Abstract
BALB/c mice repeatedly immunized with Pneumococcus R36A vaccine produce antibodies to phosphorylcholine having the TEPC-15 myeloma idiotype (murine IgA myeloma protein that binds phosphorylcholine). The plaque-forming cell response to phosphorylcholine shows a decrease with repeated immunizations. In contrast, spleen cells from multiply immunized mice responded better in vitro than spleen cells from nonimmunized mice. The serum of animals immunized four or five times agglutinates TEPC-15-coated sheep erythrocytes. Inhibition of hemagglutination shows that the agglutinating activity is directed against the TEPC-15 idiotype. Sera from these mice, when added to cultures of normal spleen cells, specifically suppress the response to phosphorylcholine. The suppressive activity in the serum can be removed by solid absorption with TEPC-15. Evidently, repeated immunization with antigen induces two kinds of antibody responses: one directed against antigen and the other directed against the antibody to the antigen. It is proposed that this “auto” antibody against receptor is involved in the regulation of the immune response.
Keywords: antibodies against phosphorylcholine, specific suppression
Full text
PDF


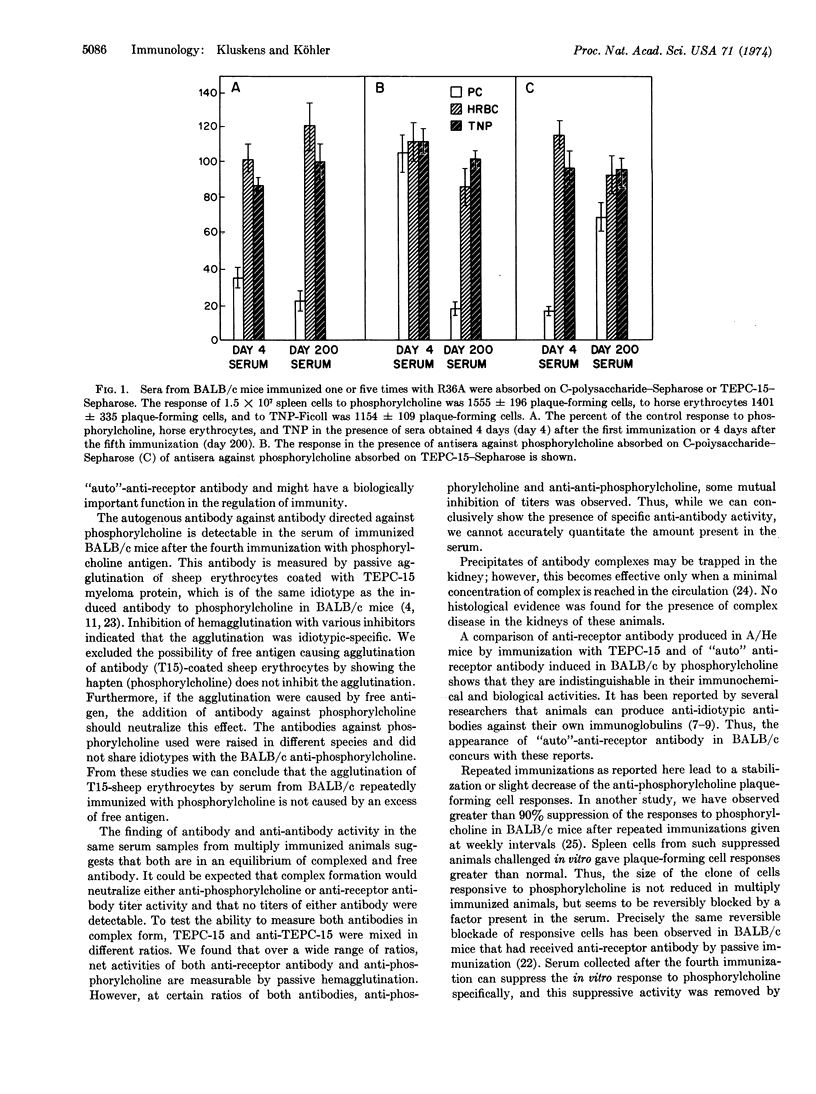

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Claflin J. L., Lieberman R., Davie J. M. Clonal nature of the immune response to phosphorylcholine. I. Specificity, class, and idiotype of phosphorylcholine-binding receptors on lymphoid cells. J Exp Med. 1974 Jan 1;139(1):58–73. doi: 10.1084/jem.139.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza H., Köhler H. Specific inhibition of plaque formation to phosphorylcholine by antibody against antibody. Science. 1972 Jun 2;176(4038):1027–1029. doi: 10.1126/science.176.4038.1027. [DOI] [PubMed] [Google Scholar]
- Cosenza H., Köhler H. Specific suppression of the antibody response by antibodies to receptors. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2701–2705. doi: 10.1073/pnas.69.9.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann K. Idiotype suppression. I. Influence of the dose and of the effector functions of anti-idiotypic antibody on the production of an idiotype. Eur J Immunol. 1974 Apr;4(4):296–302. doi: 10.1002/eji.1830040413. [DOI] [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Hart D. A., Wang A. L., Pawlak L. L., Nisonoff A. Suppression of idiotypic specificities in adult mice by administration of antiidiotypic antibody. J Exp Med. 1972 Jun 1;135(6):1293–1300. doi: 10.1084/jem.135.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU T. Y., GOTSCHLICH E. C. The chemical composition of pneumococcal C-polysaccharide. J Biol Chem. 1963 Jun;238:1928–1934. [PubMed] [Google Scholar]
