. 6, D, H, and L; p < 0.0001). Additional negative controls for PLA were done by omission of one or both antibodies (Fig. 6, M–P) or by preadsorption of anti-β2 antibody with β2a or anti-α2δ4 with α2δ4 (Fig. 6, Q and R). No PLA signals were observed under these conditions. Taken together, our results confirm that Cav1.4 channel complexes at the photoreceptor synapse are composed of Cav1.4 α1, β2, and α2δ4 and CaBP4.
FIGURE 6.
Cav1.4 α1 interacts with CaBP4, β2 and α2δ4 in mouse retina. Immunohistochemistry (IHC) or PLAs were performed in sections of WT or knock-out mouse retina using anti-Cav1.4 α1 and anti-CaBP4 (A–D), anti-Cav1.4 α1 and anti-β2 (E–H), anti-Cav1.4 α1 and anti-α2δ4 (I–L), anti-Cav1.4 (M), anti-β2 (N), anti-α2δ4 (O), no primary antibodies (P), anti-β2 preadsorbed with β2a (Q), or anti-α2δ4 preadsorbed with α2δ4 (R). For IHC, WT mouse retina sections were double-labeled with anti-Cav1.4 α1 (red) and in green anti-CaBP4 (A), anti-Cav1.4 α1 (green) and in red anti-β2 (E), or anti-α2δ4 (I). INL, inner nuclear layer. PLA was performed on retina from WT (B, F, J, and M–R), CaBP4 KO (C), or Cav1.4 KO (G and K) mice. Sites of protein interaction are visualized as red spots. Quantification of PLA spots are shown as mean ± S.E. (error bars) for Cav1.4/CaBP4 in WT (n = 7) versus CaBP4 KO mice (n = 3) (p < 0.001; Student's t test) (D), for Cav1.4-β2 in WT (n = 7) versus Cav1.4 KO mice (n = 3) (p < 0.001) (H), and for Cav1.4-α2δ4 in WT (n = 6) versus Cav1.4 KO mice (n = 3) (p < 0.001) (L). For all images, nuclei are labeled with Hoechst. Scale bar, 5 μm.
Electrophysiological Analysis of Cav1.4 Channels in HEK293T Cells
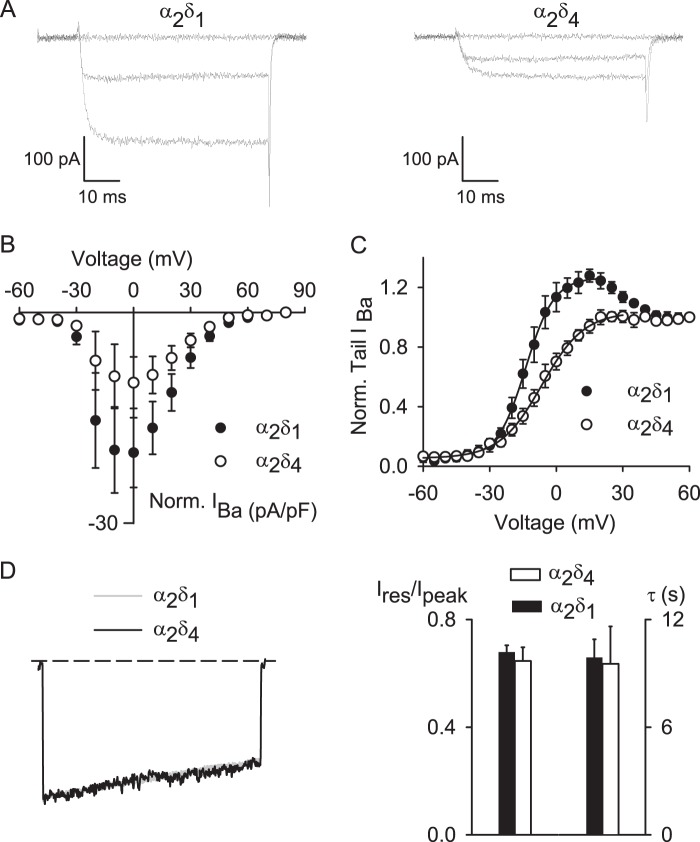
Despite the molecular characterization of α2δ4 as a Cav subunit (43), how α2δ4 affects the biophysical properties of Cav channels, particularly Cav1.4, is unknown; previous electrophysiological studies of Cav1.4 utilized the α2δ1 subunit (27, 30–32, 44). To better understand the functional properties of the native photoreceptor Cav1.4 channel complex, we performed whole-cell patch clamp recordings of Cav1.4 channels containing α2δ4 in transfected HEK293T cells. For comparison, we also carried out recordings of Cav1.4 with the α2δ1 subunit. Based on our findings that β2X13 is more highly expressed in the retina than β2a (Fig. 1), we used β2X13 in these experiments. Ba2+ was used as the charge carrier because the greater permeation of Ba2+ compared with Ca2+ increases the resolution of Cav1.4 currents, which are significantly smaller compared with those mediated by other Cav channels in HEK293T cells (31, 32). The properties of Ba2+ currents are largely similar to Ca2+ currents because Cav1.4 channels containing the distal C-terminal domain inhibitory module exhibit little Ca2+-dependent inactivation (31, 32, 44, 45).
