Abstract
Purpose.
Administration of voriconazole, an antifungal triazole, causes transient visual disturbances in patients and attenuates the b-wave of the ERG. We sought to identify the retinal target of voriconazole underlying the effect on the ERG b-wave.
Methods.
Electroretinograms were recorded from mice before and after intraperitoneal injection of voriconazole. The effect of voriconazole on ON-bipolar cells was tested by patch-clamp recordings of ON-bipolar cells in mouse retinal slices. Effects of voriconazole on mGluR6 and TRPM3 were assessed by patch-clamp recordings of Chinese hamster ovary (CHO) and HEK293 cells transfected with either TRPM3 or mGluR6 plus Kir3.1/Kir3.4.
Results.
Voriconazole attenuated the ERG b-wave in mice, and inhibited ON-bipolar cell responses evoked by application of CPPG, an mGluR6 antagonist, onto the ON-bipolar cell dendrites, indicating that voriconazole blocks a step in the mGluR6-TRPM1 signal transduction pathway. Voriconazole almost completely blocked capsaicin-activated currents in ON-bipolar cells, which have been attributed to direct activation of the TRPM1 cation channel. Furthermore, application of voriconazole to CHO cells expressing TRPM3, a closely related channel to TRPM1, showed that voriconazole reversibly blocked pregnenolone sulfate–stimulated TRPM3 currents in transfected cells. In contrast, voriconazole only slightly inhibited mGluR6-mediated activation of G-protein activated inward rectifier potassium (GIRK) currents in cotransfected cells, suggesting that mGluR6 is not the primary target of voriconazole in ON-bipolar cells.
Conclusions.
The visual disturbances associated with voriconazole are likely due to block of TRPM1 channels in retinal ON-bipolar cells. Other neurological effects of voriconazole may be due to block of TRPM3 channels expressed in the brain.
Keywords: voriconazole, TRPM1, mGluR6, bipolar cell, b-wave
Voriconazole is a highly effective antifungal triazole used to treat invasive fungal infections, but it can cause transient visual disturbances during treatment. Here we provide evidence that these adverse visual effects are due to the direct block of retinal TRPM1 channels by voriconazole.
Introduction
Voriconazole (VFEND; Pfizer, New York, NY, USA) is a triazole antibiotic used to treat serious fungal infections. It is a broad-spectrum fungal inhibitor and has become the standard of care for the treatment of invasive pulmonary aspergillosis, a life-threatening lung infection that occurs in immunocompromised patients.1 Recently, voriconazole was recommended by the Centers for Disease Control and Prevention for combating the 2012 outbreak of fungal meningitis that occurred from contaminated steroid injections.2 Other triazoles, including itraconazole, fluconazole, and posaconazole, also are prescribed for systemic fungal infections. The common mechanism of action of these triazoles is inhibition of the fungal cytochrome P450 14α-demethylase.3 Voriconazole is generally well tolerated, but is unique among the triazoles in causing transient visual disturbances, including altered light perception and photophobia.4,5 These visual side effects typically occur within 30 minutes of dosing and are correlated with plasma concentrations above 1 to 3 μg/mL. The effect is reversible, with vision usually returning to normal within an hour of intravenous administration, or when the plasma concentration falls below 1 μg/mL.5 Electroretinogram (ERG) recordings from monkeys within an hour after intravenous administration of voriconazole demonstrated a dramatic attenuation of both the photopic and scotopic b-waves, with no effect on the a-wave.6 Attenuation of the b-wave was correlated with plasma concentrations above 3 μg/mL. As in human patients, the effect was transient. Within 24 hours after voriconazole administration, the plasma concentration had fallen below 1 μg/mL, and the recorded ERGs were normal.
The selective loss of the ERG b-wave in voriconazole-treated animals is similar to that seen in ERGs from patients with complete congenital stationary night blindness (CSNB1), which is caused by mutations affecting the metabotropic glutamate receptor 6 (mGluR6) signal transduction pathway of ON-bipolar cells. In a healthy retina, deactivation of the mGluR6-based signaling pathway mediates the depolarizing light response of ON-bipolar cells by triggering the opening of the transient receptor potential melastatin 1 (TRPM1) cation channel.7–9 Mutations in either mGluR610,11 or TRPM112–14 have been shown to cause CSNB1. Although night blindness is not typically listed as a symptom of voriconazole toxicity, photophobia, a common manifestation of voriconazole toxicity, is described by approximately 20% of CSNB1 patients,15 and may be related to changes in light adaptation and contrast sensitivity associated with disruption of ON-bipolar cell signaling.8,15,16 Similar to CSNB1, the voriconazole-induced attenuation of the ERG b-wave following a normal a-wave, suggests mGluR6 or TRPM1 as possible sites of the block in visual processing; therefore, we investigated both proteins as potential targets of voriconazole inhibition.
