Abstract
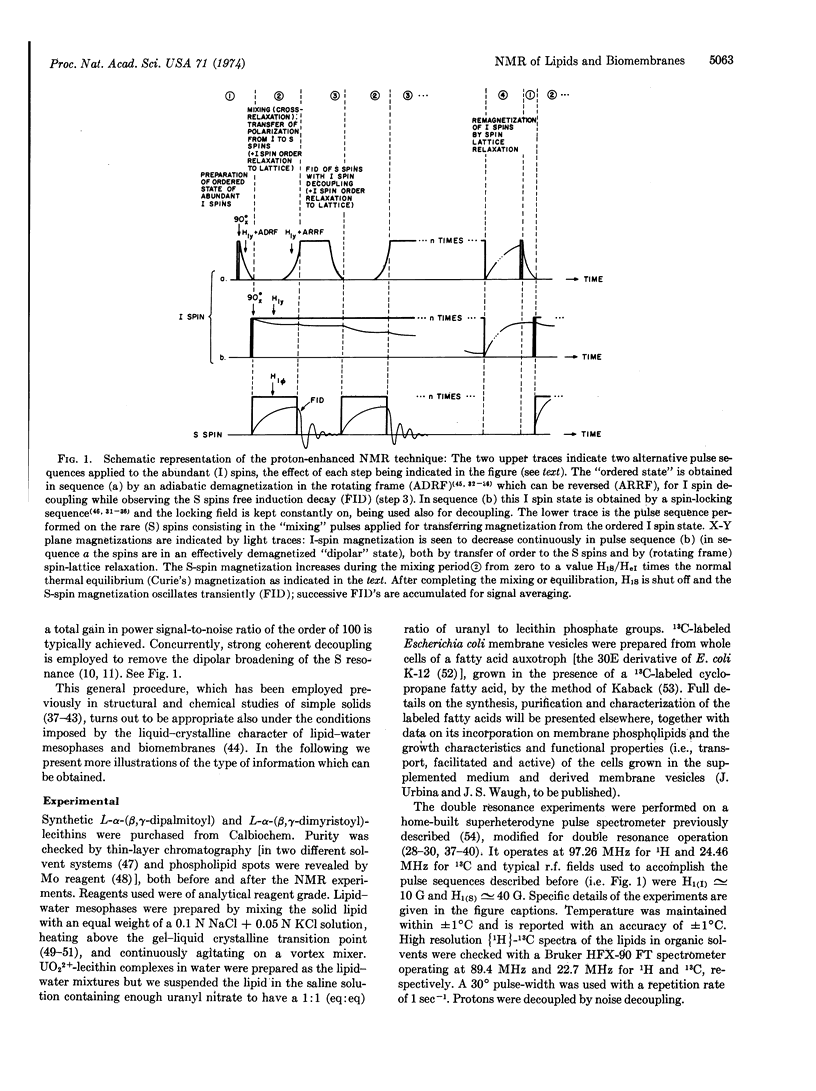
A recently developed nuclear double resonance technique which permits sensitive detection, together with high resolution, of rare spins in solids or other dipolar-coupled nuclear systems [Pines, Gibby, and Waugh (1973) J. Chem. Phys. 59, 569] has been applied to the study of natural abundance 13C-nuclear magnetic resonance in lipid mesophases and of selectively labeled carbon sites in bacterial membranes.
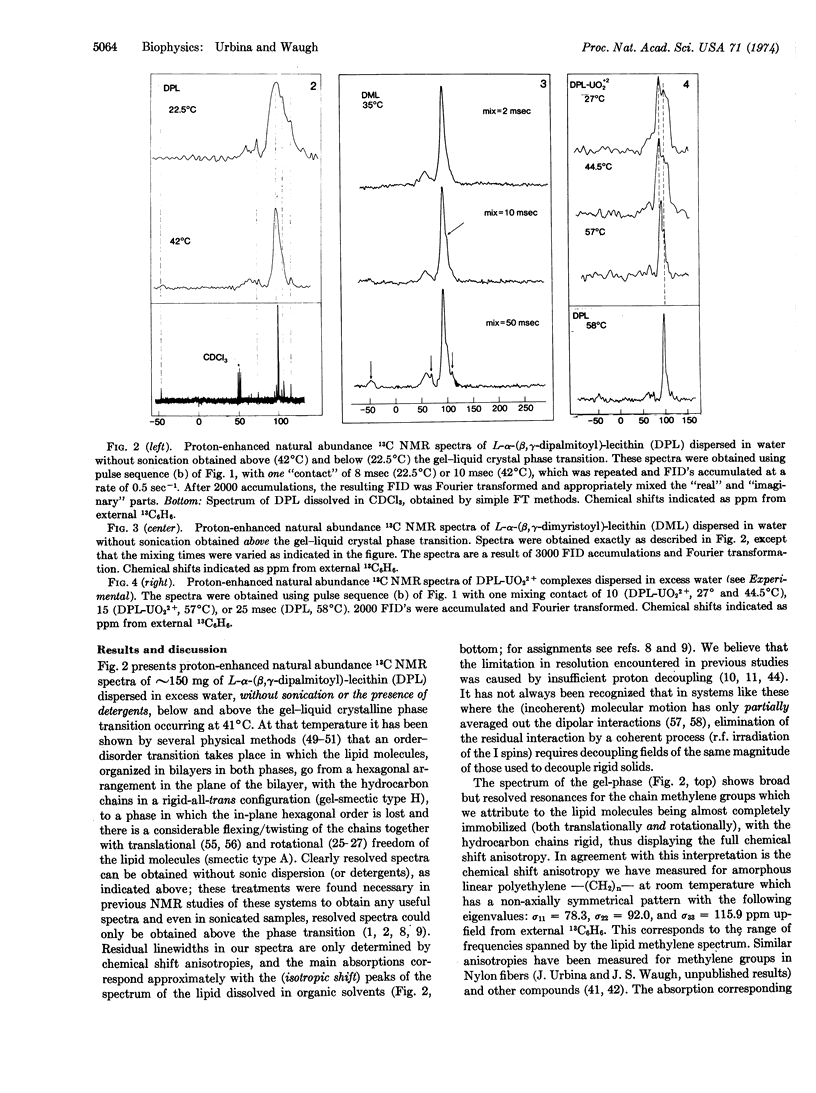
Detailed microscopic information on the molecular organization and phase transitions of the lipid phases and their interaction with ions and other molecules can be obtained from the study of the chemical shift anisotropies and dynamical aspects of the 13C NMR spectra of unsonicated lipid dispersions (liposomes). Experiments are reported which demonstrated the feasibility of quantitatively observing the 13C-nuclear magnetic resonance of specifically labeled sites in unperturbed Escherichia coli membrane vesicles for the study of the physical state of the lipids with the aim of relating it to the known lipid-dependent functional properties of the membranes.
Keywords: phospholipids, bilayers
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSON D., BLECHER M. QUANTITATIVE TWO-DIMENSIONAL THIN-LAYER CHROMATOGRAPHY OF NATURALLY OCCURRING PHOSPHOLIPIDS. J Lipid Res. 1964 Oct;5:628–631. [PubMed] [Google Scholar]
- Chan S. I., Seiter C. H., Feigenson G. W. Anisotropic and restricted molecular motion in lecithin bilayers. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1488–1492. doi: 10.1016/0006-291x(72)90775-9. [DOI] [PubMed] [Google Scholar]
- Chan S. I., Sheetz M. P., Seiter C. H., Feigenson G. W., Hsu M. C., Lau A., Yau A. Nuclear magnetic resonance studies of the structure of model membrane systems: the effect of surface curvature. Ann N Y Acad Sci. 1973 Dec 31;222:499–522. doi: 10.1111/j.1749-6632.1973.tb15283.x. [DOI] [PubMed] [Google Scholar]
- Chapman D., Urbina J. Biomembrane phase transitions. Studies of lipid-water systems using differential scanning calorimetry. J Biol Chem. 1974 Apr 25;249(8):2512–2521. [PubMed] [Google Scholar]
- Devaux P., McConnell H. M. Lateral diffusion in spin-labeled phosphatidylcholine multilayers. J Am Chem Soc. 1972 Jun 28;94(13):4475–4481. doi: 10.1021/ja00768a600. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Law J. H., Tsukagoshi N., Wilson G. A density label for membranes. Proc Natl Acad Sci U S A. 1970 Oct;67(2):598–605. doi: 10.1073/pnas.67.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R. B., Radda G. K. The interaction of 1-anilino-8-naphthalene sulphonate with erythrocyte membranes. FEBS Lett. 1969 Apr;3(2):150–152. doi: 10.1016/0014-5793(69)80121-3. [DOI] [PubMed] [Google Scholar]
