Abstract
In the last years, there has been extremely much information which reveals an alarming increase of obesity in children and, at the same time, an increase of the incidence of non-alcoholic fatty liver disease (NAFLD). NAFLD implies a wide range of affections starting from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH); the latter can evolve to cirrhosis and hepatic carcinoma. All these affections were noticed in children, too. The article presents data on the epidemiology, pathogeny, clinical and paraclinical findings, and treatment of NAFLD in children.
Keywords: non-alcoholic fatty liver disease, NAFLD, non-alcoholic steatohepatitis, obesity, child
Introduction
Hepatic steatosis is defined as a light to moderate enlargement of the liver, because of diffuse accumulation of neutral fats (triglycerides) in hepatocytes [1].
Physiologically, 5% of the liver weight is represented by lipids: triglycerides, fatty acids, phospholipids, cholesterol and cholesterol esters. The lipidic content is more than 5% in hepatic steatosis [2]. If steatosis is accompanied by an inflammatory process, steatohepatitis occurs.
In children, hepatic steatosis is met in various affections: nutritional diseases, genetic metabolism diseases, intoxications, drug consumption, and other affections (Table 1) [2].
Table 1.
Causes of hepatic steatosis in children (according to Grigorescu Sido)
| Nutritional causes | Obesity Protein-calorie malnutrition Kwashiorkor Food intake absence Essential fatty acid deficiency Zinc deficiency |
| Intoxications | Carbon tetrachloride Organic phosphates Organic solvents Alcohol |
| Drugs | Glucocorticoids Estrogens Tetracycline Methotrexate Phosphor Valproic acid Aspirin Acetaminophen Vitamin A Asparaginase |
| Metabolism diseases | Genetic: Galactosemia Fructose Intolerance Glicogenosis Tyrosinemia Homocistinuria Nieman-Pick disease Refsum disease Cystic fibrosis Multifactorial: DM Other diseases: Weber Christian |
| Other causes | Reye Syndrome VHC Infection Total parenteral nutrition Pregnancy |
The disease complex, morpho-pathologically represented by non-alcoholic fatty liver disease (NAFLD), is represented by a spectrum of lesions starting from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH) which can evolve to hepatic cirrhosis and hepatocellular carcinoma [3]. All these affections were also met during pediatric age. Recent studies describe NASH as a hepatic manifestation of the metabolic syndrome which was also described in children and teenagers in the last years [4].
Nowadays, the fatty liver disease is frequently met in obese and insulin resistance children. NAFLD in children and teenagers is considered a complication arising from obesity, the disease still being poorly diagnosed and insufficiently treated, despite an exponential increase of its prevalence [5]. There is less clinical experience regarding NAFLD/NASH in children as compared to the one in adults.
History
Ludwig, in 1980, described, for the first time, the non-alcoholic steatohepatitis in adults: a kind of hepatic affection which resembles the alcoholic hepatitis but where the alcohol consumption was infirmed. Three years later, the disease was also described, for the first time, in obese children by Moran and colab. [6,7].
Epidemiology
For pupils and teenagers all over the world, NAFLD represents the main cause of the chronic hepatic disease. In the last years, there has been noticed both an increase of the NAFLD incidence and an alarming increase of obesity prevalence in children [3].
The impact of the chronic hepatic disease upon obese children is increasing and, since they can develop cirrhosis and hepatic carcinoma, it is regarded as significant [8]. Most worryingly is the fact that cardio-vascular morbidity in children and teenagers is associated with NAFLD [8].
Due to its high prevalence and its potentially unfavorable evolution, NAFLD represents a considerable public health care issue [5].
The NAFLD prevalence varies from 3% in common children to 80% in obese children [9]. In a sequence of 742 autopsies performed in San Diego on children aged 12-19 years, dead in traffic accidents, the NAFLD prevalence, histologically speaking, was 9.6%, and the NASH prevalence was 2.96% [10].
A NAHNES study (1999-2004) showed an increased ALAT level – a surrogate marker for NAFLD, in the absence of other causes for hepatic diseases, in 8% of the teenagers between 12 and 19 years old in the USA [11].
The NAFLD prevalence increases with age: 0.7% between 2 and 4 years, 17.3% between 17 and 19 years old [10].
In children, NAFLD chiefly occurs in males, during puberty, in children of Hispanic origin and is associated with insulin resistance and with acanthosis nigricans [12].
To determine the real prevalence of NAFLD in children may be a difficult thing because the liver biopsy, the “gold standard” for diagnosis, has not been performed in large epidemiologic studies; the present studies use the ALAT and ASAT seric concentration or the abdominal ultrasound, but their specificity and predictive values are uncertain [13].