Materials and Methods
Chemicals
The VFEND IV is a white lyophilized powder containing nominally 200 mg voriconazole and 3200 mg sulfobutylether beta-cyclodextrin sodium in a 30-mL Type I clear glass vial. It is reconstituted with saline to produce a solution containing 20 mg/mL voriconazole and 320 mg/mL sulfobutylether beta-cyclodextrin sodium. Control ERG experiments were performed with a saline solution containing 320 mg/mL sulfobutylether beta-cyclodextrin sodium only. The VFEND or control solution was administered by intraperitoneal (IP) injection.
Electroretinogram Recording
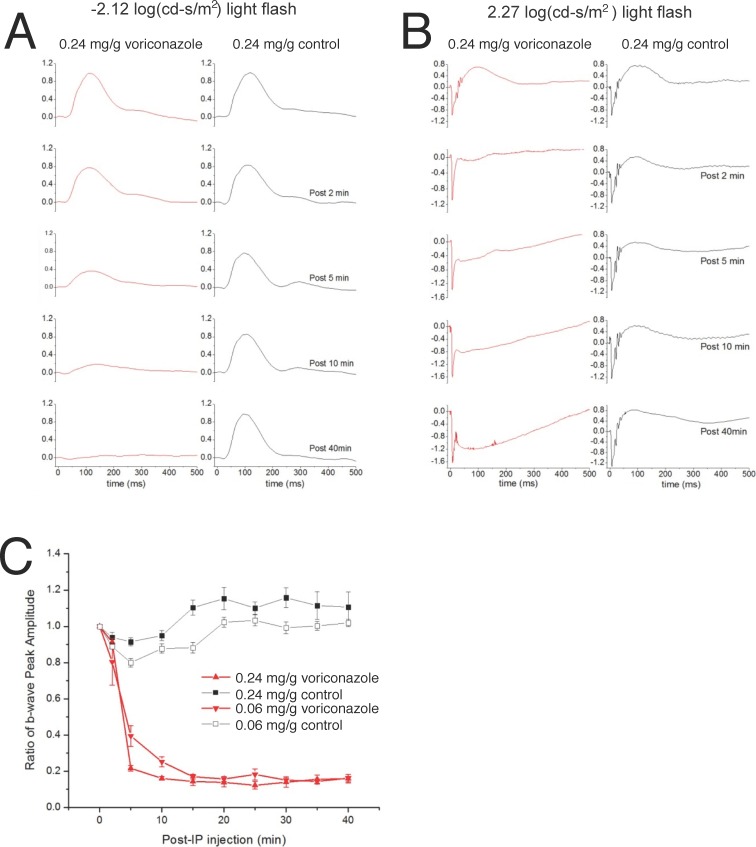
Adult C57BL/6 mice (OHSU, Portland, OR, USA) were dark-adapted overnight (>12 hours). They were anesthetized with IP injection of a ketamine:xylazine mixture and maintained at a body temperature of 36°C to 37°C measured with a rectal thermometer. Full-field scotopic ERGs were recorded with customized software (ERGTool, Richard Weleber; Casey Eye Institute, Portland, OR, USA) from the mouse cornea. Two flash intensities (−2.12 and 2.27 log [cd-s/m2]) were used, and the traces shown in Figures 1A and 1B are the averages of six responses and one response, respectively. Electroretinograms were recorded before injection of the drug/control solution, and then at 2, 5, 10, 15, 20, 25, 30, 35, and 40 minutes after IP injection.
Figure 1.
Intraperitoneal injection of voriconazole in mice selectively abolishes the ERG b-wave. (A) The average of normalized ERG recordings from three mice (six eyes) to flashes of −2.12 log (cd-s/m2) are displayed before (top traces) and after IP injection of 0.24 mg/g body weight voriconazole (red traces) or control (black traces). (B) The average of normalized ERG recordings from three mice (six eyes) to flashes of 2.27 log (cd-s/m2) are displayed before (top traces) and after IP injection of 0.24 mg/g body weight voriconazole (red traces) and control (black traces). (C) Changes in the b-wave peak amplitude normalized to their pre-injection amplitude over time following voriconazole injections of 0.06 mg/g and 0.24 mg/g, at a light intensity of 2.27 log (cd-s/m2).
