Abstract
Titanium and titanium alloys are considered to be one of the most applicable materials in medical devices because of their suitable properties, most importantly high corrosion resistance and the specific combination of strength with biocompatibility. In order to improve the biocompatibility of titanium surfaces, the current report initially focuses on specifying the topography of titanium dioxide (TiO2) nanotubes (NTs) by electrochemical anodization. The zeta potential (ζ-potential) of NTs showed a negative value and confirmed the agreement between the measured and theoretically predicted dependence of ζ-potential on salt concentration, whereby the absolute value of ζ-potential diminished with increasing salt concentrations. We investigated binding of various plasma proteins with different sizes and charges using the bicinchoninic acid assay and immunofluorescence microscopy. Results showed effective and comparatively higher protein binding to NTs with 100 nm diameters (compared to 50 or 15 nm). We also showed a dose-dependent effect of serum amyloid A protein binding to NTs. These results and theoretical calculations of total available surface area for binding of proteins indicate that the largest surface area (also considering the NT lengths) is available for 100 nm NTs, with decreasing surface area for 50 and 15 nm NTs. These current investigations will have an impact on increasing the binding ability of biomedical devices in the body leading to increased durability of biomedical devices.
Keywords: protein binding, serum amyloid A, β2-glycoprotein I, immunoglobulin G, histone IIA
Introduction
Hemocompatible nanomaterials can be used as scaffolds for various clinical devices, such as biosensors, dental devices,1,2 craniofacial implants3,4 and cardiovascular stents.5,6 In all these cases, hemocompatibility and antimicrobial/anti-inflammatory properties of these devices are prime considerations. Biocompatible devices can be made out of different materials, such as metals (stainless steel, tantalum, cobalt alloys, titanium and its alloys), ceramics (aluminum oxide, zirconia, and calcium phosphates), and synthetic or natural polymers.7 Titanium (Ti) and its alloys are considered as one of the most significant biocompatible material for medical applications due to resistance to body fluid effects, great tensile strength, flexibility, and high corrosion resistance.8 Structures present in the human body are not only in micrometer but also in nanometer scale, such as the bone, which is made up of nanostructures. To produce better biocompatible materials, the surfaces of these materials must be nanostructured.9 An easy method to obtain nanostructures and to control the obtained nanotopography is electrochemical anodization; other methods include hydrothermal treatments or sol-gel methods.10 Generally, nanoscale surfaces increase roughness and possess high effective surface area, which may lead to increasing initial protein adsorption that is a very important first step in regulating cellular interactions of the implant. Surface physical and chemical properties of the material have an impact also on adhesion, together with charge distribution.11–13
As TiO2 surfaces and cell membranes are both negatively charged, there is a need for a mediator that shields the repulsive force between the negatively charged biosurfaces.9,14 These can be proteins or divalent ions which bridge the repulsive electrostatic interactions between equally charged surfaces.9 However, other interactions, such as strong chemisorption energies,15,16 and hydrophobic interactions17 also need to be taken into consideration when trying to explain protein interactions with TiO2 surfaces. The interactions of proteins with biomaterial are of great importance because of their presence in plasma and potential functions in wound healing and inflammatory responses.
Previous reports have shown binding of different protein or peptide interlayers, such as growth factors,18 albumin, fibrinogen, IgG,13,19 fibronectin,20 collagen type 1,21 BMP-2,18,22 RGD peptide,23 and laminin-derived peptide,24 to TiO2 nanotubes (NTs), which could provide for crucial information concerning their integration into the body. The majority of the more recent investigations studied the combination of different cell types with different prebound proteins25,26 on TiO2 NTs and found that binding is cell-specific and cannot be generalized. However, reports on hemocompatibility with TiO2 nanostructured material are rare, with exceptions, such as plasma protein adsorption binding studies by Smith et al13,27 and binding of collagen type 1 by Yang et al21 with an explanation of interaction energies and main driving forces.
Proteins studied in the current report were serum amyloid A (SAA), β2-glycoprotein I (β2GPI), β2GPI-derived peptides, immunoglobulin G (IgG), oxygenized IgG (oxIgG), and a non-plasma protein, histone IIA. In addition to plasma proteins, we also examined histone IIA, a small nuclear DNA binding protein, because of its small molecular weight of 14 kDa and positive charge, due to rich lysine residues.28,29 It served as a non-plasma protein control.
SAA is a major acute phase protein which is rapidly upregulated during inflammatory events such as infections, wounds, and injuries.30,31 The role of the acute phase response is prevention of pathogen entry, neutralization and clearing of pathogens, minimization of tissue damage that leads to wound healing, and restoring of homeostasis.30,32 SAA is a relatively small protein of ~12 kDa33 with ~80% alpha helical conformation.34
β2GPI, also known as apolipoprotein H,35 is a multifunctional 53 kDa glycosylated plasma protein36 with physiological values of 20–300 mg/L.37 The protein consists of 326 amino acids folded into five domains, where the fifth domain is positively charged, with a lysine-rich region281 CKNKEKKC,288 a hydrophobic loop313 LAFW,316 and extended C-terminal domain, that enable binding to negatively charged phospholipids.38–40 After binding to phospholipids, β2GPI changes its conformation from circular to open fish-hook, exposing a cryptic epitope which is recognized by antibodies against β2GPI (anti-β2GPI) directed against domain I.41 Zager et al also reported that anti-β2GPI recognize both native and conformational epitopes located on different domains of anti-β2GPI.42 Anti-β2GPI represents one of the main subgroups of antiphospholipid antibodies and is one of the laboratory criteria for classification of antiphospholipid syndrome.43
IgG antibodies are large molecules of about 150 kDa and are the predominant antibody isotype in normal human serum. IgG accounts for 70%–75% of the total serum antibody pool and consists of a monomeric four-chain molecule. The normal concentration range is 6.0–16 g/L.44 Oxidation of normally protective polyclonal IgG antibodies isolated from healthy blood donors by various agents resulted in severe alteration of their specificity and increased affinity to self-antigens.45–47 β2GPI, one of the main pathologically relevant antigens in antiphospholipid syndrome, was reported to be recognized by oxidatively altered IgG/IgM.47,48
Peptide binding to TiO2 NTs has been used predominantly for studying the increase of cell adhesion and osteogenic gene expression22,23,49 as well as for soft tissue interactions24 and local delivery of antimicrobial peptides.50 A hexapeptide motif was found to bind electrostatically to titanium surfaces, with potential important osteogenic consequences.17
Therefore, we tested the binding of plasma proteins with different molecular weights, charges, and functions to TiO2 NTs with specific diameters. Nanotubular surfaces were designed by specific electrochemical anodization of the metallic substrate.10,14,51–55 Prepared TiO2 nanotubular structures have nanorough surfaces with sharp edges and spikes which promote the binding of proteins and thus better/stronger adhesion of certain cell types.9,14,56
Our aim was to investigate whether plasma proteins, not previously reported, can bind to TiO2 NTs and characterize if the binding is dependent on nanotube diameter, protein molecular size, charge, or conformation and determine whether the interaction is dose-dependent.
Materials and methods
Growth of titanium dioxide NTs by electrochemical anodization
To obtain different TiO2 NTs, 0.1 mm thick titanium foils (Advent Research Materials Ltd., UK) of 99.6% purity were used. Prior to anodization, titanium foils were cleaned by successive ultrasonication in acetone, ethanol, and deionized water for 5 minutes and dried in nitrogen stream. Actually, the anodization method is a two-step process. In the first step, NTs are grown in ethylene glycol (EG) plus 0.1 M NH4F plus 1 M H2O at 35 V for 2 hours. The NTs were removed by ultrasonication in water and the obtained pretreated sample (after cleaning again by ultrasonication – as initially performed, and dried in nitrogen stream) was subsequently used as substrate for obtaining the final nanostructures with the desired diameters. EG-based electrolytes, with hydrofluoric acid as the source of F− ions with specific amount of water, were used in the second anodization step, under various applied potentials for obtaining the desired different diameter NTs. Specifically, the electrolyte was EG plus 8 M water plus 0.2 M hydrofluoric acid and the anodization time of 2.5 hours was used for obtaining all nanostructures (15, 50, and 100 nm), while the applied potential was 58 V for 100 nm, 20 V for 50 nm, and 10 V for 15 nm. The NTs thus formed were kept in ethanol for 2 hours to remove all organic components from the electrolyte, washed with distilled water, and dried under nitrogen stream.57 Anodization parameters were optimized in order to get the specific morphology NTs.10 All the anodization experiments were carried out at room temperature (~20°C) in a two-electrode system with titanium foil as the anode and platinum gauze as the cathode. Freshly made and non-autoclaved NTs were used in further experiments.
Electron microscopy
The morphology of the formed TiO2 NTs was evaluated using a field-emission scanning electron microscope (SEM) (Hitachi S4800), which was used to acquire the top view of the NTs, as previously described.58 Transmission electron microscopy (TEM) (JEM 2100; JEOL, Tokyo, Japan) characterization of the NT samples was performed as follows. The NTs were collected from the titanium foil support by mechanical scratching and dispersed in distilled water. Drops of distilled water with the dispersed NTs were placed on a copper-grid-supported perforated transparent carbon foil. The high-resolution TEM images were taken at the edges of the NT clusters jutted into a hole in the carbon foil. Top and cross-section views were obtained from mechanically cracked samples. The mean standard values for diameters, spacing, and length of the nanotubular structures and the standard deviation values were determined by analyzing images from three different samples for each nanostructure.