In cells cotransfected with Cav1.4 α1-β2X13-α2δ4, Ba2+ currents were generally smaller than in cells expressing Cav1.4 α1-β2X13-α2δ1, although this difference did not reach statistical significance in plots of current density against test voltage (Fig. 7, A and B). However, analyses of normalized tail current-voltage curves revealed that Cav1.4 α1-β2X13-α2δ4 channels exhibited weaker voltage dependence of activation compared with channels containing Cav1.4 α1-β2X13-α2δ1 (Fig. 7C). Boltzmann fits indicated more positive half-maximal activation voltage (Vh) and greater slope (k) for channels containing α2δ4 compared with those with α2δ1 (Table 2). In these experiments, tail currents were normalized to those measured upon repolarization from a +80-mV step, at which channel open probability should be maximal. Interestingly, the normalized tail current amplitude exceeded 1 between 0 and +30 mV with α2δ1 but not with α2δ4 (Fig. 7C), similar to that due to Ca2+-dependent facilitation of Cav2.1 channels (46). Because we used Ba2+ as the charge carrier, this “overshoot” in the normalized tail current-voltage is probably independent of Ca2+ and may result from a current-dependent mechanism, because facilitation of the tail current was maximal at voltages evoking the greatest inward currents (Fig. 7, B and C). With respect to voltage-dependent inactivation (VDI) during a sustained depolarization, Cav1.4 α1-β2X13-α2δ1 and Cav1.4 α1-β2X13-α2δ4 exhibited similar properties (Fig. 7D). Inactivation was measured as the ratio of the residual current amplitude at the end of the pulse and the peak current amplitude (Ires/Ipeak). Because Cav1.4 channels inactivate with a very slow time course, inactivation was measured during relatively long (5-s) step depolarizations. There was no significant difference in Ires/Ipeak for Cav1.4 α1-β2X13-α2δ1 (0.7 ± 0.03, n = 5) and for Cav1.4 α1-β2X13-α2δ4 (0.7 ± 0.1, n = 6; p = 0.59; Fig. 7D). Time constants for VDI were also not different (9.9 ± 1.0 s for Cav1.4 α1-β2X13-α2δ1 versus 9.5 ± 2.1 s for Cav1.4 α1-β2X13-α2δ4, p = 0.88; Fig. 7D). These results demonstrate that for Cav1.4 channels, α2δ4 and α2δ1 differ primarily in their effects on voltage-dependent activation.
FIGURE 7.
Differential modulation of Cav1.4 properties by α2δ1 and α2δ4. A and B, Ba2+ currents (IBa) were evoked by 50-ms depolarizations from a holding voltage of −80 mV in HEK293T cells transfected with Cav1.4 containing α2δ1 (n = 8) or α2δ4 (n = 9) and β2X13. A, representative IBa traces during a 50-ms depolarization to −70, 0, and +30 mV. B, current-voltage (I-V) plots for data obtained as in A. C, tail currents were evoked after 20-ms depolarization to various voltages from a holding voltage of −80 mV and repolarization to −60 mV. Tail currents were normalized to that obtained after a +80-mV pulse and plotted against test voltage. D, inactivation of IBa evoked by 5-s pulses from −80 to 0 mV. Left, representative traces. Right, inactivation was measured by dividing residual current amplitude (Ires) by the peak amplitude (Ipeak) in cells transfected with Cav1.4 containing α2δ1 (n = 5) or α2δ4 (n = 6), p = 0.59, by Student's t test. The time constant, τ, was obtained by fitting IBa decay with a single exponential function and was not different for α2δ1 and α2δ4; p = 0.88, Student's t test. Error bars, S.E.