Electroretinogram Data Analysis
Scotopic ERGs were processed and analyzed using programs implemented with MATLAB (R2006a; MathWorks, Natick, MA, USA). Time zero of the ERG recording was set as the start of the light flash. The baseline was set to the average of the 3-ms recording before the flash, and the peak of the a-wave was measured between 5 and 30 ms after the flash with low-pass filtering of 800 Hz. For the b-wave, the oscillatory potentials were removed from the signal by a digital filter using the filtfilt function in MATLAB (low-pass filter; Fc 58 Hz). The b-wave amplitude (peak reached between 25 and 350 ms post flash) was calculated from the baseline, or from the a-wave trough if present.
In Figure 1A, b-waves were normalized to the peak amplitude of b-wave before IP injection. For ERGs in which an a-wave was evident (Fig. 1B), the a-waves were normalized to the peak amplitude of a-wave before IP injection.
Retinal Slice Recordings
Chemically simulated light responses from rod bipolar cells were recorded in mouse retinal slices perfused with bicarbonate-buffered Ames medium continuously bubbled with 95% O2, 5% CO2. All procedures were performed under normal visible-light illumination. For rod bipolar cell recordings, the chamber was heated to 32°C to 34°C, and the medium was supplemented with 4 μM L-aminophosphonobutyric acid (L-AP4; Tocris Bioscience, Ellisville, MO, USA) to maximally activate the mGluR6 receptors, thereby mimicking darkness.7 Patch electrodes contained (in millimolars) 108 K+-gluconate, 20 tetraethylammonium (TEA), 10 HEPES, 20 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), 4 Mg-ATP, and 1 Na-guanosine-5′-triphosphate (GTP), adjusted to pH 7.4 with KOH. This intracellular solution containing a high concentration of BAPTA was chosen to prolong the peak (RS)-α-cyclopropy1–4-phosphonophenylglycine (CPPG) response in rod bipolar cells.17 Rod bipolar cell bodies were identified according to their morphology and position in the slice, and by their response characteristics. Whole-cell voltage-clamp recordings were performed at a holding potential of −60 mV, at which inhibitory chloride currents in the axon terminal are negligible.18 Chemically simulated bipolar cell light responses were elicited by pressure application of the mGluR6 antagonist, CPPG (500 μM); in other experiments, the TRPM1 current was activated by application of 100 μM capsaicin. Pulses were delivered via a 5-MΩ patch pipette onto bipolar cell dendrites for 6 seconds at approximately 6 psi with a pressure ejection system (PicoSpritzer II). To investigate inhibition by voriconazole, a second puffer pipette was positioned in an orthogonal position, such that it applied solution to the identical region of the outer plexiform layer (OPL). After initial agonist application from one puffer pipette (2 seconds), the first pipette was turned off as the second pipette, containing 100 μM voriconazole in addition to the agonist, was turned on. After an additional 2 seconds, the initial condition was restored. Current responses were digitally sampled at 20 kHz and filtered at 5 kHz.
Whole-Cell Recordings From Transfected Cultured Cells
Chinese hamster ovary (CHO) cells and HEK293 cells were grown at 37°C in a humidified 5% CO2 incubator in high-glucose Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and a penicillin/streptomycin mixture.
TRPM3.
Chinese hamster ovary cells were transfected with a plasmid encoding a full-length mouse retinal TRPM3-mCherry fusion construct under control of the cytomegalovirus (CMV) promoter (originally based on pEGFP-N1 expression plasmid). Recording solutions were similar to those used by Lambert et al.19 The pipette solution contained (in millimolars) 80 to 90 CsAsp, 45 CsCl, 4 Na2ATP, 10 BAPTA, 5 EDTA, and 10 HEPES. The pH was adjusted to 7.2 with CsOH (adding 60 mM Cs+ to the solution), and the osmolarity was adjusted to values within the range of 305 to 320 mOsm; the bath solution contained (in millimolars) 145 NaCl, 3 KCl, 10 CsCl, 2 MgCl2, 2 CaCl2, and 10 HEPES, pH 7.2. Cells were held at −60 mV, and voltage ramps from −100 to +100 mV were applied every 20 seconds for a duration of 250 ms to examine current–voltage relations. The TRPM3 currents were activated by the application of 35 μM pregnenolone sulfate (PS), and the cells were subsequently exposed to a solution containing 100 μM voriconazole in addition to the PS.