- HILDEBRAND J. G., LAW J. H. FATTY ACID DISTRIBUTION IN BACTERIAL PHOSPHOLIPIDS. THE SPECIFICITY OF THE CYCLOPROPANE SYNTHETASE REACTION. Biochemistry. 1964 Sep;3:1304–1308. doi: 10.1021/bi00897a020. [DOI] [PubMed] [Google Scholar]
- Hauser H. O. The effect of ultrasonic irradiation on the chemical structure of egg lecithin. Biochem Biophys Res Commun. 1971 Nov;45(4):1049–1055. doi: 10.1016/0006-291x(71)90443-8. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric studies of dilute aqueous suspensions of bilayers formed from synthetic L- -lecithins. J Biol Chem. 1972 Oct 10;247(19):6071–6075. [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric studies of dilute aqueous suspensions of bilayers formed from synthetic L- -lecithins. J Biol Chem. 1972 Oct 10;247(19):6071–6075. [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Phospholipid requirements for (Na + + K + )-ATPase activity: head-group specificity and fatty acid fluidity. Biochim Biophys Acta. 1972 Sep 1;282(1):277–292. doi: 10.1016/0005-2736(72)90334-3. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., McConnell H. M. Lateral diffusion of phospholipids in a vesicle membrane. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2564–2568. doi: 10.1073/pnas.68.10.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. G., Birdsall N. J., Levine Y. K., Metcalfe J. C. High resolution proton relaxation studies of lecithins. Biochim Biophys Acta. 1972 Jan 17;255(1):43–56. doi: 10.1016/0005-2736(72)90006-5. [DOI] [PubMed] [Google Scholar]
- Levine Y. K., Birdsall N. J., Lee A. G., Metcalfe J. C. 13 C nuclear magnetic resonance relaxation measurements of synthetic lecithins and the effect of spin-labeled lipids. Biochemistry. 1972 Apr 11;11(8):1416–1421. doi: 10.1021/bi00758a014. [DOI] [PubMed] [Google Scholar]
- Levine Y. K., Partington P., Roberts G. C., Birdsall N. J., Lee A. G., Metcalfe J. C. 13 C nuclear magnetic relaxation times and models for chain motion in lecithin vesicles. FEBS Lett. 1972 Jun 15;23(2):203–207. doi: 10.1016/0014-5793(72)80341-7. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Peticolas W. L. Laser Raman investigation of the effect of cholesterol on conformational changes in dipalmitoyl lecithin multilayers. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1572–1576. doi: 10.1073/pnas.68.7.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavis R. D., Vagelos P. R. The effect of phospholipid fatty acid composition in membranous enzymes in Escherichia coli. J Biol Chem. 1972 Feb 10;247(3):652–659. [PubMed] [Google Scholar]
- McConnell H. M., McFarland B. G. Physics and chemistry of spin labels. Q Rev Biophys. 1970 Feb;3(1):91–136. doi: 10.1017/s003358350000442x. [DOI] [PubMed] [Google Scholar]
- Metcalfe J. C., Birdsall N. J., Feeney J., Lee A. G., Levine Y. K., Partington P. 13 C NMR spectra of lecithin vesicles and erythrocyte membranes. Nature. 1971 Sep 17;233(5316):199–201. doi: 10.1038/233199a0. [DOI] [PubMed] [Google Scholar]