Zeta potential of TiO2 NTs
The suspension of sheared TiO2 NTs was monitored with electrokinetic measurements of the zeta potential (ζ-potential) (Brookhaven Instruments Corporation, ZetaPALS). For determination of the ζ-potential, their aqueous suspension was used as a function of the pH. Prior to the experiment, the pH of the aqueous suspension was set to the desired value with the HCl or NaOH (0.01 M) solutions.
Theoretical approach for determining the ζ-potential
ζ-potential can be also determined using various theoretical approaches. In this study, the Stern–Gouy–Chapman model and Gongadze–Iglič (GI) model were combined to calculate electric potential near a negatively charged planar surface with negative surface charge density.59,60 In the Stern layer, the Gouy–Chapman equation was applied, while in the diffuse layer, the GI equation was used. As ζ-potential was experimentally measured at the distance greater than the distance of closest approach (b) – the distance of the plane dividing the Stern and diffuse layers – the electric potential was also calculated only in the diffuse layer.
Biological material
Proteins used in the NT binding experiments were either purchased as lyophilized material and reconstituted according to manufacturer’s instructions or isolated from human biological material, as described below. β2GPI was isolated from human plasma using a combination of perchloric acid precipitation and heparin affinity/cationic exchange chromatography.61 Human polyclonal IgG were isolated from sera of healthy blood donors by affinity chromatography HiTrap™ Protein G HP column (GE HealthCare Bio-Sciences AB, Uppsala, Sweden) as previously described. Oxidatively altered IgG (oxIgG) with increased immunoreactivity to β2GPI and its peptide clusters influence human coronary artery endothelial cells.62 Isolated IgG were oxidized as previously reported48 with exposure to direct current using graphite stick electrodes on a current/voltage generator. Isolated IgGs were denatured by boiling the protein samples at 95°C for 5 minutes (in 2720 Thermal Cycler; Thermo Fisher Scientific, Waltham, MA, USA) in denaturation buffer (2% sodium dodecyl sulfate [SDS]; Bio-Rad Laboratories Inc., Hercules, CA, USA), 0.01% (w/v) β-mercaptoethanol (Riedel-de Haën, Hannover, Germany), 1 mM dithiothreitol (Sigma-Aldrich Co., St Louis, MO, USA) in phosphate buffered saline (PBS), pH 7.4. Lyophilized histone from calf thymus type II-A (Sigma®, Sigma-Aldrich Co.) and lyophilized human recombinant SAA (Peprotech, Rocky Hill, NJ, USA) were resuspended and/or diluted with PBS and sterilized with filter (0.20 μm pores; Acrodisc®; Pall Corporation, Ann Arbor, MI, USA).
The sequences of peptide 1 (QGPAHSK) and peptide 2 (KMDGNHP) were found using heptapeptide phage display library by Zager et al.42 The amino acid sequence QGPAHSK represents a mimotope of the conformational epitope that resides on the third domain of β2GPI and is recognized by more than 30% share of high-avidity anti-β2GPI antibodies. The amino acid sequence KMDGNHP represents a mimotope of the conformational epitope between the third and fourth domain of β2GPI that is recognized by more than 30% share of polyclonal low avidity anti-β2GPI antibodies when β2GPI is in open conformation. Peptide 1 and peptide 2 used in the current experiments had an additional tail added at the N-terminus GGGS NH2.
Binding of different proteins to TiO2 NTs and protein quantification
For assessment of total bound proteins on NTs, a microassay procedure from the Thermo Scientific™ Pierce™ BCA protein assay (Thermo Fisher Scientific) was used. Bovine serum albumin (BSA) served as a standard, as supplied in the BCA protein assay. According to the microassay procedure, the standard curve range was 20–2,000 μg/mL.
For the TiO2 NT binding experiments (as represented by Figure 1), 5×5 mm square TiO2 NTs of different diameter – specifically 100, 50, and 15 nm – were used. As a control for nonspecific binding, titanium foil (the template on which NTs are “grown”) was used.
Figure 1.
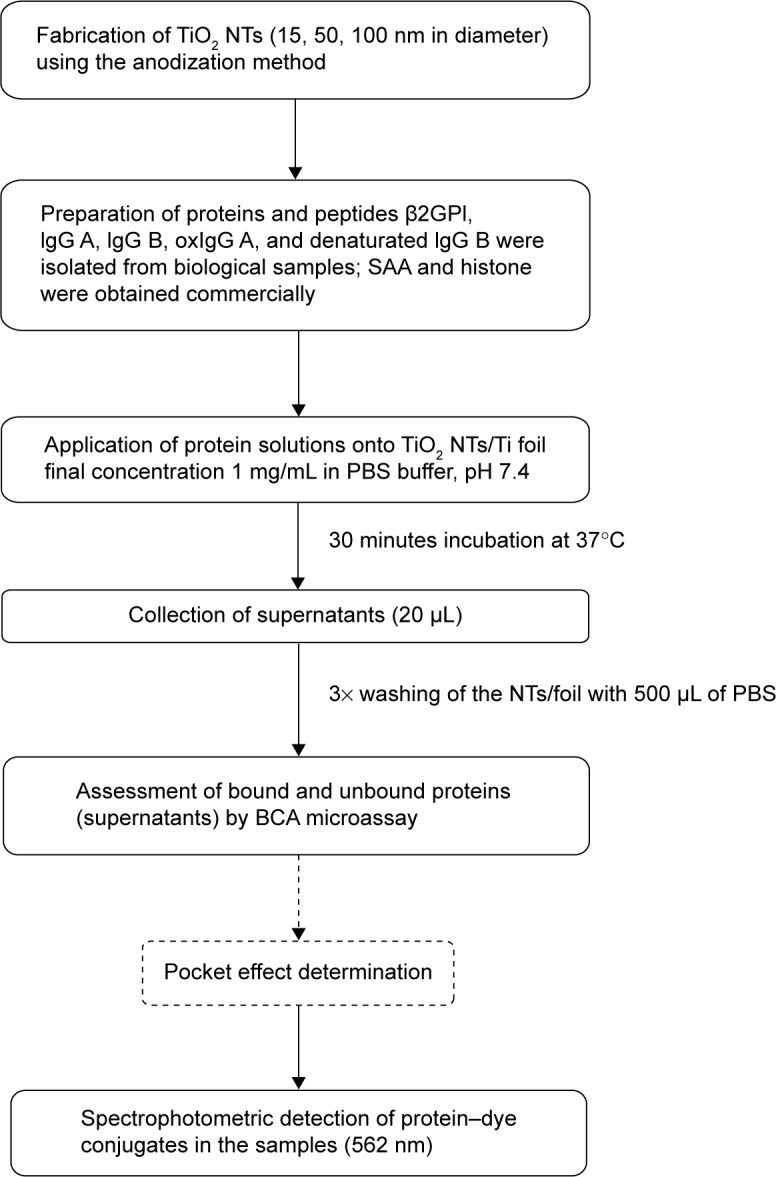
Protocol for quantification of protein binding to TiO2 NTs/Ti foil.
Abbreviations: oxIgG, oxygenized IgG; NTs, nanotubes; PBS, phosphate buffered saline; SAA, serum amyloid A.
Protein quantification was performed as described below. As the first step, 20–40 μL of protein samples (25 μg of protein if not stated otherwise) in the form of a droplet was carefully pipetted onto the surface of TiO2 NTs/Ti foil that were placed in 24-well plates (TPP Techno Plastic Products AG, Trasadingen, Switzerland) and incubated for 30 minutes at 37°C. As a negative control, PBS was used. After incubation, 20 μL samples of supernatants were collected and the NTs/foils were washed three times repeatedly with 500 μL of PBS and transferred into fresh wells. Twenty microliters of PBS and 200 μL of BCA working reagent (prepared according to the manufacturer’s instructions, by mixing 50 units of reagent A with 1 unit of reagent B) were applied into each 24-well plate with the NTs or foil. At the same time, 20 μL of BSA standards or starting protein samples (in concentrations applied on NTs/foil) with 200 μL of BCA working reagent were pipetted into 96-well plates (MaxiSorp, MicroWell, Nunc-Immuno, Thermo Fisher Scientific). After incubation for 30 minutes at 37°C, the same volume of all samples from NTs/foil in 24-well plates was transferred to 96-well plates (with BSA standards and protein starting material) and absorbance was measured at a wavelength of 562 nm with a Sunrise Tecan spectrophotometer (Tecan Trading AC, Maennedorf, Switzerland) (Figure 1). According to the instructions of Thermo Scientific™ Pierce™ BCA protein assay, the correction factors for determination of exact protein concentrations (masses) were used. Where correction factors were not known, we estimated experimental correction factors calculated from the determined concentrations in BCA protein assay based on BSA standard curve and based on the known starting concentrations of the studied proteins. The results were analyzed with two-way analysis of variance and Bonferroni posttests.