To study the current–voltage relationship of TRPM3 currents, TRPM3-EGFP plasmid was electroporated into HEK293 cells. Whole-cell patch-clamp recordings of GFP-positive cells were performed 48 hours after transfection. The bath solution (Ringer's solution) contained (in millimolars) 137 NaCl, 5 KCl, 2.5 CaCl2, 1 MgCl2, 15 glucose, and 10 HEPES, pH 7.4 adjusted with NaOH. The pipette solution contained (in millimolars) 125 CsMeSO4, 15 CsCl, 10 Na2ATP, 4 EGTA, and 10 HEPES, pH 7.4 adjusted with CsOH. The osmolality of both solutions was approximately 310 mOsm. Cells were held at −90 mV, and voltage ramps from −100 to +100 mV were applied for a duration of 2 seconds to examine current–voltage relations.
mGluR6-GIRK.
A stable mGluR6-expressing cell line was produced by Lipofectamine-based transfection (Invitrogen, Carlsbad, CA, USA) with a plasmid encoding an mGluR6-enhanced yellow fluorescent protein (EYFP) fusion protein, followed by G418 selection. To assess mGluR6 function, mGluR6-stable CHO cells were transiently transfected with plasmids encoding monomeric red fluorescent protein (mRFP) as a marker for transfected cells, and the G-protein regulated K+-channel subunits Kir3.1 and Kir3.4 (KCNJ3 + KCNJ5). One to 5 days after transfection, cells were mechanically detached from growth plates, triturated to obtain single cells, and allowed to settle on the bottom of a recording chamber. The external solution contained a relatively high K+ concentration to enhance inward currents at negative potentials (in millimolars): 125 NaCl, 25 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 5 HEPES (pH 7.4). After obtaining a whole-cell configuration, cells were lifted from the bottom and placed in front of a Warner Instruments (Hamden, CT, USA) three-barrel Quick-Change solution changer. Cells were typically held at a voltage of −100 mV, and voltage dependence was measured by 500-ms ramps from −100 to +50 mV once every 20 seconds. Currents of G-protein–activated inwardly rectifying K+ (GIRK) channels were activated via mGluR6 by application of 1 mM glutamate, followed by application of 100 μM voriconazole in 1 mM glutamate to assess mGluR6 inhibition.
Results
Voriconazole Suppresses the Mouse ERG b-Wave
We examined the ERG of mice injected with IP voriconazole (Fig. 1). Dark-adapted mice, injected with 0.24 mg/g body weight voriconazole, or control solution, were exposed to dim and bright scotopic flashes of −2.12 (Fig. 1A) and 2.27 (Fig. 1B) log(cd-s/m2), respectively. The ERG b-wave was selectively reduced at both light intensities in a time-dependent manner. The ratio of the b-wave peak amplitude at different times after IP injection normalized to the b-wave before IP injection at two drug concentrations (0.24 mg/g and 0.06 mg/g) is shown in Fig. 1C. At both drug concentrations, a reduction in the b-wave was apparent as early as 2 minutes after injection, and the b-wave was almost abolished at 15 minutes after injection for both concentrations of voriconazole.
Voriconazole Blocks Rod Bipolar Cell Responses in the Mouse Retinal Slices
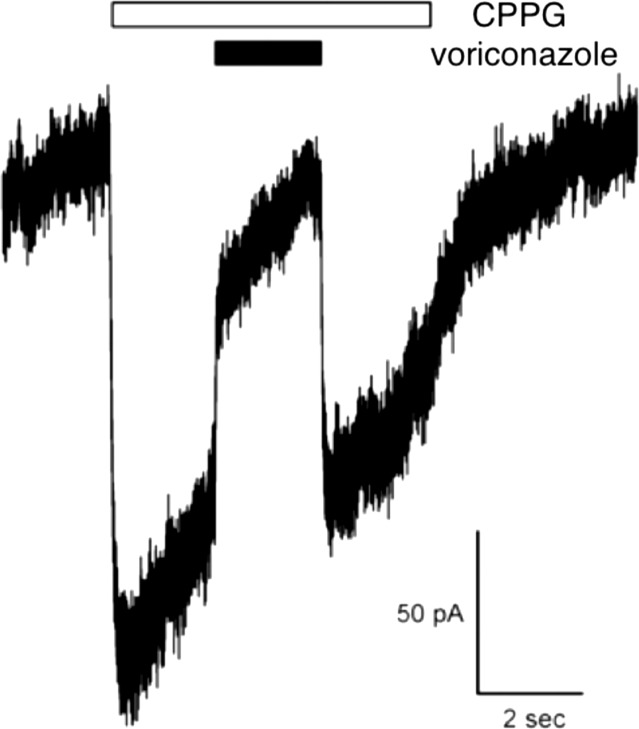
The ERG b-wave mostly reflects the depolarizing light response of ON-bipolar cells. To assess the effect of voriconazole application on ON-bipolar cell currents, we recorded chemically simulated light responses from rod bipolar cells in mouse retinal slices as described previously.8 In this method, the mGluR6 agonist, L-AP4, is applied to the bath solution, mimicking glutamate release from photoreceptors in darkness. Brief application of the mGluR6 antagonist, CPPG, to the ON-bipolar cell dendrites simulates the light-induced decrease in glutamate release from photoreceptors, giving rise to a depolarizing current similar to the ON-bipolar cell light response. One advantage of this approach is that it isolates the response of bipolar cells from that of photoreceptors. We found that voriconazole blocks CPPG responses of rod bipolar cells, indicating that it is directly inhibiting a component of the mGluR6-TRPM1 signal transduction pathway (Fig. 2; 75% inhibition ± 12% SEM, n = 5).