TABLE 2.
Parameters for voltage-dependent activation
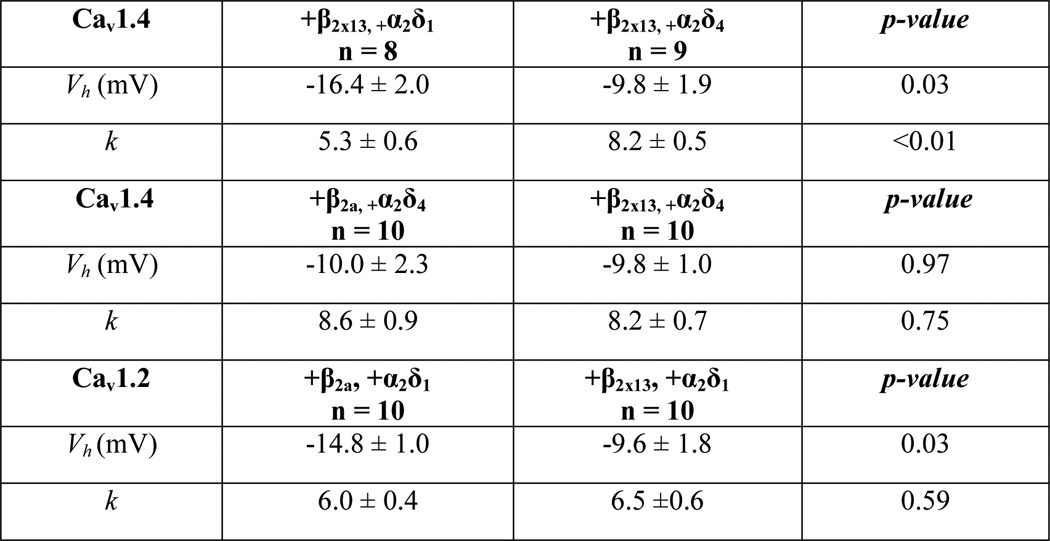
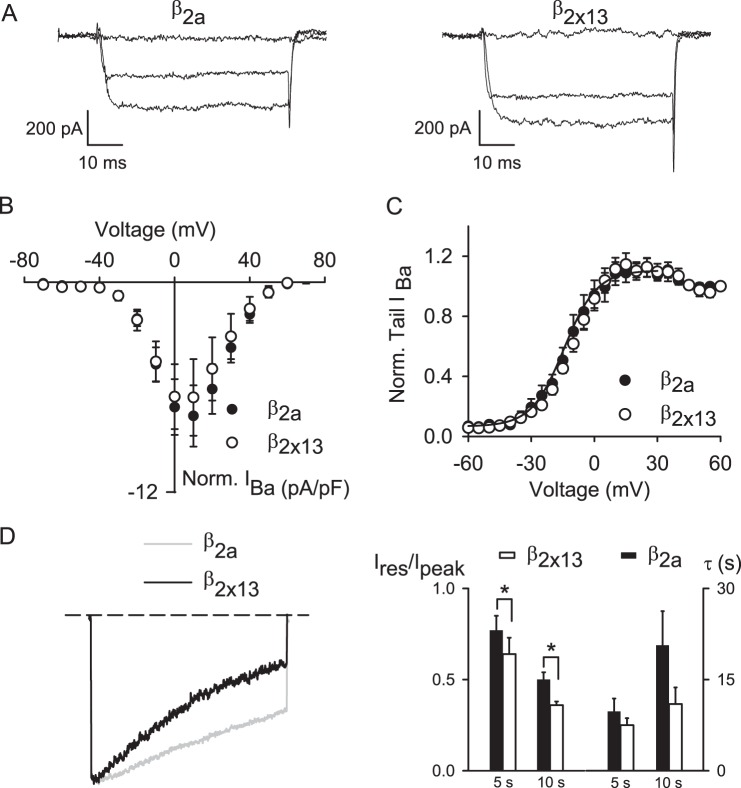
We next compared the impact of β2a and β2X13 on Cav1.4 function. Compared with other Cav β subunits, β2a significantly slows VDI of Cav channels (47). Structure/function analyses indicate that this property of β2a is mediated by the HOOK domain (48, 49). Because β2X13 and β2a differ in the HOOK domain (Fig. 1A), we predicted that these splice variants may have distinct effects on VDI of Cav1.4. Consistent with previous findings that the HOOK domain did not affect voltage-dependent activation (48), there were no differences in Vh or k obtained from normalized tail current-voltage curves in cells transfected with Cav1.4 α1-β2X13-α2δ4 or Cav1.4 α1-β2a-α2δ4 (Fig. 8, A and B, and Table 2). However, VDI during a 5- and 10-s depolarizing pulse was significantly greater for Cav1.4 α1-β2X13-α2δ4 than for Cav1.4 α1-β2a-α2δ4, although the time constants for VDI were not different (see legend to Fig. 8D for values). These results show that the β2X13 subunit influences the amount but not the rate of VDI of Cav1.4 channels.
FIGURE 8.
Differential modulation of Cav1.4 properties by β2a and β2X13. A–C, same as in Fig. 7 except for Cav1.4 containing β2a (n = 10) or β2X13 (n = 10) and α2δ4. D, same as in Fig. 7D except for Cav1.4 containing β2a (n = 7) or β2X13 (n = 7), and IBa was evoked by either 5- or 10-s pulses from −80 to +10 mV. Left, representative traces are evoked by 10-s pulses. Right, Ires/Ipeak (*, p = 0.01 for 5-s pulses; p = 0.04 for 10-s pulses, by Student's t test) and τ (p = 0.32 for 5-s pulses, whereas p = 0.16 for 10-s pulses by Student's t test). Error bars, S.E.
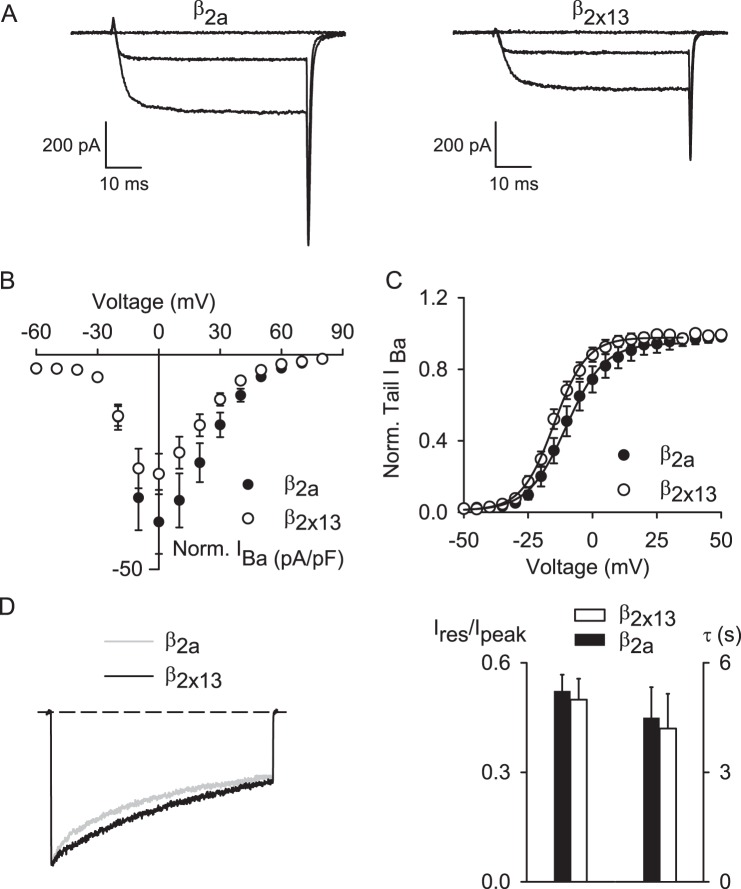
To determine whether β2X13 had similar effects on other Cav channels, we compared properties of Ba2+ currents in cells transfected with Cav1.2 α1-β2X13-α2δ1 and those transfected with Cav1.2 α1-β2a-α2δ1. In these experiments, α2δ1 was used because native Cav1.2 channels in the brain are not likely to associate with α2δ4 based on the near absence of α2δ4, but prevalence of α2δ1, in mouse brain (50). Ba2+ current density was generally lower for Cav1.2 α1-β2X13-α2δ1 than for Cav1.2 α1-β2a-α2δ1 but not significantly different. Unlike its lack of effect on Cav1.4 activation, β2X13 caused a small but significant (∼+5 mV) shift in the Vh obtained from normalized tail current curves compared with β2a for Cav1.2 channels (Fig. 9, A–C, and Table 2). However, unlike for Cav1.4, β2X13 and β2a had similar effects on VDI (Fig. 9D). Therefore, β2X13 and β2a differentially modulate the functional properties of Cav1.2 and Cav1.4.