“Pocket effect” monitoring
After initial protein determination with BCA, NTs/foil were washed three more times with 500 μL of PBS and three times with 500 μL of dH2O (first washing). Bound protein concentration was again measured with BCA as described in “Biological material”, using the same BSA standard dilutions. Afterwards, the NTs/foil were washed again, first briefly with 1,000 μL of PBS and twice by flushing rigorously four to six times. This washing step was repeated with dH2O (second washing). Protein concentration was measured for the third time as described before with the same BSA standards.
Immunofluorescent microscopy and intensity determination
For immunochemistry determination, different doses of SAA (20, 2, and 0.2 μg) were used on NTs with different diameter pore sizes (100, 50, and 15 nm) and on foil. After binding of SAA, NTs and foil were washed three times with PBS buffer and incubated in blocking buffer (1% BSA and 5% milk) for 30 minutes. NTs and foil were washed again three times with PBS and incubated with primary antibody against human SAA (Reu86.1; Santa Cruz Biotechnology Inc., Dallas, TX, USA). After 1 hour of incubation, the samples were washed three times with PBS and incubated with secondary goat anti-mouse antibody labeled with FITC (Santa Cruz Biotechnology Inc.) for 30 minutes (Figure 2). After washing with PBS, the samples were observed using a fluorescent microscope (Nikon eclipse E400) and images were taken with a digital camera (Nikon Instruments, Melville, NY, USA) and Nikon ACT-1 imaging software. Four different measurements of fluorescence intensity were made for each sample with ImageJ software. An average of four replicates were normalized with an average of background fluorescence (0 μg SAA). The intensity of fluorescent signal of different doses of SAA on different pore size of NTs and foils was compared. The results were analyzed with two-way analysis of variance and Bonferroni posttests.
Figure 2.
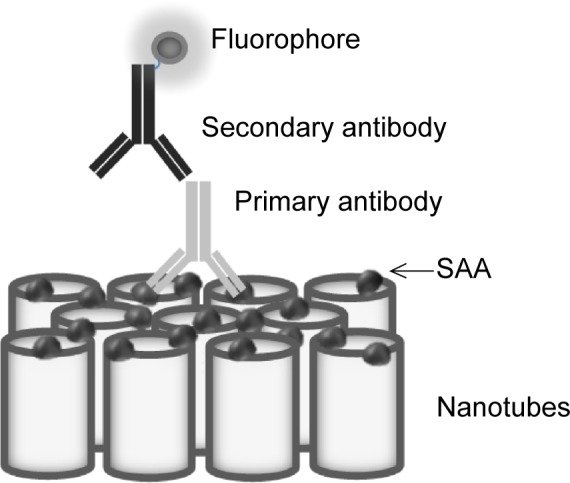
Schematic representation of immunofluorescent detection of SAA protein binding to TiO2 nanotubes.
Abbreviation: SAA, serum amyloid A.
Results
Morphology of titanium dioxide NTs as determined by SEM and TEM
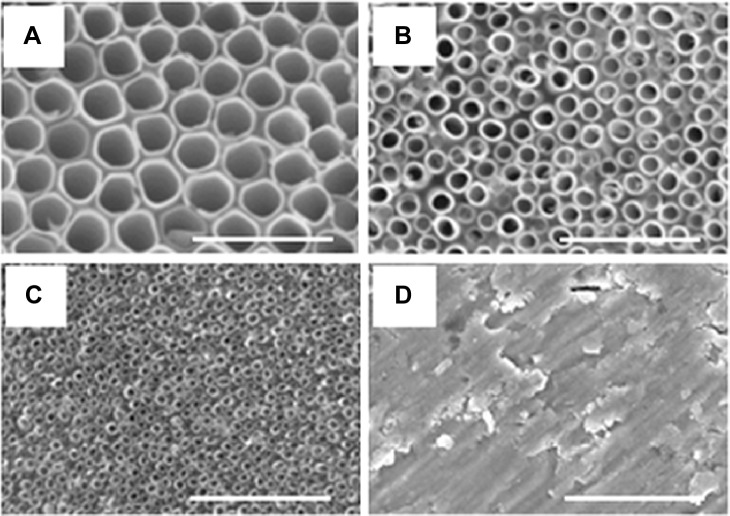
By tailoring the anodization conditions (applied voltage, anodization time, and electrolyte composition), vertically oriented NTs with different diameters were obtained. The use of the double anodization method leads to a clean top surface, ie, no initiation layer or nanograss formation.10 The average diameters of the obtained nanotubular structures were 15 nm, 50 nm, and 100 nm, while the standard deviations for each type of diameters were ±13%, ±10%, and ±5%, respectively. The length of the NTs was determined from cross-section images for each diameter of NTs (images not shown); that is, for 15 nm, 50 nm, and 100 nm diameters, the length was 370 nm, 1 μm, and 3.7 μm with standard deviations of ±8%, ±5%, and ±2.7%, respectively. Top-view SEM images of the obtained nanotubular surfaces, indicating well-controlled diameters and an open top of the NTs, are shown in Figure 3.
Figure 3.
Scanning electron microscope images of the top surface of TiO2 nanotubes of different diameters.
Notes: (A) 100 nm, (B) 50 nm, (C) 15 nm, and (D) titanium foil. Scale bar is 500 nm.
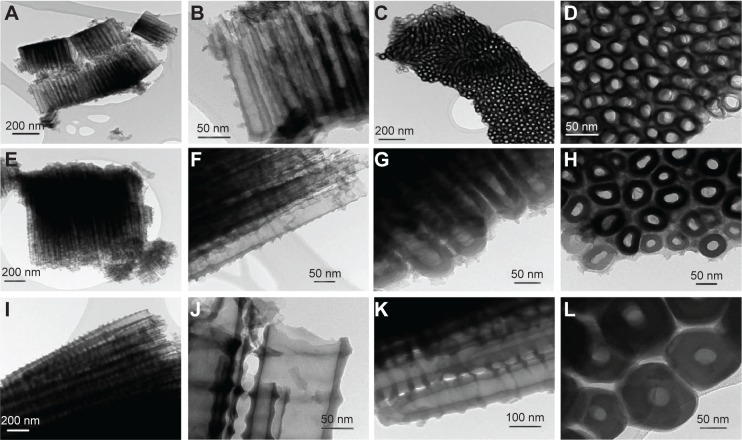
TEM images (shown in Figure 4) confirmed the diameters and alignments of NT clusters. Furthermore, the TEM images put into evidence the inner shape of the nanotubular structure, which is a ‘V-shape’ meaning that the inner diameter decreases along the length from top to bottom, while the tube wall thickness increases.10 For example, comparing TEM images in Figure 4B (15 nm) with Figure 4F and G (50 nm), and with Figure 4J and K (100 nm), a difference in both the tube wall thickness and inner diameter between top and bottom of each nanostructure is observed. It follows that, due to the higher values, it is easier to observe the “V-shape” for larger-diameter NTs (100 nm) than for smaller-diameter NTs (50 or 15 nm diameter); nevertheless, this “V-shape” is present in all obtained nanostructures.
Figure 4.
Transmission electron microscopy images of the TiO2 NTs.
Notes: (A–D) Represents the NTs with 15 nm diameters; (E–H) represents the NTs with 50 nm diameters; and (I–L) represents the NTs with 100 nm diameters.
Abbreviation: NTs, nanotubes.
ζ-potential of TiO2 NTs
The changes in the NTs’ surface charge were followed by measurements of the ζ-potential of their aqueous suspension as a function of the applied pH (Figure 5). The TiO2 NTs show a relatively acidic character for the whole range of considered pH values. The NTs have an isoelectric point (IEP) at a pH of 4.9. The ζ-potential curve reached negative values lower than −25 mV at pH values above 6.5. The high absolute values of the NTs’ ζ-potential in neutral and basic conditions provide strong electrostatic repulsive forces with the negatively charged colloids and strong attraction forces with the positively charged colloids. We measured ζ-potential for all three diameters of NTs (15, 50, and 100 nm). The ζ-potential values of all three diameters of NTs were not significantly different and the ζ-potential curves of the TiO2 NTs (with 15, 50, and 100 nm diameter) as a function of pH in water are shown in Figure 5.
Figure 5.
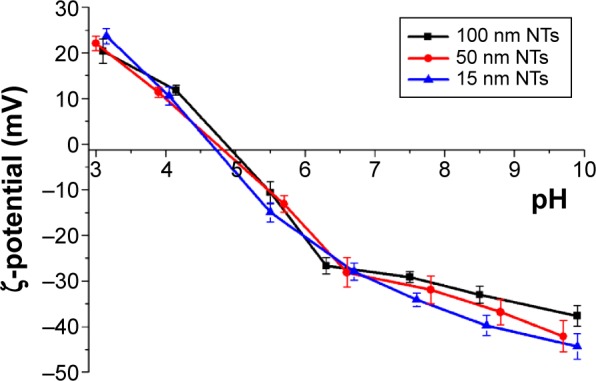
ζ-potential curves of the TiO2 NTs (with 15 nm, 50 nm, and 100 nm diameters) as a function of pH in water.
Abbreviation: NTs, nanotubes.