Figure 2.
Voriconazole blocks CPPG responses of rod bipolar cells in the mouse retinal slice, but fails to block mGluR6 activation of GIRK currents in transfected CHO cells. Puff application of the mGluR6 antagonist, CPPG, onto rod bipolar cell dendrites displaces bath-applied L-AP4, thereby activating an inward current carried by TRPM1 channels. The inward current is inhibited by co-application of voriconazole with CPPG (75% inhibition ± 12% SEM, n = 5). The inward current is quickly restored in the presence of CPPG following washout of voriconazole.
Voriconazole Blocks TRPM1 and TRPM3 Currents
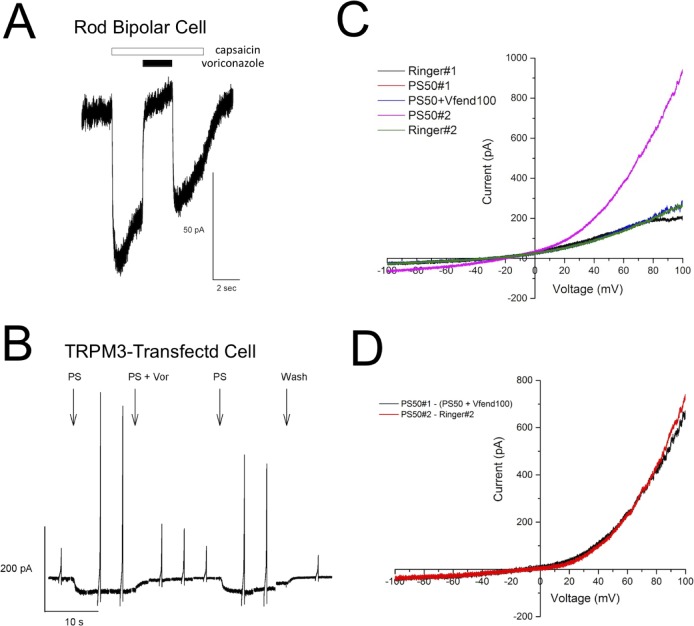
We tested whether voriconazole blocks the TRPM1 cation channel directly. The TRPM1 currents in ON-bipolar cells can be activated by application of capsaicin.7,20 We recorded rod bipolar cell currents in mouse retinal slices in response to capsaicin puffed over the dendrites, then switched to capsaicin plus voriconazole, then back to capsaicin alone (Fig. 3A). Capsaicin activated an inward current that was blocked by voriconazole (90% inhibition ± 4% SEM, n = 7). Washout of voriconazole in the continued presence of capsaicin restored the inward current, indicating that the block is reversible. Because of the difficulty with heterologous expression of TRPM1, we tested voriconazole on TRPM3, the most closely related channel to TRPM1 (70% amino acid sequence identity). Plasmids encoding a fusion of mouse TRPM3 to either mCherry or EGFP were transiently transfected into CHO cells (TRPM3-mCherry) or HEK293 cells (TRPM3-EGFP). Transfected cells were identified by fluorescence and currents recorded in response to application of the TRPM3 activator, PS.19,21 To test for the effect of voriconazole on the PS-activated current, the PS solution was switched to PS plus voriconazole (100 μM), and then back to PS alone. As seen in Figures 3B through 3D, voriconazole dramatically inhibits PS-activated TRPM3 currents (92.3% inhibition ± 6.3% SEM, n = 4).
Figure 3.