FIGURE 9.
Unlike their distinct effects on Cav1.4, β2a and β2X13 do not differentially modulate Cav1.2 inactivation. A–C, same as in Fig. 7 except for Cav1.2 channels containing β2a (n = 10) or β2X13 (n = 10) and α2δ4. D, same as in Fig. 7D except for Cav1.2 containing β2a (n = 9) or β2X13 (n = 9), and IBa was evoked by 5-s pulses from −80 to 0 mV. Left, representative traces are evoked by 5-s pulses. Right, Ires/Ipeak (*, p = 0.75, by Student's t test) and τ (p = 0.81 by Student's t test). Error bars, S.E.
DISCUSSION
In this paper, we extend previous work indicating the importance of the auxiliary Cav subunits, β2 and α2δ4, in the retina. First, we provide the first evidence that Cav1.4 α1, β2, and α2δ4 physically and functionally interact both in transfected HEK293T cells and in photoreceptor synaptic terminals in the retina. Second, we identify the β2X13 splice variant, which is distinct from the β2a subunit expressed in the brain, as the major β2 subunit in human retina. Finally, we show that both β2X13 and α2δ4 have distinct effects on the biophysical properties of Cav1.4 compared with β2a and α2δ1. Our results reveal unexpected differences in the modulatory capabilities of Cav β and α2δ subunits, which may influence the properties of native Cav1.4 channels in photoreceptors.
Role of Cav β and α2δ4 as Auxiliary Subunits of Cav1.4 in the Retina
The notion that β2 and α2δ4 associate with Cav1.4 channels in the retina is supported by immunochemical and genetic evidence. First, antibodies against β2 and α2δ4 strongly label the OPL of the retina, where photoreceptor synapses are localized (51–53). In contrast, antibodies against β1, β3, and β4 label other regions in the retina but not the OPL (51). Second, mice lacking expression of β2, but not mice lacking β1, β3, or β4, exhibited strongly reduced b-waves in electroretinograms (25). The b-wave indicates efficiency of transmission from photoreceptors to bipolar neurons and is highly dependent on proper function of Cav1.4. The b-wave is absent in Cav1.4 KO mice (15) and in mice expressing a mutation in the α2δ4 gene, Cacna2d4, in which a truncated α2δ4 protein is expressed at very low levels compared with the full-length protein in wild-type mice (26). In the α2δ4 mutant mice, there is also delayed degeneration of cones, and human mutations in CACNA2D4 are associated with slowly progressive cone dystrophy (54). Our findings that β2 and α2δ4 colocalize with Cav1.4 α1 at structures resembling photoreceptor synaptic ribbons (Fig. 4) and interact with Cav1.4 α1 in transfected cells (Fig. 5) and in the OPL (Fig. 6) verify that these auxiliary subunits are primary components of Cav1.4 channel complexes at the photoreceptor synapse.
Molecular and Functional Characterization of a New Retinal Cav β2X13 Splice Variant
Cav β subunits regulate multiple aspects of Cav channel function, including their gating properties and levels at the cell surface (reviewed in Refs. 1 and 55). Of the four classes of Cav β that have been characterized, β2 is subject to the most significant alternative splicing, with 13 variants thus far identified (1, 41). Despite the evidence in favor of β2 as a Cav1.4 subunit, there has been no characterization of Cav β2 variants expressed in human retina. In our RT-PCR analyses of human retina, we detected the β2a variant that is present in the brain (56) as well as a β2 splice variant, β2X13, which has not been previously reported in the retina. This new β2X13 variant has the unique combination of containing the two N-terminal cysteines shown to be palmitoylated in β2a (57) and containing a shorter HOOK domain partially encoded by exon 7B (Fig. 1A). Whereas β2X13 was consistently detected at higher levels by qPCR than β2a in RNA isolated from three different individuals, there was large variability in the ratio between β2X13 and β2a (Fig. 1B and Table 1). Previous analyses indicate that β2 splice variant expression can vary significantly during development (58). Because we did not have information regarding the age of the individuals from which the samples were isolated, it is possible that our results reflect age-related differences in β2X13 and β2a levels. We detected β2X13 in both mouse and human retina but not in mouse brain (Fig. 1C), which further supports the physiological importance of β2X13 as a partner for photoreceptor Cav1.4 channels. In addition, RNA-seq analysis of the retinal β2 transcripts showed that β2X13 is the major transcript in the cone-rich macula and in the peripheral retina, indicating that β2X13 is the main isoform in both rods and cones.
The inclusion of exon 7B in β2X13 creates a shorter HOOK domain (7 amino acids) compared with the exon 7A-containing β2a (45 amino acids; Fig. 1A). The HOOK domains of β1b, β1c, β3, and β4 are the same length as (and contain the same sequence (AKQKQK) that is present in) the β2X13 exon 7B (1). Transfer of the HOOK domain from β1b to β2a causes strong VDI of Cav2.1 channels, typical of β1b (48). Moreover, deletion of the HOOK domain from β2a enhances VDI of Cav2.2 channels (49). Consistent with these findings, we found that, compared with β2a, β2X13 causes stronger VDI of Cav1.4 channels (Fig. 8D). Curiously, this was not the case for Cav1.2 (Fig. 9D). The effect of β2a in slowing VDI of Cav1.2 is less profound than observed for other Cav channels (59), so it may be that Cav1.2 VDI is somehow more resistant to modulation by β2a subunits than Cav1.4, such that the distinct effects of β2a and β2X13 on VDI are more readily resolved in Cav1.4 than Cav1.2.