ζ-potential measurements of NTs’ surfaces were also measured at different concentrations of salt in PBS buffer solution (Table 1). For comparison, the ζ-potential dependence on salt concentration in PBS was also predicted theoretically (Figure 6) using the combination of Stern and GI models.59,60,63 As expected, we observed the decrease in negative ζ-potential values (ie, increase in the absolute value of ζ-potential) with increase in dilution of PBS solution (ie, with a decrease in salt concentrations) in experiments and theory. The agreement between the measured (Table 1) and theoretically predicted (Figure 6) dependence of ζ-potential on salt concentration is better for larger salt concentrations.
Table 1.
Zeta potential values of NT 100 nm in various concentrations of salts in PBS
| Dilutions of PBS | ζ-potential (mV) |
|---|---|
| 1–1 PBS | −25.7±3.0 |
| 1–1.3 PBS | −27.6±3.7 |
| 1–1.5 PBS | −36.5±3.5 |
| 1–2 PBS | −39.8±3.3 |
| 1–10 PBS | −48.6±1.9 |
| 1–100 PBS | −55.1±1.3 |
Notes: The numbers in the sample represent dilutions (PBS - distilled water). Zeta potential values are shown as mean ± standard deviation.
Abbreviations: PBS, phosphate buffered saline; NT, nanotube.
Figure 6.
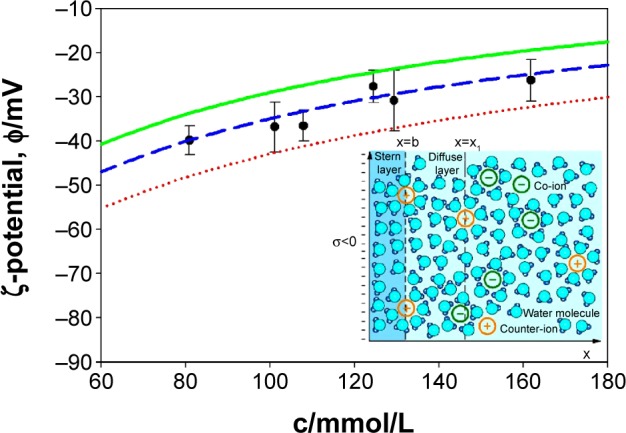
ζ-potential of NTs obtained from experiments (points) and theoretical (curves) consideration in dependence of monovalent salt concentration in PBS buffer solution.
Notes: The theoretical values of ζ-potential were calculated at three different values of the distance from the charged plane (x1): 1.0 nm (red dotted line), 1.2 nm (blue dashed line), and 1.4 nm (green full line). Other model parameters are: water dipole moment p0 =3.1 D, bulk concentration of water now/NA =55 mol/L, temperature =298 K, surface charge density σ=−0.1 As/m2, and distance of closest approach =0.4 nm.60,63 Inset: graphical presentation of Stern and diffuse electric double layer. Outer Helmholtz plane is located at x=b (distance of closest approach) which is approximately equal to the hydrated radius of the counter-ions. ζ-potential plane is calculated at the distance x=x1 from the negatively charged surface located at x=0.
Abbreviations: c, salt concentration; PBS, phosphate buffered saline.
Experimental binding of different proteins and peptides to NTs
Binding of SAA, β2GPI, IgG (from two different donors, namely IgG A and IgG B), oxIgG A, and histone IIA proteins which possess different characteristics was studied on 100, 50, and 15 nm NT surfaces and titanium foil. We observed that proteins of different sizes and characteristics (11–150 kDa; different charges and IEP) can all bind to NTs of different diameters; however, the binding of all those proteins significantly decreases with decreasing diameter of the NTs. The highest binding for all proteins observed was for 100 nm NT a and the binding decreased with smaller NTs’ pore diameter (100 nm NT >50 nm NT >15 nm NT >Ti foil) (Table 2; Figure 7). We examined the highest relative fold changes of bound mass on 100 nm NTs, compared to Ti foil. The highest change was observed with histone IIA, where the bound mass was 8.2-times higher for 100 nm NTs as compared to foil, followed by plasma proteins SAA (7.2×), oxIgG A (5.0×), IgG A (4.8×), and β2GPI (4.5×). With lowering of NT diameter, bound protein mass almost linearly decreased. Interestingly, with 50 nm NTs, the amount of bound proteins was decreased by half (49%–62%) (for all proteins), while on 15 nm NTs, around one-third (29%–41%) of proteins were bound in comparison to 100 nm NTs. On Ti foil, only a small amount (12%–24%) of proteins was bound; the binding did not exceed 25%, which clearly indicates that the titanium foil is less eligible for protein binding. Surprisingly, all the examined proteins showed the same trends and ratios of binding on different NTs (Figure 7). Even histones IIA did not seem to be an exception, although they are small, basic proteins not found in plasma.
Table 2.
Binding of different proteins/peptides to 100, 50, and 15 nm NTs
| Protein | Mass of bound proteins/peptides
|
||||
|---|---|---|---|---|---|
| Initial mass of the protein on NTs (μg) | 100 nm NTs (μg) | 50 nm NTs (μg) | 15 nm NTs (μg) | Foil (μg) | |
| β2GPI | 20.73 | 3.92 | 2.05 | 1.38 | 0.88 |
| SAA | 22.19 | 5.26 | 2.60 | 2.16 | 0.73 |
| IgG A | 25.29 | 3.38 | 2.09 | 1.40 | 0.71 |
| oxIgG A | 24.83 | 3.83 | 2.07 | 1.45 | 0.76 |
| Histone IIA | 14.50 | 4.65 | 2.79 | 1.83 | 0.57 |
| Peptide 1 of β2GPI | 24.85 | 2.26 | 2.08 | 2.32 | 1.84 |
| Peptide 2 of β2GPI | 25.82 | 8.52 | 6.14 | 5.90 | 5.99 |
Abbreviations: β2GPI, β2-glycoprotein I; NT, nanotube; oxIgG A, oxidized IgG A; SAA, serum amyloid A.
Figure 7.
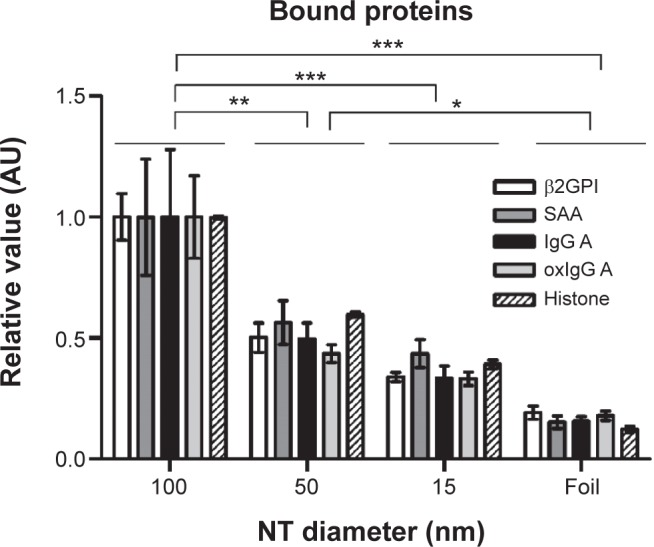
Relative value (normalized to 100 nm NT) of bound protein mass of different plasma proteins and histone IIA.
Notes: Three independent experiments for all proteins were performed. The results were analyzed with two-way analysis of variance and Bonferroni posttests, and significance was determined as follows: *P<0.05, **P<0.01, ***P<0.001.
Abbreviations: β2GPI, β2-glycoprotein I; NT, nanotube; oxIgG A, oxidized IgG A; SAA, serum amyloid A.
In order to determine whether different domains of β2GPI antigen were important for binding to NTs, we used two different peptides representing surface amino acid clusters on β2GPI. Interestingly, the peptides significantly differ from the binding trend of the proteins. Peptide 1 equally bound to all NTs, regardless of the diameter, with approximately the same low amount. Peptide 2, however, showed high binding to all NTs, with highest binding to 100 nm NTs. The amount of bound Peptide 2 on 100 nm NTs exceeded the general amounts of bound proteins on NTs of the same diameter. Moreover, on the NTs with smaller diameters (50 and 15 nm) and foil, the amount of bound peptide 2 was still higher than the average amount of any bound proteins on 100 nm NTs.
Pocket effect and protein denaturation
In order to determine if the proteins are being adsorbed into the NTs interior (ie, pocket effect), we repeated the washing of the NTs several times (see “Binding of different proteins to TiO2 NTs and protein quantification”). Standard washing was applied the first time and more rigorous washing the second time to flush out the proteins that might still be bound in the interior of the NTs. To examine if the structure of the protein affects the extent of binding on NTs of different diameters, we bound the same quantity of native and denaturated IgGs. Denaturation was achieved using temperature, as well as SDS, β-mercaptoethanol, and dithiothreitol.
The highest amount of bound native IgG B protein was observed on 100 nm NTs. Interestingly, the denaturated protein also has the highest amount of binding on 100 nm NTs and it follows the same pattern as other examined proteins (100 nm NT >50 nm NT >15 nm NT >foil) (Figure 8). Each washing step expectedly reduced the amount of bound proteins, but the pattern remained comparable. Interestingly, the highest amount of washed protein was observed on 100 nm NTs (~70% native and denaturated IgG B). After the second washing, the mass of bound protein on average decreased by ~15% as compared to the first washing. A similar decrease of bound proteins was observed on 15 nm NTs and foil, presumably indicating that IgGs were not bound inside the 15 nm NTs; however the fact that the second wash was observed at the limit of the BCA technique has to be taken into account.