Pathogeny
The adipose tissue, considered just a passive deposit for excessive energy, is nowadays recognized as an active hormonal system, producing molecules which exert central and peripheral effects, regulate the metabolism of glucose and lipids, and modulate the immune response.
There is a series of mechanisms involved in producing NAFLD:
Decrease of mitochondrial oxidation;
Increase of fatty acid level in liver, either by endogenous synthesis, or by fatty acid influx, moved from the adipose tissue;
Triglycerides excess and dysfunctions in their metabolism [1].
A series of cytokines and hormones which are secreted at the level of adipocytes (leptin, resistin, adipokines, TNF-α) constitute the link between the accumulation of adipose tissue (especially the visceral one) and the progression of the hepatic inflammation, having as consequence the increase of insulin resistance [14,15,16].
A study which was carried out in children with NAFLD showed that the severity of the hepatic lesions is strongly associated with the presence of an aterogenous lipidic profile, this lesion association having potential implications and significances for diagnosis and treatment [14].
Some studies demonstrated that NAFLD was present in 2 or more generations of the same family, suggesting that it has a genetic component [13].
Clinical Manifestations
The NAFLD onset is insidious. Children with NAFLD are often asymptomatic. The most frequent symptoms are: pain in the right hypochondriac region, fatigue, generally altered state. Other clinical manifestations which are described in children: irritability, sadness, lack of concentration, nausea, diarrhea, abdominal distension, muscle cramps, cephalea [17].
Most of the children with NAFLD/NASH are overweight (specific sex/age) percentile 85≤IMC< percentile 95 or obese (IMC≥ percentile 95), with mainly abdominal adipose tissue distribution [12].
Hepatomegaly is often present.
Acanthosis nigricans can be noticed in 30-50% of the children with NAFLD, associated with insulin resistance. It is characterized by the pigmentation of the flexion areas: cervical region, axillary and inguinal areas [3].
Signs and symptoms of the chronic hepatic disease (ascites, esophageal varices) are very rare.
Sometimes, children have a positive NAFLD familial history, insulin resistance or diabetes mellitus type II [13].
Paraclinical Investigations
Biochemical
Serum transaminases may vary from normal to high values, sometimes rather severely (6-7 times more than normal values), however, the sensitivity of these laboratory analyses remains poor [14]. Alkaline phosphatase can have elevated values.
Other laboratory parameters which deal with hepatic function are usually within normal limits.
Dyslipidemia is present in most of the children with NAFLD, with abnormal levels of triglycerides and/or low HDL cholesterol. To diagnose NAFLD, we need investigations to exclude other multiple causes for hepatic steatosis (described in Table 1) and some other causes for chronic hepatic diseases.
Imagistic investigations
Abdominal ultrasound is often used in screening for NAFLD. Fat storage in liver makes the liver look “shiny”, as compared to the kidney. Abdominal ultrasound has a predictive value of 87-94%. One cannot detect, from an ultrasound point of view, the fat load of the liver under 30% as compared to the histological result [13].
The computed tomography, magnetic resonance and spectroscopy are imagistic techniques of high precision in determining the liver fat content; besides the fatty liver, other characteristics of NASH cannot be evaluated. There is no other imagistic technique that can render a possible difference between simple steatosis and NASH [3].
Liver biopsy represents the gold standard to diagnose NAFLD.
It is essential to establish a diagnosis, in order to eliminate some untraceable pathology through other methods. It is the only method which can diagnose a hepatitis whose etiology could not be established on clinical and paraclinical basis. This method can be used for the symptomatic patients with increased transaminases for more than 6 months. It is not regarded as a diagnosis screening test. It allows the liver disease staging taking into account that simple steatosis is a reversible disease with a good prognostic, whereas steatohepatitis can evolve into cirrhosis.
The histological lesions in the case of NAFLD [15] are:
-hepatic steatosis (present in 100% of the cases), which can be:
-macrovesicular, the most frequent type, with one vacuole, with lipids, which pushes the cytoplasm and the hepatocyte nucleus to periphery;
-microvesicular, characterized by the presence of several vacuoles with lipids within the hepatocyte cytoplasm;
-lymphocytary lesions, with various degrees, most frequently in the portal areas, but also intralobular;
-hepatic cell balonization; hepatocyte ballooning is the most characteristic feature of steatohepatitis and is typically associated with formation of Malory bodies.
-fibrosis.
Other lesions with a lower frequency: pigmented macrophages [3].