Voriconazole blocks TRPM1 currents in rod bipolar cells and TRPM3 currents in transfected CHO cells. (A) The TRPM1 currents in rod bipolar cells activated by puff application of 100 μM capsaicin were inhibited by co-application of voriconazole (90% inhibition ± 4% SEM, n = 7). Washout of voriconazole restored the capsaicin-activated current. (B) The TRPM3 currents were elicited by application of 35 μM PS in CHO cells transiently transfected with a plasmid encoding a TRPM3-mCherry fusion protein. Co-application of 100 μM voriconazole with PS dramatically reduced the TRPM3 current at both negative and positive voltages. Return to PS alone restored the TRPM3 current. Similar to the effect on TRPM1 in rod bipolar cells, voriconazole resulted in a near complete block of the TRPM3 current. Vertical spikes represent currents elicited in response to voltage ramps. (C) HEK293 cells transiently transfected to express EGFP-TRPM3 were stepped sequentially through the following solutions: Ringer's solution, 50 μM PS, 50 μM PS plus 100 μM voriconazole, 50 μM PS, and Ringer's solution. Currents were recorded to a voltage ramp for each solution. (D) The I-V relationship for the PS-induced current was calculated by subtracting the current recorded in Ringer's solution from the one recorded in 50 μM PS, as shown in red. The current induced by PS but blocked by voriconazole was calculated by subtracting the current in 50 μM PS plus 100 μM voriconazole from the one in 50 μM PS, as shown in black. The two subtracted currents look almost identical.
Voriconazole Does Not Block mGluR6 Activity
To test whether voriconazole also acts on mGluR6, we produced a CHO-derived cell line stably expressing mGluR6-EYFP. This cell line was then transiently transfected with the GIRK channels Kir3.1 and Kir3.4, which can be opened following glutamate activation of mGluR6, and with mRFP to allow for identification of transfected cells by red fluorescence. Patch-clamp recordings of red fluorescent cells confirmed that an mGluR6-coupled inward current could be activated by application of 1 mM glutamate in a high-potassium external solution (Fig. 4); this current was not present in cells that had not been transfected with the GIRK channels. A small decrease in the potassium current (2%–9%, n = 6) was observed when the glutamate solution was replaced by glutamate plus voriconazole (100 μM; Fig. 4). Thus, voriconazole was found to only slightly inhibit glutamate-activated mGluR6-coupled GIRK currents, suggesting that mGluR6 is not the primary target of voriconazole in ON-bipolar cells.
Figure 4.
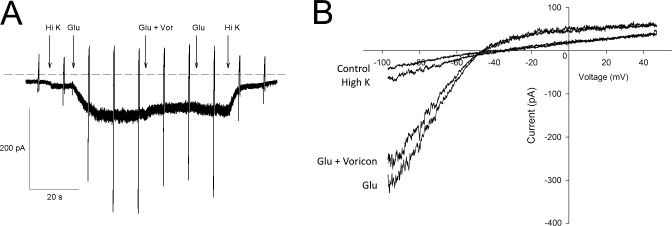
Voriconazole has little effect on mGuR6-mediated activation of GIRK currents. (A) Patch-clamp recordings of CHO cells expressing mGluR6-EYFP and GIRK potassium channels demonstrated that an mGluR6-coupled GIRK current could be activated by application of 1 mM glutamate in a high-potassium (High K) external solution. Only a very slight decrease in the current was observed when the glutamate solution was replaced by glutamate plus voriconazole (100 μM). Return to glutamate led to a slight increase in the current. The effect of voriconazole on mGluR6 is mild. Vertical spikes represent current elicited in response to voltage ramps. (B) Individual currents in Ringer's solution (control), High-potassium solution (High K), 1 mM glutamate in high-potassium solution (Glu), and 100 μM voriconazole in 1 mM glutamate high-potassium solution (Glu + Voricon) were recorded during the voltage ramp and plotted versus voltage, showing that voriconazole has only a minor effect on GIRK currents evoked by mGluR6 receptor activation.
Discussion
Voriconazole is approved as an antifungal treatment, despite a relatively high risk (30%) of visual adverse events. These events are reversible on therapy reduction or cessation, but their mechanism remains obscure. When injected systemically in monkeys6 and rats,22 voriconazole selectively attenuates the ERG b-wave. Here, we demonstrate that systemically injected voriconazole similarly attenuates the b-wave in mice. Interestingly, voriconazole, when injected directly into the vitreous, does not effect the b-wave amplitude in rabbits23 or rats,24 suggesting that intravitreally injected voriconazole does not reach ON-bipolar cell dendrites due to a barrier to diffusion, such as the inner limiting membrane.