Figure 8.
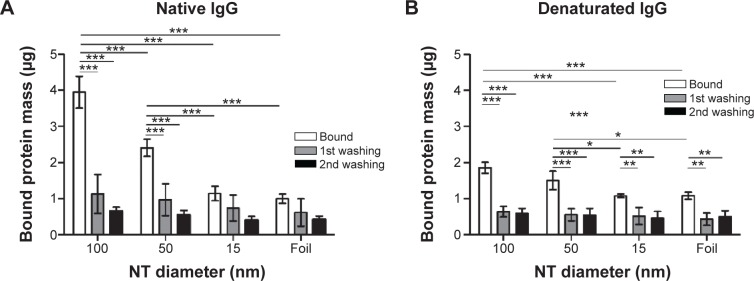
Bound protein mass of native and denaturated IgG B on 100, 50, and 15 nm NTs and foil.
Notes: (A) Native IgG B bound protein mass. (B) Denatured IgG B bound protein mass. Two-way analysis of variance and Bonferroni posttests were used to determine significance: ***P<0.001, **P<0.01, *P<0.05. Five independent experiments for native IgG B and four for denaturated IgG B were performed.
Abbreviation: NT, nanotube.
SAA dose-dependent binding to NTs
In previous experiments we used relatively high amounts of initial protein concentration due to an optimal range of sensitivity of the BCA method. In order to prevent any results due to supersaturation, we examined the binding of different concentrations of SAA on 100 nm NTs. Of all non-histone proteins tested, SAA was chosen due to presenting the highest change of bound mass to 100 nm NTs as compared to foil. We observed that binding of SAA to 100 nm NTs is dependent on the initial concentrations. The observed bound mass was highest when the highest amount of SAA was applied, and it decreased with lowering the starting concentrations, while no or negligible binding was noted for 30 μg of SAA applied to foil (Figure 9). The results were consistent with dose dependency of histone IIA (data not shown). The amount of bound SAA with an initial mass of 30 μg was 7.7-fold higher on 100 nm NTs than on foil.
Figure 9.
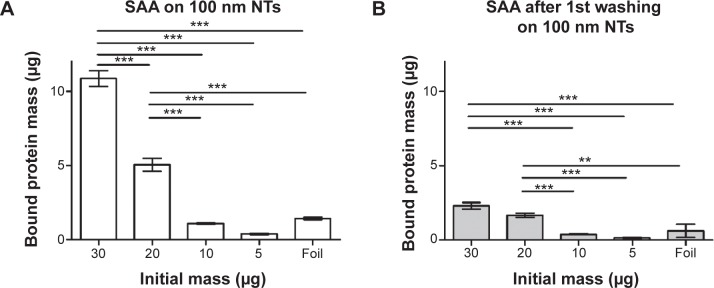
Dose-dependent binding of SAA to 100 nm NTs and foil using BCA protein assay.
Notes: (A) SAA protein bound to 100 nm NTs. (B) SAA protein bound following the first washing of the protein indicating the pocket effect. Two-way analysis of variance and Bonferroni posttests were used to determine significance: ***P<0.001, **P<0.01. The bars represent the average of three independent experiments performed.
Abbreviations: NTs, nanotubes; SAA, serum amyloid A.
Immunofluorescent detection of dose-dependent SAA binding to NTs with different diameters
To fully confirm dose-dependent binding of SAA on 100 nm NTs, immunofluorescence was used with different concentrations (20, 2, 0.2, and 0 μg) of SAA (Figure 10). Immunochemistry was performed by staining, using SAA-specific primary antibodies and fluorophore-conjugated secondary antibodies. We measured the fluorescence intensity on the surface of NTs with different diameters and confirmed the results obtained with BCA assay. Besides the trend of decreased SAA binding with smaller diameter of NTs, we also confirmed a dose-dependent effect of SAA on the NTs with different diameters (Figure 10).
Figure 10.
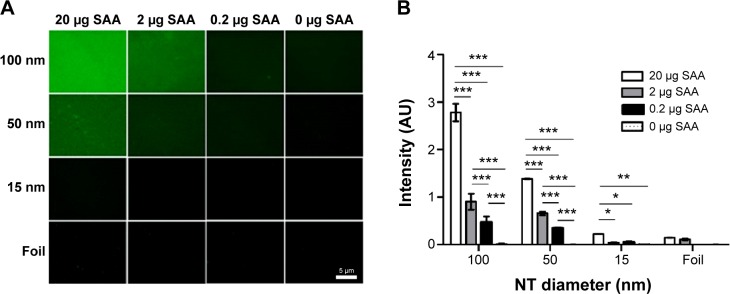
SAA dose dependently binds to NTs with different diameters.
Notes: (A) Immunofluorescence of bound SAA. (B) Average of intensity in arbitrary units was measured and compared with different NT diameters. Two way analysis of variance and Bonferroni posttests were used to determine significance: ***P<0.001, **P<0.01, *P<0.05. Four independent experiments for each concentration and NT diameter were performed.
Abbreviations: NTs, nanotubes; SAA, serum amyloid A.
Theoretical properties of NTs and proteins/peptides
For the quantification of protein binding on the NTs, one factor often considered is the area of top surface of NTs as well as the total available surface area of the NTs, with consideration of the length of NTs.
The surface areas of the tops of NTs were calculated by using the formula:
| (1) |
The values of surface areas of the tops of NTs are given in Table 3. Our calculations showed that 15 nm NTs have more top surface available than 100 nm NTs. So, logically, 15 nm NTs should show more protein binding surface than 100 nm NTs. However, length of the NTs is another important factor which needs to be taken into consideration when calculating the total surface area available for protein binding (Table 3). To confirm the effect of the length of NTs, we calculated the total surface area of the NTs by summation of the outer surface areas of the NTs (cylindrical), inner surface area of NTs (“V-shape”, ie, cone), and the top edges of the NTs. The total surface area is then given by the following formula:
| (2) |
where, R denotes the radius of the outer cylinder, r the radius of the inner cone, and h the height of the nanotube. By counting the number of NTs in the original small area, we calculated the total surface area of NTs in an area of 1 cm2. For the three regimes of different diameters of NTs, the values of total surface areas are given in Table 3.
Table 3.
Calculated NT surface and edges area, and distances between NTs with different diameters
| 100 nm NTs | 50 nm NTs | 15 nm NTs | |
|---|---|---|---|
| Calculated edges area (cm2) | 0.31 | 0.35 | 0.39 |
| Calculated total surface area (cm2) | 142.83 | 48.58 | 20.01 |
| Measured distance between the NTs (nm) | 30.8 | 21.4 | 6.3 |
Abbreviation: NT, nanotube.
Theoretical calculation confirms that the 100 nm diameter NTs have the largest theoretically calculated available surface area for potential protein binding, due to the longer lengths of the NTs.
Spacing between the NTs is a further consideration for protein binding, since smaller-sized proteins can enter inside the space between the NTs. For determination of the spacing between NTs, an average value was obtained from statistical measurements performed on SEM images. The average distance/spacing between NTs is taken into account in the modeling. Our calculated theoretical data show that with increasing diameter of the NTs, the spacing between them is also increased, and for the 100 nm diameter NTs, the observed spacing is approximately five-times greater than the spacing observed for 15 nm diameter NTs (Table 3).
The plasma proteins used in the current report have different biochemical characteristics important for their binding to NTs’ surfaces, such as IEP, charge at pH 7.4, and wettability properties (Table 4). The plasma proteins β2GPI, SAA, and IgG were less basic than histone IIA, where the highest change of binding was also observed experimentally. Although the IEP and charge of the proteins were relatively different, the trend of their binding to NTs of different diameters was similar (Table 2; Figure 7).
Table 4.
Protein IEPs, charge, wettability, and other attributes as determined by IEP calculator, Peptide Property Calculator GenScript2014, and literature
| Proteins and peptides | IEP
|
Charge and attribute64
|
Hydrophilic/hydrophobic properties [%]64
|
||||
|---|---|---|---|---|---|---|---|
| IEP calculators64,65 | IEP from literature | Charge at pH 7.4 | Attribute | Hydrophilic | Hydrophobic | Others | |
| β2GPI | 7.8/8.0 | 5–766 | 12 | Basic | 23 | 40 | 37 |
| SAA | 6.1/6.3 | 7.9–9.367 | 1 | Basic | 28 | 42 | 31 |
| IgG | 8.5/8.8 | 6.4–9.068 | 7 | Basic | 15 | 38 | 47 |
| Histone IIA | 11.5/11.7 | 11.1369 | 22 | Basic | 32 | 41 | 26 |
| Peptide 1 of β2GPI | 9.6/9.7 | / | 2 | Basic | 18 | 18 | 64 |
| Peptide 2 of β2GPI | 7.4/7.6 | / | 1 | Basic | 27 | 18 | 55 |
Note: / indicates that there is no existing data in current literature.
Abbreviations: β2GPI, β2-glycoprotein I; IEP, isoelectric point; SAA, serum amyloid A.