The distinct exon 7B present in β2X13 is orthologous to exon 9 of the zebrafish β2.1 gene, which is found in a number of β2.1 variants that are expressed at distinct developmental ages and in different tissues, including the eye (60). Whereas β2X13 includes the two palmitoylated N-terminal cysteines present in β2a (57), these β2.1 variants do not. Although it is tempting to speculate on the physiological significance of β2X13-containing Cav1.4 channels, it is important to note that VDI is still relatively limited for these channels, with ∼35% of the current remaining after 10 s of depolarization (Fig. 9D). Moreover, maximal depolarization of photoreceptors is likely not to exceed ∼−40 mV, which should not induce significant VDI of Cav1.4 channels. Therefore, whether the difference in VDI due to β2X13 and β2a is physiologically relevant for photoreceptor synaptic transmission is debatable. An alternate possibility is that β2X13 may affect modulation of Cav1.4 by other factors. For example, Cav1.3 channels containing β2a but not other β subunits are relatively resistant to inhibition by arachidonic acid (61). Because synaptic Ca2+ currents in salamander photoreceptors are strongly inhibited by arachidonic acid (62), it will be of interest to determine whether β2X13 alters the sensitivity of Cav1.4 channels to this arachidonic acid or other neuromodulators.
Functional Characterization of Cav1.4 Channels Containing the α2δ4 Subunit
Cav α2δ subunits are composed of α2 and δ proteins that are encoded by a single gene; proteolytic cleavage of the α2-δ preprotein separates α2 and δ proteins, which remain linked under native conditions by disulfide bonds (reviewed in Ref. 3). Cav α2δ subunits enhance the cell surface trafficking and decrease the turnover of Cav1 and Cav2 channels (63, 64). These effects are due in part to a von Willebrand factor A (VFA) domain and in particular a metal ion adhesion motif (MIDAS), the mutation of which prevents the cell surface trafficking function of α2δ2 on Cav2.1 currents (64). In addition, glycosylation of the extracellular domain is important for the current-enhancing effects of α2δ (65). α2δ4 contains fewer glycosylation sites compared with the three other α2δ subunits (43), which may explain why Cav1.4 current densities were generally smaller with α2δ4 than with α2δ1 (Fig. 7, A and B). Notably, α2δ4 does not have the asparagine corresponding to residue 184, which, when mutated to glutamine, inhibits the enhancement of Cav2.2 channels caused by α2δ1 (66).
Cav α2δ subunits have been shown to have variable effects on voltage-dependent activation of Cav channels, which may depend on the particular Cav α1 subunit with which they associate (reviewed in Ref. 3). For Cav1.2 channels, α2δ1 causes a negative shift in the voltage dependence of activation, which depends largely on the δ-encoding sequence (67). Our findings that Cav1.4 channels containing α2δ4 exhibit weaker voltage dependence of activation than with α2δ1 may result from relatively weak conservation (∼28% sequence identity) in the δ-encoding sequence for α2δ4 and α2δ1. A further distinction between α2δ4 and α2δ1 was in the facilitation of currents evoked by moderate voltages that was unique to α2δ1-containing Cav1.4 channels (Fig. 7C). Although further analyses are required, this facilitation may result from the binding of permeant Ba2+ ions in the channel protein, similar to what has been proposed for ion-dependent inactivation of Ba2+ currents through Cav1.2 channels (68). The absence of this facilitation, in addition to relatively weak voltage dependence of activation, in α2δ4-containing Cav1.4 channels might be considered maladaptive in terms of supporting synaptic Ca2+ signals that drive photoreceptor transmission. However, native Cav1.4 channels would also be associated with CaBP4, which significantly enhances voltage-dependent activation of Cav1.4 (28, 42). Thus, native α2δ4-containing Cav1.4 channels in photoreceptors may be more strongly modulated by CaBP4 than α2δ1-containing Cav1.4 channels. α2δ subunits also play roles in regulating neurotransmitter release probability (69) as well as synaptogenesis (70, 71), independent of effects on Cav function. Understanding the precise roles of α2δ4 in regulating photoreceptor signaling and how its dysregulation leads to vision impairment in humans and mice (26, 54) remains an important challenge for future studies.
Acknowledgments
We thank Dr. Annette Dolphin for the kind gift of plasmids encoding α2δ1, α2δ2, and α2δ3 subunits, Dr. Sharona Gordon and the members of her group for helpful discussions, and the Lions Eye Bank of Oregon for providing human retinas.
This work was supported, in whole or in part, by National Institutes of Health (NIH), NEI, Grant R01-EY020850 (to F. H.); NIH Grants DC009433 and NS084190 (to A. L.); NIH Grant T32 NS007421 (to B. W.); and NIH, NCRR, Grant S10 RR025429 (to Sharona Gordon). This work was also supported by a Carver Research Program of Excellence Award (to A. L.).
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) KJ789960.
- qPCR
- quantitative PCR
- RNA-seq
- RNA sequencing
- PLA
- proximity ligation assay
- OPL
- outer plexiform layer
- ONL
- outer nuclear layer
- VDI
- voltage-dependent inactivation.