A histological study on NAFLD/NASH described two distinct methods of lesions which are compatible to steatohepatitis. The first pattern is consistent with NASH as described in adults. We termed this pattern “type 1” NASH and defined it as the presence of steatosis with ballooning degeneration and/or perisinusoidal fibrosis in the absence of portal lesions (Table 2, Fig.1) [18]. The second pattern was termed “type 2” NASH and was defined as the association of steatosis with portal inflammation and/or fibrosis in the absence of ballooning degeneration and perisinusoidal fibrosis (Table 2, Fig.2, Fig.3) [18].
Table 2.
Definition for NASH Types (according to Schwimmer JB, 2005)
| Type 1 | Type 2 | |||||
| Ballooning through degeneration | + | + | - | - | ||
| Perisinusoidal fibrosis | - | + | + | - | ||
| Steatosis | + | + | ||||
| Portal inflammation | - | + | + | - | ||
| Portal fibrosis | - | - | + | + | ||
Fig.1.
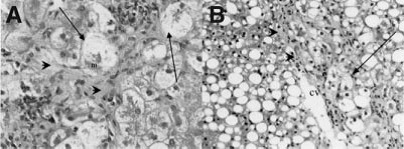
Typical histological appearance of type 1 NASH in children (according to Schwimmer JB, 2005). These photomicrographs illustrate the typical features of classic or type 1 NASH in biopsies from two different obese children. Ballooned hepatocytes are conspicuous (arrows) through the existence of the hyaline (Mallory bodies), identifiable in the cytoplasm of the ballooned hepatocyte (panel A, m). Perisinusoidal fibrosis is notable (arrowheads). m=Mallory; CV=central vein.
Fig.2.
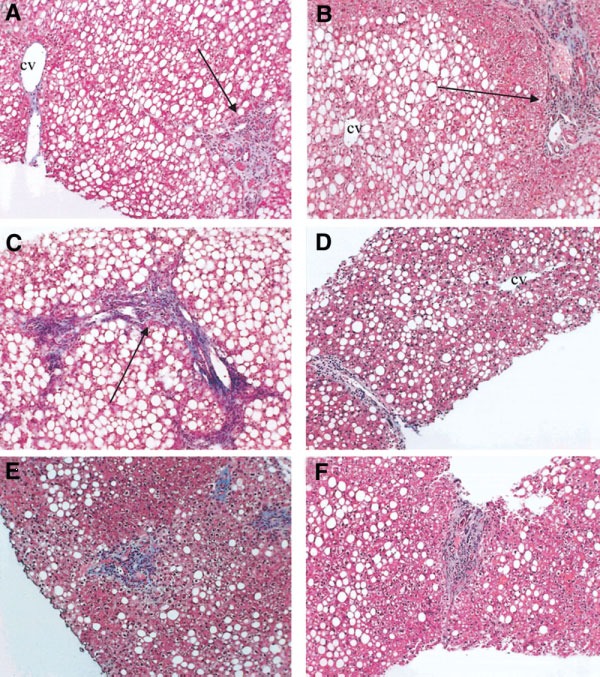
Typical histological appearance of type 2 NASH in children (according to Schwimmer JB, 2005). These panels represent histological findings from six different obese children with nonalcoholic fatty liver disease. The identifiable pattern consists of moderate to marked steatosis, with portal inflammation or fibrosis and the absence of vacuolar degeneration and perisinusoidal/perivenular fibrosis. (A-C) Portal fibrous expansion (arrows) with fibrous septa present in panel C. The center-lobular veins are free of fibrosis. Magnification, 100x. cv=central vein.
Fig.3.
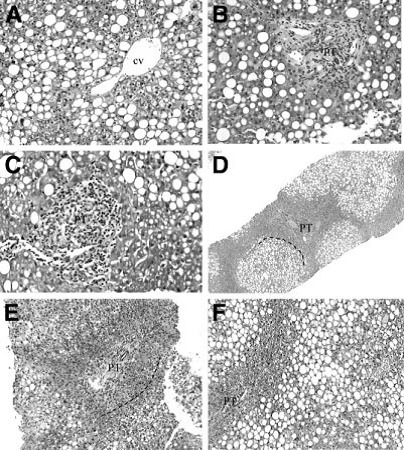
Specific histological findings in children with type 2 NASH (according to Schwimmer JB, 2005). These photomicrographs further illustrate findings in liver biopsies from children with the type 2 pattern. (A) The center-lobular veins consistently show no evidence of injury or fibrosis. (B-E) Predominately lymphocytic portal inflammation, (B-C) with early portal fibrous expansion (D-E). Some cases exhibit a conspicuous periportal (acinar zone 1) sparing of steatosis (D-F). Dashed lines indicate approximate demarcation between hepatocytes with and without fat droplets. CV=central vein; PT= portal area.