Discussion
It is evident that the response of the surrounding tissue to biomaterials fully depends on its biocompatibility to the material.70 Surface properties, surface charge distribution, and submicron structure are some of the key factors in the biological acceptance of the implants.9,71–74 To produce better biocompatible materials, the surfaces of these materials must be nanostructured by increasing their roughness in the nanometer scale.9,75 TiO2 NTs meet these criteria on the technical side of nanorough materials. It was our aim to examine which diameter of NTs is more appropriate for protein binding and more suitable (long-term) as nanostructured implant material. Implanted material in contact with blood becomes adhered to plasma proteins13,76 once embedded in the body.13 Plasma is rich in proteins, carbohydrates, and lipids, as well as lipoprotein and other combinations of complexes; however, when studying clinically relevant proteins, our aim was to first determine their interactions with NTs’ surfaces. Lipids can also directly adsorb/bind to the outer surface of TiO2 NTs. This process can be chemisorption with energies stronger than charge interactions. Neutral membrane lipids with zwitterionic headgroups may be electrostatically attracted to negatively charged titanium surfaces as recently shown experimentally and theoretically.77 However, in cellular systems, the sugar residues attached to proteins and lipids on the outer cell membrane surface prevent the direct membrane lipid bilayer–titanium interactions, and also abolish short-range attractive van der Waals force and the short range attractive or oscillatory hydration forces between the cell membrane, outer lipid bilayer surfaces, and titanium surface.78
As TiO2 surfaces and the cell membranes are both negatively charged,9 there is a need for a mediator that shields the repulsive force between the negatively charged biosurfaces. These can be proteins or divalent ions, which can bridge the repulsive electrostatic interactions between equally charged surfaces. Gongadze et al showed that the attraction between the negatively charged TiO2 surface and negatively charged cells is mediated by charged proteins with a distinctive quadrupolar internal charge distribution.9
One of the bridging proteins, which directly mediates attractive interactions between charged–charged, charged– neutral, and neutral–neutral phospholipid membranes, is β2GPI.79,80 It was found that β2GPI binds to structures with negatively charged phospholipid molecules, thrombocytes,81 erythrocytes,80 and serum lipoproteins;82,83 moreover, it binds to phospholipid layers with electrostatic and also hydrophobic interactions.79,80,84 β2GPI has a positive ζ-potential of 17.80 mV. Several domains of β2GPI are highly positively charged,85,86 which probably contributes to binding to negatively charged TiO2 surfaces. However, we need to consider that other energies, such as chemisorption and hydrophobic interactions, could also be responsible for the interactions.
The binding of both peptides derived from β2GPI is different from the binding of the total protein β2GPI. There is a binding difference between peptide 1 and peptide 2, indicating that peptides were not bound with a common N-terminal GGGS NH2 tail that they share. Peptide 1 showed low binding to all diameters of NTs, where the average mass of bound protein complies with the masses of other proteins bound on 50 nm NTs. However, peptide 2 highly bound to 100 nm NTs (in fact, it showed the highest binding of all proteins) and also highly bound on all other diameters. Peptide 2 has a higher percent of hydrophilic residues, as compared to peptide 1, presumably making greater binding possible.
IgG is the most abundant antibody isotype composed of two heavy and two light polypeptide chains forming a dimer with particular internal charge distribution.79 Even though IgG possess a unique quaternary structure,87 the binding ratio on NTs remained comparable with other studied proteins. With denaturated IgG, however, the three dimensional conformation was destroyed and, consequentially, the binding was reduced, with the trend of binding with regard to the NTs diameters remaining unchanged. This indicates that the three-dimensional conformation is not the main factor required for binding.
Histone IIA has a ζ-potential of 7.40 mV and is positively charged in physiological conditions,28 which is responsible for the tight binding to negatively charged DNA. Gongadze et al proposed that nanorough regions cause an increase of electric field strength and an aggregation of charge which enhances the binding of molecules to the titanium implant surface.9
SAA is important in inflammation, infections, and tissue injury during the acute phase response where it can be quickly increased up to 1,000-fold33 and be readily available in the circulation. It is the smallest protein examined in our study with an Mr ~12 kDa, as estimated by SDS-PAGE. It is a globular protein, consisting of 104–112 amino acids,32 and containing positively charged proteoglycan-binding domains.88
TiO2 NTs present a relatively acidic character for the whole range of considered pH values. The NTs have an IEP at a pH of 4.9. The ζ-potential curve reached negative values lower than −25 mV at pH values above 6.5. As expected, we observed a decrease in negative ζ-potential values (ie, increase in the absolute value of ζ-potential) with an increase in dilution of PBS solution (ie, with a decrease in salt concentrations) in experiments and theory.
The proteins tested in the present manuscript showed comparable adsorption to NTs (Figure 7). This could be the consequence of many factors, among which are: protein size, charge, conformation, electrostatic interactions, chemisorption, hydrophobic interactions, etc.
It should be stressed that in biological systems, the media in contact with the surface of the titanium implant does not contain only proteins. Inflamed medium is also rich in chelating carboxylates (lactate, citrate) that may change the charge density of the TiO2 surface and also slowly digest TiO2, partially changing its morphology and the surface’s chemical and physical (binding) properties.
It is clear that theoretical physical and electrochemical properties of the proteins/peptides, such as surface area, IEPs, molecule size, ζ-potential, charge distribution, and NT wettability, are important and could influence the binding of plasma proteins to TiO2 NTs of different diameters. One of the important issues can be the contact area between the buffer/water on TiO2 NTs, which changes in regard to TiO2 NT diameter,89 as well as wettability of the NTs. More hydrophilic NTs enable a better connection with buffer/water, which is reflected also in the pocket effect and better protein binding on the 100 nm diameter NTs. However, the current study indicates lower protein binding on smaller-diameter NTs and NTs with shorter lengths, independent of protein size, charge, or conformation, for reasons we have yet to determine.
The pocket effect (defined as the consequence of protein binding to inner nanotube surfaces in depth, both between NTs as well as within each nanotube, following the initial washing steps) could represent an explanation of why proteins bind to a higher degree to 100 nm NTs.
From the examined literature, it is known that the diameter of the NTs can be a decisive factor for cellular binding, independent of the specific cell type.20,90,91 Regardless of the protein’s IEP, conformation, charge, or percentage of either hydrophilic or hydrophobic amino residues, the general trend of protein binding remains unchanged (Table 4). The mechanism of protein binding has yet to be elucidated.
Smith et al reported that higher protein binding on NTs was observed due to larger surface area of the NTs, which possess multiple functional sites for the proteins to adsorb.13 The protein binding may be changed/optimized by changing the size parameters of TiO2 NT arrays.13 Another research group obtained similar results with binding of collagen type 1 on surfaces of NTs, where faster and higher binding was observed on NTs with larger diameters (100 nm). The main driving force for collagen type 1 binding was physical adsorption, including van der Waals force and hydrogen bonds between the proline residues of collagen type 1 and NTs.21 The authors also calculated the interaction energies, which indicated that TiO2 NTs with larger diameter possess higher interaction energies leading to higher protein adsorption.21,92 On the other hand, we need to take into account that 100 nm NTs also possess space between the tubes, which is much smaller than the original diameter. This difference in inter-space size decreases with smaller diameters of the NTs. NTs thus possess two different microenvironments for binding, which is an important factor in calculating the total available surface area for binding of proteins, and should be considered due to the possibility that proteins can also go inside the NTs. In the current study, 100 nm NTs showed comparatively more SAA binding than 15 nm NTs since the total available surface area of 100 nm NTs is almost seven-times larger than 15 nm NTs. Spacing between the NTs is particularly important for the smaller-sized proteins, which can enter inside the space between the NTs more readily. Our theoretically calculated data indicated that with increasing diameter of the NTs, the spacing between the NTs also increases. For example, the spacing between 100 nm NTs is almost five-times larger than the spacing between 15 nm NTs. This could prove to be of importance because TiO2 NTs possess great potential for drug-delivery applications. Antibacterial agent release studies showed that with controlling the dimensions of NTs it is possible to prolong the release of antibacterial agent.93 NTs loaded with antibiotics have a great potential to become orthopedic implants, with decreased implanted infection rates along with increased osteoblast function.94 Different diameters of NTs, as well as their different lengths, can have a consequence on hemocompatibility responses13 and on how physiological plasma proteins bind at the blood–nanomaterial interface.