Fourgeux et al.22 propose that the effect of systemically injected voriconazole on the ERG b-wave is due to inhibition of cholesterol 24S-hydroxylase (CYP46A1) and a consequent reduction of 24S-hydroxycholesterol that negatively affects retinal function. Our finding that CPPG responses of ON-bipolar cells in a retinal slice preparation are rapidly and reversibly inhibited by voriconazole perfusion (Fig. 2) suggests the ERG effect is due to a direct block of the mGluR6-TRPM1 signal transduction pathway in ON-bipolar cells. Furthermore, voriconazole strongly blocked capsaicin-activated currents in ON-bipolar cells, implicating TRPM1, rather than mGluR6 as the target. Heterologous expression studies confirmed that mGluR6 is very little affected by voriconazole, but that TRPM3, a close homolog of TRPM1, is blocked by voriconazole.
Mutations in the TRPM1 gene are a major cause of CSNB1, in which affected individuals experience difficulty in seeing at night and in very dim light.12–15 Surprisingly, night blindness is rarely listed among the visual problems associated with voriconazole. Rather, the visual side effects most commonly reported by patients include photophobia, photopsia, and enhanced perception (i.e., objects appearing brighter).4,5 These visual symptoms are similar to those described by patients with melanoma-associated retinopathy (MAR), an autoimmune syndrome associated with cutaneous melanoma. Melanoma-associated retinopathy is caused by the production of autoantibodies against TRPM1,25–28 which is normally expressed not only in the eye but also by melanocytes in the skin.29,30 The ERGs of MAR patients typically show a normal a-wave, but a reduced or absent b-wave.31 Injection of serum from MAR patients into the vitreous in mice results in a decrease in the amplitude of the ERG b-wave and immunohistological labeling of ON-bipolar cells, which is absent in TRPM1 knockout mice.27 The differences in reported visual symptoms between CSNB1 patients and those with MAR or voriconazole toxicity may partly be due to somewhat different visual effects of a congenital absence of TRPM1 versus an acute loss of TRPM1 function.
Together, our findings indicate that the cause of the transient visual disturbances reported by patients receiving voriconazole treatment for invasive fungal infections may be due to direct inhibition of ON-bipolar cell TRPM1 currents by voriconazole. Furthermore, the ability of voriconazole to inhibit TRPM3, in addition to TRPM1, may underlie some of the other transient neurological side affects sometimes associated with voriconazole treatment, such as visual and auditory hallucinations,32 as TRPM3 is more widely expressed in the brain.33 In addition, our findings identify a novel pharmacological agent capable of inhibiting TRPM1 and TRPM3, channels that have been difficult to study given the limited agonists and antagonists available.
Acknowledgments
The authors thank John Adelman for the gift of plasmids encoding GIRK-channel subunits.
Supported by grants from the National Eye Institute (EY019907 [RMD, RLB] and EY018625 [CWM]).
Disclosure: W.-H. Xiong, None; R.L. Brown, None; B. Reed, None; N.S. Burke, None; R.M. Duvoisin, None; C.W. Morgans, None
References
- 1. Thompson GR, Patterson TF. Pulmonary aspergillosis: recent advances. Semin Respir Crit Care Med. 2011; 32: 673–681. [DOI] [PubMed] [Google Scholar]
- 2. Pappas PG. Lessons learned in the multistate fungal infection outbreak in the United States. Curr Opin Infect Dis. 2013; 26: 545–550. [DOI] [PubMed] [Google Scholar]
- 3. Scott LJ, Simpson D. Voriconazole: a review of its use in the management of invasive fungal infections. Drugs. 2007; 67: 269–298. [DOI] [PubMed] [Google Scholar]
- 4. Ghannoum MA, Kuhn DM. Voriconazole—better chances for patients with invasive mycoses. Eur J Med Res. 2002; 7: 242–256. [PubMed] [Google Scholar]
- 5. Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J Clin Pharmacol. 2006; 46: 235–243. [DOI] [PubMed] [Google Scholar]
- 6. Kinoshita J, Iwata N, Ohba M, Kimotsuki T, Yasuda M. Mechanism of voriconazole-induced transient visual disturbance: reversible dysfunction of retinal ON-bipolar cells in monkeys. Invest Ophthalmol Vis Sci. 2011; 52: 5058–5063. [DOI] [PubMed] [Google Scholar]
- 7. Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009; 29: 6088–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morgans CW, Zhang J, Jeffrey BG, et al. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A. 2009; 106: 19174–19178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koike C, Obara T, Uriu Y, et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010; 107: 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeitz C, van Genderen M, Neidhardt J, et al. Mutations in GRM6 cause autosomal recessive congenital stationary night blindness with a distinctive scotopic 15-Hz flicker electroretinogram. Invest Ophthalmol Vis Sci. 2005; 46: 4328–4335. [DOI] [PubMed] [Google Scholar]