REFERENCES
- 1. Buraei Z., Yang J. (2010) The β subunit of voltage-gated Ca2+ channels. Physiol. Rev. 90, 1461–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dolphin A. C. (2012) Calcium channel auxiliary α2δ and β subunits: trafficking and one step beyond. Nat. Rev. Neurosci. 13, 542–555 [DOI] [PubMed] [Google Scholar]
- 3. Dolphin A. C. (2013) The α2δ subunits of voltage-gated calcium channels. Biochim. Biophys. Acta 1828, 1541–1549 [DOI] [PubMed] [Google Scholar]
- 4. Simms B. A., Zamponi G. W. (2014) Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron 82, 24–45 [DOI] [PubMed] [Google Scholar]
- 5. Catterall W. A. (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555 [DOI] [PubMed] [Google Scholar]
- 6. Catterall W. A., Perez-Reyes E., Snutch T. P., Striessnig J. (2005) International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 57, 411–425 [DOI] [PubMed] [Google Scholar]
- 7. Lipscombe D., Andrade A., Allen S. E. (2013) Alternative splicing: functional diversity among voltage-gated calcium channels and behavioral consequences. Biochim. Biophys. Acta 1828, 1522–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matthews G., Fuchs P. (2010) The diverse roles of ribbon synapses in sensory neurotransmission. Nat. Rev. Neurosci. 11, 812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krizaj D., Copenhagen D. R. (2002) Calcium regulation in photoreceptors. Front. Biosci. 7, d2023–d2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barnes S., Kelly M. E. M. (2002) Calcium channels at the photoreceptor synapse. Adv. Exp. Med. Biol. 514, 465–476 [DOI] [PubMed] [Google Scholar]
- 11. Berntson A., Taylor W. R., Morgans C. W. (2003) Molecular identity, synaptic localization, and physiology of calcium channels in retinal bipolar cells. J. Neurosc. Res. 71, 146–151 [DOI] [PubMed] [Google Scholar]
- 12. Morgans C. W. (2001) Localization of the α(1F) calcium channel subunit in the rat retina. Invest. Ophthalmol. Vis. Sci. 42, 2414–2418 [PubMed] [Google Scholar]
- 13. Liu X., Kerov V., Haeseleer F., Majumder A., Artemyev N., Baker S. A., Lee A. (2013) Dysregulation of Ca(v)1.4 channels disrupts the maturation of photoreceptor synaptic ribbons in congenital stationary night blindness type 2. Channels 7, 514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knoflach D., Kerov V., Sartori S. B., Obermair G. J., Schmuckermair C., Liu X., Sothilingam V., Garrido M. G., Baker S. A., Glösmann M., Schicker K., Seeliger M., Lee A., Koschak A. (2013) Cav1.4 IT mouse as model for vision impairment in human congenital stationary night blindness type 2. Channels 7, 503–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mansergh F., Orton N. C., Vessey J. P., Lalonde M. R., Stell W. K., Tremblay F., Barnes S., Rancourt D. E., Bech-Hansen N. T. (2005) Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum. Mol. Genet. 14, 3035–3046 [DOI] [PubMed] [Google Scholar]
- 16. Zabouri N., Haverkamp S. (2013) Calcium channel-dependent molecular maturation of photoreceptor synapses. PLoS One 8, e63853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Strom T. M., Nyakatura G., Apfelstedt-Sylla E., Hellebrand H., Lorenz B., Weber B. H. F., Wutz K., Gutwillinger N., Rüther K., Drescher B., Sauer C., Zrenner E., Meitinger T., Rosenthal A., Meindl A. (1998) An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat. Genet. 19, 260–263 [DOI] [PubMed] [Google Scholar]
- 18. Wutz K., Sauer C., Zrenner E., Lorenz B., Alitalo T., Broghammer M., Hergersberg M., de la Chapelle A., Weber B. H. F., Wissinger B., Meindl A., Pusch C. M. (2002) Thirty distinct CACNA1F mutations in 33 families with incomplete type of XLCSNB and Cacna1f expression profiling in mouse retina. Eur. J. Hum. Genet. 10, 449–456 [DOI] [PubMed] [Google Scholar]
- 19. Bech-Hansen N. T., Naylor M. J., Maybaum T. A., Pearce W. G., Koop B., Fishman G. A., Mets M., Musarella M. A., Boycott K. M. (1998) Loss-of-function mutations in a calcium-channel α(1)-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat. Genet. 19, 264–267 [DOI] [PubMed] [Google Scholar]
- 20. Boycott K. M., Maybaum T. A., Naylor M. J., Weleber R. G., Robitaille J., Miyake Y., Bergen A. A., Pierpont M. E., Pearce W. G., Bech-Hansen N. T. (2001) A summary of 20 CACNA1F mutations identified in 36 families with incomplete X-linked congenital stationary night blindness, and characterization of splice variants. Hum. Genet. 108, 91–97 [DOI] [PubMed] [Google Scholar]
- 21. Taylor W. R., Morgans C. (1998) Localization and properties of voltage-gated calcium channels in cone photoreceptors of Tupaia belangeri. Vis. Neurosci. 15, 541–552 [DOI] [PubMed] [Google Scholar]
- 22. Morgans C. W. (1999) Calcium channel heterogeneity among cone photoreceptors in the tree shrew retina. Eur. J. Neurosc. 11, 2989–2993 [DOI] [PubMed] [Google Scholar]
- 23. Ko M. L., Liu Y., Dryer S. E., Ko G. Y. P. (2007) The expression of L-type voltage-gated calcium channels in retinal photoreceptors is under circadian control. J. Neurochem. 103, 784–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Busquet P., Nguyen N. K., Schmid E., Tanimoto N., Seeliger M. W., Ben-Yosef T., Mizuno F., Akopian A., Striessnig J., Singewald N. (2010) CaV1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int. J. Neuropsychopharmacol. 13, 499–513 [DOI] [PubMed] [Google Scholar]
- 25. Ball S. L., Powers P. A., Shin H. S., Morgans C. W., Peachey N. S., Gregg R. G. (2002) Role of the β(2) subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest. Ophthalmol. Vis. Sci. 43, 1595–1603 [PubMed] [Google Scholar]