In conclusion, type 1 and type 2 NASH are distinct subtypes of pediatric NAFLD, and type 2 is the most common pattern in children. NASH subtypes should be considered when interpreting liver biopsies and planning studies of the pathophysiology, genetics, natural history, or response to treatment in pediatric NAFLD [15].
Studies which were carried out in North America and in Australia showed differences between the histological lesions in children, in the case of NAFLD, as compared to those noticed in adults [11].
These differences include: more severe steatosis, less balonization, less lobular inflammation, less PMN infiltration, absence of portal chronic inflammation, more portal fibrosis without accompanying perisinusoidal fibrosis [3]. The differences between the histological aspects which were noticed in children and adults are due to the fact that the liver answers in a different way, to an aggression which was recently installed as compared to the adult where there is a long-term aggression action.
Since not everybody consuming larger quantities of alcohol develops hepatic affections, multiple genetic factors of the host and genetic polymorphisms are recognized as significant factors able to modify the individual risk to develop metabolic and inflammatory processes in NASH [3].
Establishing an early diagnosis, by using some non-invasive screening methods in high risk groups, represents the most important strategy against NAFLD [9].
FibroScan (transient elastography) is a new, non-invasive method used to explore the liver stiffness. Although this method is particularly used in adults, recent studies pointed out the benefits of the fibroscan technique in order to discover fibrosis in pediatric age [19].
Treatment
Within the therapeutic behavior in NAFLD, a pediatric, endocrinological, dietary, kinotherapeutic and psycho-social multidisciplinary approach is necessary [20].
The treatment strategies for NAFLD during childhood consist of a lifestyle change and pharmacologic treatment.
Lifestyle changes
Losing weight with physical activity and going on a diet represent the only effective treatment of NASH during childhood, at present [12,21]. Losing weight has to be progressive, because a rapid process can accelerate the hepatic disease. The effect of losing weight consists in the normalization of the transaminases and of ultrasound changes and in the remission of the steatosis and micro-inflammation but not of the fibrosis [13].
When promoting a healthy lifestyle, the child’s environment has an important role: family, colleagues, neighbors, school. To achieve this, it is necessary to [8]:
Develop a normal alimentary behavior and lifestyle;
Change alimentary habits;
Increase the level of public awareness;
Integrate educative measures within the curriculum;
Eradicate children’s poverty.
At present, in highly industrialized countries, there are perspectives to develop some National Programmes to promote education and encourage physical activities, to prevent obesity in children with risk [21]. Other studies suggest setting up some National Healthy Programmes which should include the Screening Programme for obesity in children and teenagers [4].
Drug treatment is being developed and has as objectives:
The decrease of insulin resistance, with benefic effects upon the increased levels of transaminases, as well as a steatosis decrease (metformin);
The decrease of oxidative stress (ursodeoxycholic acid, vitamin E) [10,22].
The studies on the results obtained after the pharmacological treatment are not encouraging [11].
Research has been carried out regarding the effects of probiotics for the treatment of NAFLD [20, 23]. Bariatric surgery was used in the treatment of obesity in teenagers [5,24]. We need large, multicentric studies to assess the efficiency of the developing pharmacological therapies; however, the most important action regarding NASH in children should be the prevention of childhood obesity [13].
Conclusions
It is necessary to develop sensitive and specific methods, which are non-invasive for the NAFLD/NASH diagnosis in order to permit the diagnosis in large groups of patients with risk. There are also necessary longitudinal studies to trace the disease for a better understanding of the NAFLD natural history and evolution in children.