Acknowledgments
The authors would like to acknowledge Slovenian Research Agency (ARRS) grants J1-4109, J1-4136, J3-4108, P3-0314, and P2-0232 for financial support. The authors also acknowledge the use of equipment in the Center of Excellence on Nanoscience and Nanotechnology – Nanocenter at Jožef Stefan Institute, Ljubljana SI-1000, Slovenia and Mitja Drab for his help in calculating the surface areas of nanotubes. The contribution of A. Artenjak preceded his employment at the current institution/company.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wang RR, Fenton A. Titanium for prosthodontic applications: a review of the literature. Quintessence Int. 1996;27(6):401–408. [PubMed] [Google Scholar]
- 2.Jokstad A, Braegger U, Brunski JB, Carr AB, Naert I, Wennerberg A. Quality of dental implants. Int J Prosthodont. 2004;17(6):607–641. doi: 10.1111/j.1875-595x.2003.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 3.Heo SJ, Sennerby L, Odersjö M, Granström G, Tjellström A, Meredith N. Stability measurements of craniofacial implants by means of resonance frequency analysis. A clinical pilot study. J Laryngol Otol. 1998;112(6):537–542. doi: 10.1017/s0022215100141039. [DOI] [PubMed] [Google Scholar]
- 4.Eufinger H, Wehmöller M. Individual prefabricated titanium implants in reconstructive craniofacial surgery: clinical and technical aspects of the first 22 cases. Plast Reconstr Surg. 1998;102(2):300–308. doi: 10.1097/00006534-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Peng L, Eltgroth ML, LaTempa TJ, Grimes CA, Desai TA. The effect of TiO2 nanotubes on endothelial function and smooth muscle proliferation. Biomaterials. 2009;30(7):1268–1272. doi: 10.1016/j.biomaterials.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Fine E, Zhang LJ, Fenniri H, Webster TJ. Enhanced endothelial cell functions on rosette nanotube-coated titanium vascular stents. Int J Nanomedicine. 2009;4:91–97. doi: 10.2147/ijn.s5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mihov D, Katerska B. Some biocompatible materials used in medical practice. Trakia Journal of Sciences. 2009;8(2):119–125. [Google Scholar]
- 8.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Gongadze E, Kabaso D, Bauer S, et al. Adhesion of osteoblasts to a nanorough titanium implant surface. Int J Nanomedicine. 2011;6:1801–1816. doi: 10.2147/IJN.S21755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee K, Mazare A, Schmuki P. One-dimensional titanium dioxide nanomaterials: nanotubes. Chem Rev. 2014;114(19):9385–9454. doi: 10.1021/cr500061m. [DOI] [PubMed] [Google Scholar]
- 11.Diebold U. The surface science of titanium dioxide. Surf Sci Rep. 2003;48(5–8):53–229. [Google Scholar]
- 12.Puckett SD, Taylor E, Raimondo T, Webster TJ. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials. 2010;31(4):706–713. doi: 10.1016/j.biomaterials.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 13.Smith BS, Yoriya S, Grissom L, Grimes CA, Popat KC. Hemocompatibility of titania nanotube arrays. J Biomed Mater Res A. 2010;95(2):350–360. doi: 10.1002/jbm.a.32853. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni M, Mazare A, Gongadze E, et al. Titanium nanostructures for biomedical applications. Nanotechnology. 2015;26:062002. doi: 10.1088/0957-4484/26/6/062002. [DOI] [PubMed] [Google Scholar]
- 15.Casarin M, Maccato C, Vittadini A. Molecular chemisorption on TiO2(110): A local point of view. J Phys Chem B. 1998;102(52):10745–10752. [Google Scholar]
- 16.Linsebigler A, Lu G, Yates JT., Jr CO chemisorption on Tio2(110) – oxygen vacancy site influence on CO adsorption. J Chem Phys. 1995;103(21):9438–9443. [Google Scholar]
- 17.Sano KI, Shiba K. A hexapeptide motif that electrostatically binds to the surface of titanium. J Am Chem Soc. 2003;125(47):14234–14235. doi: 10.1021/ja038414q. [DOI] [PubMed] [Google Scholar]
- 18.Bauer S, Park J, Pittrof A, Song YY, von der Mark K, Schmuki P. Covalent functionalization of TiO2 nanotube arrays with EGF and BMP-2 for modified behavior towards mesenchymal stem cells. Integr Biol (Camb) 2011;3(9):927–936. doi: 10.1039/c0ib00155d. [DOI] [PubMed] [Google Scholar]
- 19.Deng ZJ, Mortimer G, Schiller T, Musumeci A, Martin D, Minchin RF. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology. 2009;20(45):455101. doi: 10.1088/0957-4484/20/45/455101. [DOI] [PubMed] [Google Scholar]
- 20.Park J, Bauer S, Schlegel KA, Neukam FW, von der Mark K, Schmuki P. TiO2 nanotube surfaces: 15 nm – an optimal length scale of surface topography for cell adhesion and differentiation. Small. 2009;5(6):666–671. doi: 10.1002/smll.200801476. [DOI] [PubMed] [Google Scholar]
- 21.Yang W, Xi X, Ran Q, Liu P, Hu Y, Cai K. Influence of the titania nanotubes dimensions on adsorption of collagen: an experimental and computational study. Mater Sci Eng C Mater Biol Appl. 2014;34:410–416. doi: 10.1016/j.msec.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 22.Balasundaram G, Yao C, Webster TJ. TiO2 nanotubes functionalized with regions of bone morphogenetic protein-2 increases osteoblast adhesion. J Biomed Mater Res A. 2008;84(2):447–453. doi: 10.1002/jbm.a.31388. [DOI] [PubMed] [Google Scholar]
- 23.Cao X, Yu WQ, Qiu J, Zhao YF, Zhang YL, Zhang FQ. RGD peptide immobilized on TiO2 nanotubes for increased bone marrow stromal cells adhesion and osteogenic gene expression. J Mater Sci Mater Med. 2012;23(2):527–536. doi: 10.1007/s10856-011-4479-0. [DOI] [PubMed] [Google Scholar]
- 24.Werner S, Huck O, Frisch B, et al. The effect of microstructured surfaces and laminin-derived peptide coatings on soft tissue interactions with titanium dental implants. Biomaterials. 2009;30(12):2291–2301. doi: 10.1016/j.biomaterials.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Reinhardt M, Giri S, Bader A. Preclinical Challenges and Clinical Target of Nanomaterials in Regenerative Medicine. In: Suar M, Salhotra A, Soni S, editors. Handbook of Research on Diverse Applications of Nanotechnology in Biomedicine, Chemistry, and Engineering. Hershey: IGI Global; 2014. pp. 350–371. [Google Scholar]
- 26.Kulkarni M, Mazare A, Schmuki P, Iglic A. Biomaterial surface modification of titanium and titanium alloys for medical applications. In: Seifalian A, editor. Nanomedicine. UK Central Press; 2014. pp. 11–136. [Google Scholar]
- 27.Smith BS, Popat KC. Titania nanotube arrays as interfaces for blood-contacting implantable devices: a study evaluating the nanotopography-associated activation and expression of blood plasma components. J Biomed Nanotechnol. 2012;8(4):642–658. doi: 10.1166/jbn.2012.1421. [DOI] [PubMed] [Google Scholar]
- 28.Katsani KR, Arredondo JJ, Kal AJ, Verrijzer CP. A homeotic mutation in the trithorax SET domain impedes histone binding. Genes Dev. 2001;15(17):2197–2202. doi: 10.1101/gad.201901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elgin SC, Weintraub H. Chromosomal proteins and chromatin structure. Annu Rev Biochem. 1975;44:724–774. doi: 10.1146/annurev.bi.44.070175.003453. [DOI] [PubMed] [Google Scholar]
- 30.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 31.Lakota K, Thallinger GG, Cucnik S, et al. Could antibodies against serum amyloid A function as physiological regulators in humans? Autoimmunity. 2011;44(2):149–158. doi: 10.3109/08916934.2010.487504. [DOI] [PubMed] [Google Scholar]
- 32.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265(2):501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 33.Upragarin N, Landman WJ, Gaastra W, Gruys E. Extrahepatic production of acute phase serum amyloid A. Histol Histopathol. 2005;20(4):1295–1307. doi: 10.14670/HH-20.1295. [DOI] [PubMed] [Google Scholar]
- 34.Stevens FJ. Hypothetical structure of human serum amyloid A protein. Amyloid. 2004;11(2):71–80. doi: 10.1080/13506120412331272296. [DOI] [PubMed] [Google Scholar]
- 35.Chiu WC, Chiou TJ, Chiang AN. β2-Glycoprotein I inhibits endothelial cell migration through the nuclear factor κB signalling pathway and endothelial nitric oxide synthase activation. Biochem J. 2012;445(1):125–133. doi: 10.1042/BJ20111383. [DOI] [PubMed] [Google Scholar]
- 36.Lozier J, Takahashi N, Putnam FW. Complete amino acid sequence of human plasma beta 2-glycoprotein I. Proc Natl Acad Sci U S A. 1984;81(12):3640–3644. doi: 10.1073/pnas.81.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin F, Murphy R, White B, et al. Circulating levels of beta2-glycoprotein I in thrombotic disorders and in inflammation. Lupus. 2006;15(2):87–93. doi: 10.1191/0961203306lu2270oa. [DOI] [PubMed] [Google Scholar]