- 11. Dryja TP, McGee TL, Berson EL, et al. Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proc Natl Acad Sci U S A. 2005; 102: 4884–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Z, Sergouniotis PI, Michaelides M, et al. Recessive mutations of the gene TRPM1 abrogate ON bipolar cell function and cause complete congenital stationary night blindness in humans. Am J Hum Gen. 2009; 85: 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Audo I, Kohl S, Leroy BP, et al. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2009; 85: 720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Genderen MM, Bijveld MMC, Claassen YB, et al. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet. 2009; 85: 730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bijveld MMC, van Genderen MM, Hoeben FP, et al. Assessment of night vision problems in patients with congenital stationary night blindness. PLoS One. 2013; 8: e62927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sarnaik R, Chen H, Liu X, Cang J. Genetic disruption of the On visual pathway affects cortical orientation selectivity and contrast sensitivity in mice. J Neurophys. 2014; 111: 2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bernston A, Smith RG, Taylor WR. Postsynaptic calcium feedback between rods and rod bipolar cells in the mouse retina. Vis Neurosci. 2004; 21: 913–924. [DOI] [PubMed] [Google Scholar]
- 18. Pang JJ, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol. 2004; 558: 897–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lambert S, Drews A, Rizun O, et al. Transient receptor potential melastatin 1 (TRPM1) is an ion-conducting plasma membrane channel inhibited by zinc ions. J Biol Chem. 2011; 286: 12221–12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peachey NS, Pearring JN, Bojang P, et al. Depolarizing bipolar cell dysfunction due to a Trpm1 point mutation. J Neurophysiol. 2012; 108: 2442–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner TFJ, Loch S, Lambert S, et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nature Cell Biol. 2008; 10: 1421–1430. [DOI] [PubMed] [Google Scholar]
- 22. Fourgeux C, Martine L, Acar N, Bron AM, Creuzot-Garcher CP, Bretillon L. In vivo consequences of cholesterol-24S-hydroxylase (CYP46A1) inhibition by voriconazole on cholesterol homeostasis and function in the rat retina. Biochem Biophys Res Com. 2014; 446: 775–781. [DOI] [PubMed] [Google Scholar]
- 23. Harrison JM, Glickman RD, Ballentine CS, et al. Retinal function assessed by ERG before and after induction of ocular aspergillosis and treatment by the anti-fungal, micafungin, in rabbits. Doc Ophthalmol. 2005; 110: 37–55. [DOI] [PubMed] [Google Scholar]
- 24. Gao H, Pennesi ME, Shah K, et al. Intravitreal voriconazole: an electroretinographic and histopathologic study. Arch Ophthalmol. 2004; 122: 1687–1692. [DOI] [PubMed] [Google Scholar]
- 25. Dhingra A, Fina ME, Neinstein A, et al. Autoantibodies in melanoma-associated retinopathy target TRPM1 cation channels of retinal ON bipolar cells. J Neurosci. 2011; 31: 3962–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kondo M, Sanuki R, Ueno S, et al. Identification of autoantibodies against TRPM1 in patients with paraneoplastic retinopathy associated with ON bipolar cell dysfunction. PLoS One. 2011; 6: e19911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiong WH, Duvoisin RM, Adamus G, Jeffrey BG, Gellman C, Morgans CW. Serum TRPM1 autoantibodies from melanoma associated retinopathy patients enter retinal on-bipolar cells and attenuate the electroretinogram in mice. PLoS One. 2013; 8: e69506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dalal MD, Morgans CW, Duvoisin RM, et al. Diagnosis of occult melanoma using transient receptor potential melastatin 1 (TRPM1) autoantibody testing: a novel approach. Ophthalmol. 2013; 120: 2560–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morgans CW, Brown RL, Duvoisin RM. TRPM1: the endpoint of the mGluR6 signal transduction cascade in retinal ON-bipolar cells. BioEssays. 2010; 32: 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oancea E, Wicks NL. TRPM1: new trends for an old TRP. Adv Exp Med Biol. 2011; 704: 135–145. [DOI] [PubMed] [Google Scholar]
- 31. Lu Y, Jia L, He S, et al. Melanoma-associated retinopathy: a paraneoplastic autoimmune complication. Arch Ophthalmol. 2009; 127: 1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zonios DI, Gea-Banacloche J, Childs R, Bennett JE. Hallucinations during voriconazole therapy. Clin Infect Dis. 2008; 47: e7–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in mouse and its variation in three different mouse strains. BMC Genom. 2006; 7: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]