- 26. Wycisk K. A., Budde B., Feil S., Skosyrski S., Buzzi F., Neidhardt J., Glaus E., Nürnberg P., Ruether K., Berger W. (2006) Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest. Ophthalmol. Vis. Sci. 47, 3523–3530 [DOI] [PubMed] [Google Scholar]
- 27. Doering C. J., Hamid J., Simms B., McRory J. E., Zamponi G. W. (2005) Ca(v)1.4 encodes a calcium channel with low open probability and unitary conductance. Biophys. J. 89, 3042–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haeseleer F., Imanishi Y., Maeda T., Possin D. E., Maeda A., Lee A., Rieke F., Palczewski K. (2004) Essential role of Ca2+-binding protein 4, a Ca(v)1.4 channel regulator, in photoreceptor synaptic function. Nat. Neurosci. 7, 1079–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koschak A., Reimer D., Walter D., Hoda J. C., Heinzle T., Grabner M., Striessnig J. (2003) Ca(v)1.4 α 1 subunits can form slowly inactivating dihydropyridine-sensitive L-type Ca2+ channels lacking Ca2+-dependent inactivation. J. Neurosci. 23, 6041–6049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peloquin J. B., Doering C. J., Rehak R., McRory J. E. (2008) Temperature dependence of Ca-v 1.4 calcium channel gating. Neuroscience 151, 1066–1083 [DOI] [PubMed] [Google Scholar]
- 31. Baumann L., Gerstner A., Zong X., Biel M., Wahl-Schott C. (2004) Functional Characterization of the L-type Ca2+ channel Cav1.4α1 from mouse retina. Invest. Ophthalmol. Vis. Sci. 45, 708–713 [DOI] [PubMed] [Google Scholar]
- 32. McRory J. E., Hamid J., Doering C. J., Garcia E., Parker R., Hamming K., Chen L., Hildebrand M., Beedle A. M., Feldcamp L., Zamponi G. W., Snutch T. P. (2004) The CACNA1F gene encodes an L-type calcium channel with unique biophysical properties and tissue distribution. J. Neurosci. 24, 1707–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Specht D., Wu S. B., Turner P., Dearden P., Koentgen F., Wolfrum U., Maw M., Brandstätter J. H., tom Dieck S. (2009) Effects of presynaptic mutations on a postsynaptic Cacna1s calcium channel colocalized with mGluR6 at mouse photoreceptor ribbon synapses. Invest. Ophthalmol. Vis. Sci. 50, 505–515 [DOI] [PubMed] [Google Scholar]
- 34. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 35. Whitmore S. S., Wagner A. H., DeLuca A. P., Drack A. V., Stone E. M., Tucker B. A., Zeng S., Braun T. A., Mullins R. F., Scheetz T. E. (2014) Transcriptomic analysis across nasal, temporal, and macular regions of human neural retina and RPE/choroid by RNA-Seq. Exp. Eye Res. 129C, 93–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D. R., Pimentel H., Salzberg S. L., Rinn J. L., Pachter L. (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thorvaldsdóttir H., Robinson J. T., Mesirov J. P. (2013) Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 14, 178–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cui G., Meyer A. C., Calin-Jageman I., Neef J., Haeseleer F., Moser T., Lee A. (2007) Ca2+-binding proteins tune Ca2+-feedback to Cav1.3 Ca2+ channels in auditory hair cells. J. Physiol. 585, 791–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davies A., Kadurin I., Alvarez-Laviada A., Douglas L., Nieto-Rostro M., Bauer C. S., Pratt W. S., Dolphin A. C. (2010) The α2δ subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc. Natl. Acad. Sci. U.S.A. 107, 1654–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bolte S., Cordelières F. P. (2006) A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 [DOI] [PubMed] [Google Scholar]
- 41. Lyford G. L., Strege P. R., Shepard A., Ou Y., Ermilov L., Miller S. M., Gibbons S. J., Rae J. L., Szurszewski J. H., Farrugia G. (2002) α(1C) (Ca(V)1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am. J. Physiol. Cell Physiol. 283, C1001–C1008 [DOI] [PubMed] [Google Scholar]
- 42. Shaltiel L., Paparizos C., Fenske S., Hassan S., Gruner C., Rötzer K., Biel M., Wahl-Schott C. A. (2012) Complex regulation of voltage-dependent activation and inactivation properties of retinal voltage-gated Cav1.4 L-type Ca2+ channels by Ca2+-binding protein 4 (CaBP4). J. Biol. Chem. 287, 36312–36321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qin N., Yagel S., Momplaisir M. L., Codd E. E., D'Andrea M. R. (2002) Molecular cloning and characterization of the human voltage-gated calcium channel α(2)δ-4 subunit. Mol. Pharmacol. 62, 485–496 [DOI] [PubMed] [Google Scholar]
- 44. Singh A., Hamedinger D., Hoda J. C., Gebhart M., Koschak A., Romanin C., Striessnig J. (2006) C-terminal modulator controls Ca2+-dependent gating of Ca(v)1.4 L-type Ca2+ channels. Nat. Neurosci. 9, 1108–1116 [DOI] [PubMed] [Google Scholar]
- 45. Wahl-Schott C., Baumann L., Cuny H., Eckert C., Griessmeier K., Biel M. (2006) Switching off calcium-dependent inactivation in L-type calcium channels by an autoinhibitory domain. Proc. Natl. Acad. Sci. U.S.A. 103, 15657–15662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chaudhuri D., Issa J. B., Yue D. T. (2007) Elementary mechanisms producing facilitation of Cav2.1 (P/Q-type) channels. J. Gen. Physiol. 129, 385–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stea A., Tomlinson W. J., Soong T. W., Bourinet E., Dubel S. J., Vincent S. R., Snutch T. P. (1994) Localization and functional properties of a rat brain α 1A calcium channel reflect similarities to neuronal Q- and P-type channels. Proc. Natl. Acad. Sci. U.S.A. 91, 10576–10580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. He L. L., Zhang Y., Chen Y. H., Yamada Y., Yang J. (2007) Functional modularity of the β-subunit of voltage-gated Ca2+ channels. Biophys. J. 93, 834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Richards M. W., Leroy J., Pratt W. S., Dolphin A. C. (2007) The HOOK-domain between the SH3 and the GK domains of Cavβ subunits contains key determinants controlling calcium channel inactivation. Channels 1, 92–101 [DOI] [PubMed] [Google Scholar]