- Lee W., Cosenza H., Köhler H. Clonal restriction of the immune response to phosphorylcholine. Nature. 1974 Jan 4;247(5435):55–57. doi: 10.1038/247055a0. [DOI] [PubMed] [Google Scholar]
- McKearn T. J. Antireceptor antiserum causes specific inhibition of reactivity to rat histocompatibility antigens. Science. 1974 Jan 11;183(4120):94–96. doi: 10.1126/science.183.4120.94. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of normal mouse spleen cell suspensions in vitro. Science. 1966 Aug 26;153(3739):1004–1006. doi: 10.1126/science.153.3739.1004. [DOI] [PubMed] [Google Scholar]
- Plotz P. H., Talal N., Asofsky R. Assignment of direct and facilitated hemolytic plaques in mice to specific immunoglobulin classes. J Immunol. 1968 Apr;100(4):744–751. [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Potter M., Lieberman R. Common individual antigenic determinants in five of eight BALB-c IgA myeloma proteins that bind phosphoryl choline. J Exp Med. 1970 Oct 1;132(4):737–751. doi: 10.1084/jem.132.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodkey L. S. Studies of idiotypic antibodies. Production and characterization of autoantiidiotypic antisera. J Exp Med. 1974 Mar 1;139(3):712–720. doi: 10.1084/jem.139.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D. A., Fitch F. W., Stuart F. P., Köhler H., Cosenza H. Specific suppression of immune responses. Science. 1973 Sep 21;181(4105):1133–1141. doi: 10.1126/science.181.4105.1133. [DOI] [PubMed] [Google Scholar]
- Sirisinha S., Eisen H. N. Autoimmune-like antibodies to the ligand-binding sites of myeloma proteins. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3130–3135. doi: 10.1073/pnas.68.12.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H. Antibody synthesis at the cellular level. Antibody-induced suppression of 7S antibody synthesis. J Exp Med. 1966 Nov 1;124(5):953–969. doi: 10.1084/jem.124.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A. R., Askonas B. A. Senescence of an antibody-forming cell clone. Nature. 1972 Aug 11;238(5363):337–339. doi: 10.1038/238337a0. [DOI] [PubMed] [Google Scholar]