References
- 1.Fauci A.S., Braunwald E., Isselbacher K.J., Wilson J.D., Martin J.B., Kasper D.L., Hauser S.L., Longo D.L., editors. Harisson, Principiile medicinei interne. 14th; ed. a II-a in lb.romana. Vol. 2. Bucuresti: Ed. Teora; 2003. pp. 1893–1189. [Google Scholar]
- 2.Grigorescu-Sido Paula. Tratat elementar de Pediatrie. I. Cluj Napoca: Ed. Casa Cărţii de Ştiinţă; 1997. pp. 353–355. [Google Scholar]
- 3.Brunt E. Nonalcoholic steatohepatitis. Semin Liver Dis. 2004;24(1):3–20. doi: 10.1055/s-2004-823098. [DOI] [PubMed] [Google Scholar]
- 4.Kiess W, Blüher S, Kapellen T, Körner A. Metabolic syndrome in children and adolescents: Prevalence, Public Health Issue and Time for Initiative. Journal of Pediatric Gastroenterology and Nutrition. 2009;;49(3):268–271. doi: 10.1097/MPG.0b013e31819a4e9d. [DOI] [PubMed] [Google Scholar]
- 5.Pardee PE, Lavine E, Schwimmer JB. Diagnosis and treatment of pediatric nonalcoholic steatohepatitis and the implications for bariatric surgery. Semin Pediatr. Surg. 2009;18:144–151. doi: 10.1053/j.sempedsurg.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran JR, et al. Steatohepatitis in obese children: a cause of chronic liver disfunction. Am J Gastroenterol. 1983;78:374–377. [PubMed] [Google Scholar]
- 7.Manco Melania. Nonalcoholic faty liver disease in children. European Endocrinology. 2010;6(1):60–63. [Google Scholar]
- 8.Loomba R., Sirlin CB., Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009;50(4):1282–1293. doi: 10.1002/hep.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Widhalm K, Ghods E. Nonalcoholic faty liver diseases: a chalange for pediatricians. Int J Obes (Lond) 2010;34(10):1451–1467. doi: 10.1038/ijo.2010.185. [DOI] [PubMed] [Google Scholar]
- 10.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 11.Manco Melania, Battazzo GF, De Vito Rita, et al. Nonalcoholic fatty liver diseases in children. Journal of the American College of Nutrition. 2008;27(6):667–676. doi: 10.1080/07315724.2008.10719744. [DOI] [PubMed] [Google Scholar]
- 12.Nanda K. Non-alcoholic steatohepatitis in children. Pediatr Transplant. 2004 Dec;8(6):613–618. doi: 10.1111/j.1399-3046.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 13.De Bruyne Ruth M.L., Fitzpatrick Emer, Dhawan Anil. Fatty liver disease in children: eat now pay later. Hepatol Int. 2010 Mar;4(1):375–385. doi: 10.1007/s12072-009-9160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts EA. Non-alcoholic steatohepatitis in children. Clin. Liver Dis. 2007;11(1):155–172. doi: 10.1016/j.cld.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Nobili V, Manco M, Ciampalini P, Diciommo V, Devito R, Pimonte F, Comparcola D, Guidi R, Marcellini M. Leptin, free leptin index, insulin resistence and liver fibrosis in children with non-alcoholic fatty liver disease. Eur J Endocrinol. 2006;155(5):735–743. doi: 10.1530/eje.1.02288. [DOI] [PubMed] [Google Scholar]
- 16.Nobili V, Alisi A, Vania A, Tiribelli C, Pietrobattista A, Bedgni G. The pediatric NAFLD fibrosis index: a predictor of liver fibrosis in children with non-alcoholic fatty liver diseases. BMC Med. 2009;7(21) doi: 10.1186/1741-7015-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kistler K.D., Molleston J., Unalp A., Abrams SW.H., Behling C., Schwimmer J.B. Symptoms and Quality of Life in Obese Children and adolescents with Non-alcoholic Fatty Liver Disease. Aliment Pharmacol Ther. 2010;31(3):396–406. doi: 10.1111/j.1365-2036.2009.04181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42(3):641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 19.Mazhar SM, Shiehmorteza M, Siorlin CB. Noninvazive assesment of hepatic steatosis. Clin. Gastroenterology Hepatol. 2009;7;7(2):135–140. doi: 10.1016/j.cgh.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moraru Evelina, Rusu Tania, Bozomitu Laura. Actualităţi în investigarea şi terapia hepatopatiilor cronice la copil. Vol Referate Generale; Lucrările Congresului Al IX-lea Congres Naţional de Pediatrie; 2009 Oct 21-24; Iaşi, Romania. pp. 346–356. [Google Scholar]
- 21.Nobili V., Alisi A., Raponi M. Pediatric non-alcoholic fatty liver disease:preventive and therapeutic value of lifestyle intervention. World J. Gastroenterol. 2009;15(48):6017–6022. doi: 10.3748/wjg.15.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanyal AJ, Chalasani N, Kowdley KV, et al. NASH CRN. Pioglitazone, vitamine E or palcebo, for nonalcoholic steatohapatitis. N Engl J Med. 2010;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavine JE, Schwimmer JB, Van Natta M. Effect of vitamin E or metformin for treatment of nonalcoholic faty liver disease in children and adolescents. JAMA. 2011;305(16):1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xanthakos S, Daniels SR, Inge TH. Bariatric surgery in adolescents: an update. Adolesc Med Clin. 2006;17:589–612. doi: 10.1016/j.admecli.2006.06.001. [DOI] [PubMed] [Google Scholar]