- Metcalfe J. C., Birdsall N. J., Lee A. G. NMR studies of lipids in bilayers and membranes. Ann N Y Acad Sci. 1973 Dec 31;222:460–467. doi: 10.1111/j.1749-6632.1973.tb15280.x. [DOI] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Träuble H. Phase transitions in cells, membranes, and lipids of Escherichia coli. Detection by fluorescent probes, light scattering, and dilatometry. Biochemistry. 1973 Jul 3;12(14):2625–2634. doi: 10.1021/bi00738a012. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN A. Functional implications of interactions of extracellular ions with ligands of the cell membrane. Circulation. 1962 Nov;26:1189–1200. doi: 10.1161/01.cir.26.5.1189. [DOI] [PubMed] [Google Scholar]
- Robinson J. D., Birdsall N. J., Lee A. G., Metcalfe J. C. 13 C and 1 H nuclear magnetic resonance relaxation measurements of the lipids of sarcoplasmic reticulum membranes. Biochemistry. 1972 Jul 18;11(15):2903–2909. doi: 10.1021/bi00765a025. [DOI] [PubMed] [Google Scholar]
- Rottem S., Hubbell W. L., Hayflick L., McConnell H. M. Motion of fatty acid spin labels in the plasma membrane of mycoplasma. Biochim Biophys Acta. 1970;219(1):104–113. doi: 10.1016/0005-2736(70)90065-9. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Adenosine triphosphatase activity of mycoplasma membranes. J Bacteriol. 1966 Sep;92(3):714–722. doi: 10.1128/jb.92.3.714-722.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann E., Träuble H., Galla H. J., Overath P. Lateral diffusion, protein mobility, and phase transitions in Escherichia coli membranes. A spin label study. Biochemistry. 1973 Dec 18;12(26):5360–5369. doi: 10.1021/bi00750a020. [DOI] [PubMed] [Google Scholar]
- Seelig J., Niederberger W. Two pictures of a lipid bilayer. A comparison between deuterium label and spin-label experiments. Biochemistry. 1974 Apr 9;13(8):1585–1588. doi: 10.1021/bi00705a005. [DOI] [PubMed] [Google Scholar]
- Shah D. O. Interaction of uranyl ions with phospholipid and cholesterol monolayers. J Colloid Interface Sci. 1969 Feb;29(2):210–215. doi: 10.1016/0021-9797(69)90188-x. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Vagelos P. R. Fatty acid mutant of E. coli lacking a beta-hydroxydecanoyl thioester dehydrase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1579–1586. doi: 10.1073/pnas.58.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina J., Waugh J. S. Application of proton-enhanced nuclear induction spectroscopy to the study of membranes. Ann N Y Acad Sci. 1973 Dec 31;222:733–739. doi: 10.1111/j.1749-6632.1973.tb15300.x. [DOI] [PubMed] [Google Scholar]
- Wilson G., Rose S. P., Fox C. F. The effect of membrane lipid unsaturation on glycoside transport. Biochem Biophys Res Commun. 1970 Feb 20;38(4):617–623. doi: 10.1016/0006-291x(70)90625-x. [DOI] [PubMed] [Google Scholar]



