Abstract
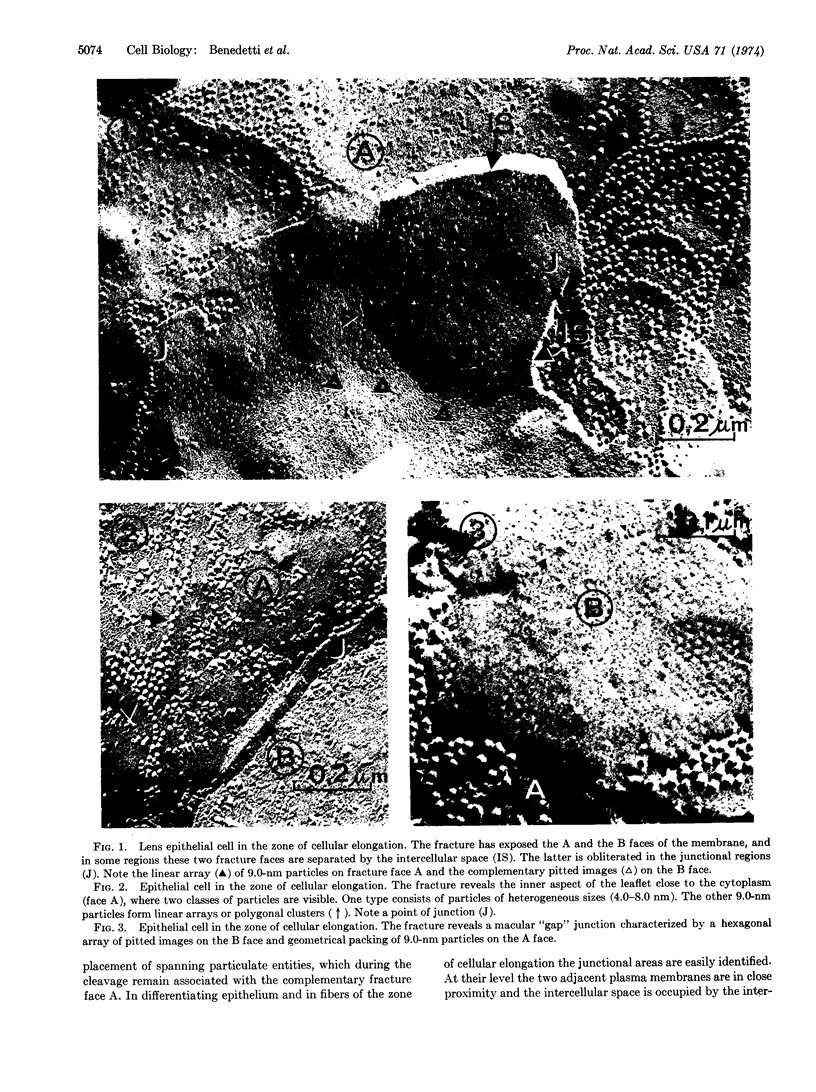
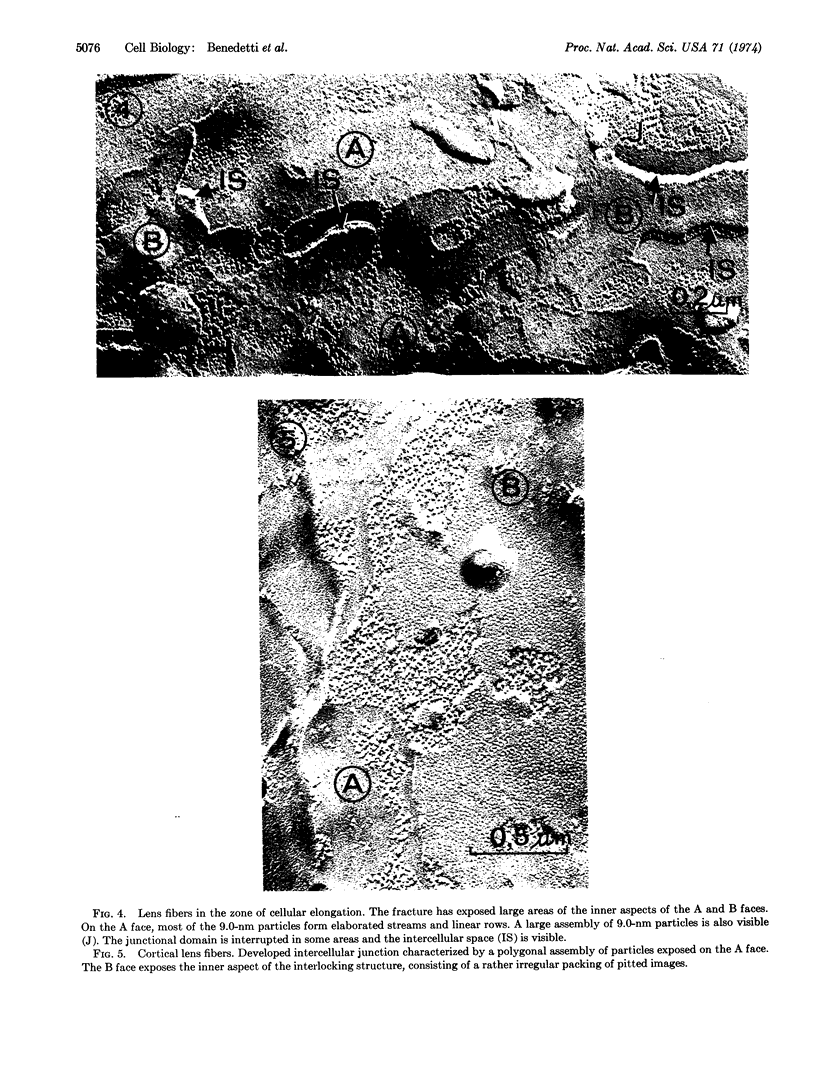
Throughout the differentiation of eye lens epithelium into fibers, an extensive system of intercellular junctions develops. The junctional assembly is initially characterized by the accumulation of 9.0-nm intramembranous particles, forming linear rows in the matching plasma membranes of adjoining fibers. At the final stage of the fiber differentiation, the junctional particles are assembled in geometrically packed arrays. The formation of linear rows and bidimensional lattices of intramembranous particles probably favors reciprocal recognition of cell surfaces and specific cell-to-cell interlocking. Moreover, the existence of a rather rigid lipid core of the plasma membrane of eye lens fiber may promote the clustered distribution of intramembranous particles and facilitate the junctional assembly.