- 50. Schlick B., Flucher B. E., Obermair G. J. (2010) Voltage-activated calcium channel expression profiles in mouse brain and cultured hippocampal neurons. Neuroscience 167, 786–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ball S. L., McEnery M. W., Yunker A. M., Shin H. S., Gregg R. G. (2011) Distribution of voltage gated calcium channel β subunits in the mouse retina. Brain Res. 1412, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mercer A. J., Chen M., Thoreson W. B. (2011) Lateral mobility of presynaptic L-type calcium channels at photoreceptor ribbon synapses. J. Neurosci. 31, 4397–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. De Sevilla Müller L. P., Liu J., Solomon A., Rodriguez A., Brecha N. C. (2013) Expression of voltage-gated calcium channel α(2)δ(4) subunits in the mouse and rat retina. J. Comp. Neurol. 521, 2486–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wycisk K. A., Zeitz C., Feil S., Wittmer M., Forster U., Neidhardt J., Wissinger B., Zrenner E., Wilke R., Kohl S., Berger W. (2006) Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. Am. J. Hum. Genet. 79, 973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Buraei Z., Yang J. (2013) Structure and function of the beta subunit of voltage-gated Ca2+ channels. Biochim. Biophys. Acta 1828, 1530–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perez-Reyes E., Castellano A., Kim H. S., Bertrand P., Baggstrom E., Lacerda A. E., Wei X. Y., Birnbaumer L. (1992) Cloning and expression of a cardiac/brain β subunit of the L-type calcium channel. J. Biol. Chem. 267, 1792–1797 [PubMed] [Google Scholar]
- 57. Chien A. J., Carr K. M., Shirokov R. E., Rios E., Hosey M. M. (1996) Identification of palmitoylation sites within the L-type calcium channel β2a subunit and effects on channel function. J. Biol. Chem. 271, 26465–26468 [DOI] [PubMed] [Google Scholar]
- 58. Chu P. J., Larsen J. K., Chen C. C., Best P. M. (2004) Distribution and relative expression levels of calcium channel β subunits within the chambers of the rat heart. J. Mol. Cell Cardiol. 36, 423–434 [DOI] [PubMed] [Google Scholar]
- 59. Yasuda T., Chen L., Barr W., McRory J. E., Lewis R. J., Adams D. J., Zamponi G. W. (2004) Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur. J. Neurosci. 20, 1–13 [DOI] [PubMed] [Google Scholar]
- 60. Ebert A. M., McAnelly C. A., Srinivasan A., Mueller R. L., Garrity D. B., Garrity D. M. (2008) The calcium channel β2 (CACNB2) subunit repertoire in teleosts. BMC Mol. Biol. 9, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Roberts-Crowley M. L., Rittenhouse A. R. (2009) Arachidonic acid inhibition of L-type calcium (CaV1.3b) channels varies with accessory CaVβ subunits. J. Gen. Physiol. 133, 387–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vellani V., Reynolds A. M., McNaughton P. A. (2000) Modulation of the synaptic Ca2+ current in salamander photoreceptors by polyunsaturated fatty acids and retinoids. J. Physiol. 529, 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bernstein G. M., Jones O. T. (2007) Kinetics of internalization and degradation of N-type voltage-gated calcium channels: role of the α2/δ subunit. Cell Calcium 41, 27–40 [DOI] [PubMed] [Google Scholar]
- 64. Cantí C., Nieto-Rostro M., Foucault I., Heblich F., Wratten J., Richards M. W., Hendrich J., Douglas L., Page K. M., Davies A., Dolphin A. C. (2005) The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of α2δ subunits is key to trafficking voltage-gated Ca2+ channels. Proc. Natl. Acad. Sci. U.S.A. 102, 11230–11235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gurnett C. A., De Waard M., Campbell K. P. (1996) Dual function of the voltage-dependent Ca2+ channel α 2 δ subunit in current stimulation and subunit interaction. Neuron 16, 431–440 [DOI] [PubMed] [Google Scholar]
- 66. Sandoval A., Oviedo N., Andrade A., Felix R. (2004) Glycosylation of asparagines 136 and 184 is necessary for the α2δ subunit-mediated regulation of voltage-gated Ca2+ channels. FEBS Lett. 576, 21–26 [DOI] [PubMed] [Google Scholar]
- 67. Felix R., Gurnett C. A., De Waard M., Campbell K. P. (1997) Dissection of functional domains of the voltage-dependent Ca2+ channel α2δ subunit. J. Neurosci. 17, 6884–6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ferreira G., Yi J., Ríos E., Shirokov R. (1997) Ion-dependent inactivation of barium current through L-type calcium channels. J. Gen. Physiol. 109, 449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hoppa M. B., Lana B., Margas W., Dolphin A. C., Ryan T. A. (2012) α2δ expression sets presynaptic calcium channel abundance and release probability. Nature 486, 122–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Eroglu C., Allen N. J., Susman M. W., O'Rourke N. A., Park C. Y., Ozkan E., Chakraborty C., Mulinyawe S. B., Annis D. S., Huberman A. D., Green E. M., Lawler J., Dolmetsch R., Garcia K. C., Smith S. J., Luo Z. D., Rosenthal A., Mosher D. F., Barres B. A. (2009) Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139, 380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kurshan P. T., Oztan A., Schwarz T. L. (2009) Presynaptic α2δ-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat. Neurosci. 12, 1415–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]