Keywords: intramembranous particles, membrane differentiation, low resistance junctions, eye lens
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett R. E., Furcht L. T., Scott R. E. Differences in membrane fluidity and structure in contact-inhibited and transformed cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1992–1994. doi: 10.1073/pnas.71.5.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti E. L., Dunia I., Diawara A. The organization of the plasma membrane in mammalian cells. Eur J Cancer. 1973 Apr;9(4):263–272. doi: 10.1016/0014-2964(73)90092-3. [DOI] [PubMed] [Google Scholar]
- Bennett M. V. Function of electrotonic junctions in embryonic and adult tissues. Fed Proc. 1973 Jan;32(1):65–75. [PubMed] [Google Scholar]
- Bloemendal H., Zweers A., Vermorken F., Dunia I., Benedetti E. L. The plasma membranes of eye lens fibres. Biochemical and structural characterization. Cell Differ. 1972 Jun;1(2):91–106. doi: 10.1016/0045-6039(72)90032-2. [DOI] [PubMed] [Google Scholar]
- DeHaan R. L., Sachs H. G. Cell coupling in developing systems: the heart-cell paradigm. Curr Top Dev Biol. 1972;7:193–228. doi: 10.1016/s0070-2153(08)60072-1. [DOI] [PubMed] [Google Scholar]
- Decker R. S., Friend D. S. Assembly of gap junctions during amphibian neurulation. J Cell Biol. 1974 Jul;62(1):32–47. doi: 10.1083/jcb.62.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunia I., Sen Ghosh C., Benedetti E. L., Zweers A., Bloemendal H. Isolation and protein pattern of eye lens fiber junctions. FEBS Lett. 1974 Sep 1;45(1):139–144. doi: 10.1016/0014-5793(74)80831-8. [DOI] [PubMed] [Google Scholar]
- Flaxman B. A., Revel J. P., Hay E. D. Tight junctions between contact-inhibited cells in vitro. Exp Cell Res. 1969 Dec;58(2):438–443. doi: 10.1016/0014-4827(69)90528-x. [DOI] [PubMed] [Google Scholar]
- Flower N. E. A new junctional structure in the epithelia of insects of the order Dictyoptera. J Cell Sci. 1972 May;10(3):683–691. doi: 10.1242/jcs.10.3.683. [DOI] [PubMed] [Google Scholar]
- Friend D. S., Gilula N. B. Variations in tight and gap junctions in mammalian tissues. J Cell Biol. 1972 Jun;53(3):758–776. doi: 10.1083/jcb.53.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin C., Zachowski A., Prigent B., Paraf A., Dunia I., Diawara M. A., Benedetti E. L. Correlation between the mobility of inner plasma membrane structure and agglutination by concanavalin A in two cell lines of MOPC 173 plasmocytoma cells. Proc Natl Acad Sci U S A. 1974 Jan;71(1):114–117. doi: 10.1073/pnas.71.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaconstantinou J. Molecular aspects of lens cell differentiation. Science. 1967 Apr 21;156(3773):338–346. doi: 10.1126/science.156.3773.338. [DOI] [PubMed] [Google Scholar]
- Rash J. E., Fambrough D. Ultrastructural and electrophysiological correlates of cell coupling and cytoplasmic fusion during myogenesis in vitro. Dev Biol. 1973 Jan;30(1):166–186. doi: 10.1016/0012-1606(73)90055-9. [DOI] [PubMed] [Google Scholar]
- Raviola E., Gilula N. B. Gap junctions between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1677–1681. doi: 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Yip P., Chang L. L. Cell junctions in the early chick embryo--a freeze etch study. Dev Biol. 1973 Dec;35(2):302–317. doi: 10.1016/0012-1606(73)90026-2. [DOI] [PubMed] [Google Scholar]
- Satir B., Schooley C., Satir P. Membrane fusion in a model system. Mucocyst secretion in Tetrahymena. J Cell Biol. 1973 Jan;56(1):153–176. doi: 10.1083/jcb.56.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P., Gilula N. B. The fine structure of membranes and intercellular communication in insects. Annu Rev Entomol. 1973;18:143–166. doi: 10.1146/annurev.en.18.010173.001043. [DOI] [PubMed] [Google Scholar]
- Scott R. E., Furcht L. T., Kersey J. H. Changes in membrane structure associated with cell contact. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3631–3635. doi: 10.1073/pnas.70.12.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socolar S. J. Cell coupling in epithella. Exp Eye Res. 1973 May 24;15(6):693–698. doi: 10.1016/0014-4835(73)90003-1. [DOI] [PubMed] [Google Scholar]
- Wade J. B., Karnovsky M. J. The structure of the zonula occludens. A single fibril model based on freeze-fracture. J Cell Biol. 1974 Jan;60(1):168–180. doi: 10.1083/jcb.60.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]