- 38.Lozier J, Takahashi N, Putnam FW. Complete amino acid sequence of human plasma beta 2-glycoprotein I. Proc Natl Acad Sci U S A. 1984;81(12):3640–3644. doi: 10.1073/pnas.81.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwarzenbacher R, Zeth K, Diederichs K, et al. Crystal structure of human beta2-glycoprotein I: implications for phospholipid binding and the antiphospholipid syndrome. EMBO J. 1999;18(22):6228–6239. doi: 10.1093/emboj/18.22.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunt JE, Simpson RJ, Krilis SA. Identification of a region of beta 2-glycoprotein I critical for lipid binding and anti-cardiolipin antibody cofactor activity. Proc Natl Acad Sci U S A. 1993;90(6):2141–2145. doi: 10.1073/pnas.90.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Laat B, Derksen RH, van Lummel M, Pennings MT, de Groot PG. Pathogenic anti-beta2-glycoprotein I antibodies recognize domain I of beta2-glycoprotein I only after a conformational change. Blood. 2006;107(5):1916–1924. doi: 10.1182/blood-2005-05-1943. [DOI] [PubMed] [Google Scholar]
- 42.Zager U, Kveder T, Cučnik S, Božič B, Lunder M. Anti-β2-glycoprotein I paratopes and β2-glycoprotein I epitopes characterization using random peptide libraries. Autoimmunity. 2014;47(7):438–444. doi: 10.3109/08916934.2014.914176. [DOI] [PubMed] [Google Scholar]
- 43.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 44.Jefferis R. Autoantibodies. In: Male D, Brostoff J, Roth DB, Roitt IM, editors. Immunology. 8th ed. Elsevier; 2013. pp. 51–70. [Google Scholar]
- 45.Dimitrov JD, Planchais C, Kang J, et al. Heterogeneous antigen recognition behavior of induced polyspecific antibodies. Biochem Biophys Res Commun. 2010;398(2):266–271. doi: 10.1016/j.bbrc.2010.06.073. [DOI] [PubMed] [Google Scholar]
- 46.McIntyre JA, Faulk WP. Redox-reactive autoantibodies: biochemistry, characterization, and specificities. Clin Rev Allergy Immunol. 2009;37(1):49–54. doi: 10.1007/s12016-008-8093-y. [DOI] [PubMed] [Google Scholar]
- 47.Omersel J, Jurgec I, Cucnik S, et al. Autoimmune and proinflammatory activity of oxidized immunoglobulins. Autoimmun Rev. 2008;7(7):523–529. doi: 10.1016/j.autrev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Omersel J, Avberšek-Lužnik I, Grabnar PA, Kveder T, Rozman B, Božič B. Autoimmune reactivity of IgM acquired after oxidation. Redox Rep. 2011;16(6):248–256. doi: 10.1179/174329211X13190184351680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun S, Yu W, Zhang Y, Zhang F. Increased preosteoblast adhesion and osteogenic gene expression on TiO2 nanotubes modified with KRSR. J Mater Sci Mater Med. 2013;24(4):1079–1091. doi: 10.1007/s10856-013-4869-6. [DOI] [PubMed] [Google Scholar]
- 50.Ma MH, Kazemzadeh-Narbat M, Hui Y, et al. Local delivery of antimicrobial peptides using self-organized TiO2 nanotube arrays for peri-implant infections. J Biomed Mater Res A. 2012;100(2):278–285. doi: 10.1002/jbm.a.33251. [DOI] [PubMed] [Google Scholar]
- 51.Assefpour-Dezfuly M, Vlachos C, Andrews EH. Oxide morphology and adhesive bonding on titanium surfaces. J Mater Sci. 1984;19(11):3626–3639. [Google Scholar]
- 52.Kowalski D, Kim D, Schmuki P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today. 2013;8(3):235–264. [Google Scholar]
- 53.Macak JM, Tsuchiya H, Ghicov A, et al. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr Opin Solid State Mater Sci. 2007;11(1–2):3–18. [Google Scholar]
- 54.Macak JM, Taveira LV, Tsuchiya H, Sirotna K, Macak J, Schmuki P. Influence of different fluoride containing electrolytes on the formation of self-organized titania nanotubes by Ti anodization. Journal of Electroceramics. 2006;16(1):29–34. [Google Scholar]
- 55.Zhou XM, Nguyen NT, Ozkan S, Schmuki P. Anodic TiO2 nanotube layers: Why does self-organized growth occur-A mini review. Electrochem Commun. 2014;46:157–162. [Google Scholar]
- 56.Smith BS, Yoriya S, Johnson T, Popat KC. Dermal fibroblast and epidermal keratinocyte functionality on titania nanotube arrays. Acta Biomater. 2011;7(6):2686–2696. doi: 10.1016/j.actbio.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 57.Kim D, Fujimoto S, Schmuki P, Tsuchiya H. Nitrogen doped anodic TiO2 nanotubes grown from nitrogen-containing Ti alloys. Electrochem Commun. 2008;10(6):910–913. [Google Scholar]
- 58.Paulose M, Prakasam HE, Varghese OK, et al. TiO2 nanotube arrays of 1000 μm length by anodization of titanium foil: Phenol red diffusion. J Phys Chem C. 2007;111(41):14992–14997. [Google Scholar]
- 59.Gongadze E, Iglič A. Decrease of permittivity of an electrolyte solution near a charged surface due to saturation and excluded volume effects. Bioelectrochemistry. 2012;87:199–203. doi: 10.1016/j.bioelechem.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Velikonja A, Gongadze E, Kralj-Iglič V, Iglič A. Charge dependent capacitance of Stern layer and capacitance of electrode/electrolyte interface. International Journal of Electrochemical Science. 2014;9:5885–5894. [Google Scholar]
- 61.Artenjak A, Leonardi A, Križaj I, et al. Optimization of unnicked β2-glycoprotein I and high avidity anti-β2-glycoprotein I antibodies isolation. J Immunol Res. 2014;2014:195687. doi: 10.1155/2014/195687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Artenjak A, Kozelj M, Lakota K, Cucnik S, Bozic B, Sodin-Semrl S. High avidity andi-beta 2-glycoprotein I antibodies activate human coronary artery endothelial cells and trigger peripheral blood mononuclear cell migration. Eur J Inflamm. 2013;11(2):385–396. [Google Scholar]
- 63.Gongadze E, Velikonja A, Perutkova Š, et al. Ions and water molecules in an electrolyte solution in contact with charged and dipolar surfaces. Electrochim Acta. 2014;126:42–60. [Google Scholar]
- 64.Peptide Property Calculator [webpage on the Internet] Piscataway: Genscript, Inc; 2014. [Accessed November 12, 2014]. [cited September 10, 2014]. Avaiable from: https://www.genscript.com/ssl-bin/site2/peptide_calculation.cgi. [Google Scholar]
- 65.Calculation of protein isoelectric point [webpage on the Internet] Poland: Kozlowski LP; 2013. [Accessed October 2, 2014]. [cited October 2, 2014]. Available from: http://isoelectric.ovh.org/ [Google Scholar]
- 66.Buttari B, Profumo E, Mattei V, et al. Oxidized beta2-glycoprotein I induces human dendritic cell maturation and promotes a T helper type 1 response. Blood. 2005;106(12):3880–3887. doi: 10.1182/blood-2005-03-1201. [DOI] [PubMed] [Google Scholar]
- 67.Jacobsen S, Niewold TA, Halling-Thomsen M, et al. Serum amyloid A isoforms in serum and synovial fluid in horses with lipopolysaccharide-induced arthritis. Vet Immunol Immunopathol. 2006;110(3–4):325–330. doi: 10.1016/j.vetimm.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 68.Griffin:Antibody Basics [webpage on the Internet] OpenWetWare; 2014. [Accessed November 6, 2014]. [updated March 18, 2014; cited September 3, 2014]. Available from: http://openwetware.org/wiki/Griffin:Antibody_Basics. [Google Scholar]
- 69.Protein Page: H3 (human) [webpage on the Internet] Cell Signaling Technology, Inc; 2013. [Accessed November 12, 2014]. [cited September 4, 2014]. Available from: http://www.phosphosite.org/proteinAction.do?id=7541&showAllSites=true. [Google Scholar]
- 70.Nuss KM, von Rechenberg B. Biocompatibility issues with modern implants in bone – a review for clinical orthopedics. Open Orthop J. 2008;2:66–78. doi: 10.2174/1874325000802010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kasemo B. Biological surface science. Surf Sci. 2002;500(1–3):656–677. [Google Scholar]
- 72.Nebe JG, Luethen F, Lange R, Beck U. Interface interactions of osteoblasts with structured titanium and the correlation between physicochemical characteristics and cell biological parameters. Macromol Biosci. 2007;7(5):567–578. doi: 10.1002/mabi.200600293. [DOI] [PubMed] [Google Scholar]
- 73.Anselme K, Bigerelle M, Noel B, et al. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J Biomed Mater Res. 2000;49(2):155–166. doi: 10.1002/(sici)1097-4636(200002)49:2<155::aid-jbm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 74.Mrksich M, Whitesides GM. Using self-assembled monolayers to understand the interactions of man-made surfaces with proteins and cells. Annu Rev Biophys Biomol Struct. 1996;25:55–78. doi: 10.1146/annurev.bb.25.060196.000415. [DOI] [PubMed] [Google Scholar]
- 75.Vasilev K, Poh Z, Kant K, Chan J, Michelmore A, Losic D. Tailoring the surface functionalities of titania nanotube arrays. Biomaterials. 2010;31(3):532–540. doi: 10.1016/j.biomaterials.2009.09.074. [DOI] [PubMed] [Google Scholar]
- 76.Tang L, Eaton JW. Natural responses to unnatural materials: A molecular mechanism for foreign body reactions. Mol Med. 1999;5(6):351–358. [PMC free article] [PubMed] [Google Scholar]
- 77.Velikonja A, Santhosh PB, Gongadze E, et al. Interaction between dipolar lipid headgroups and charged nanoparticles mediated by water dipoles and ions. Int J Mol Sci. 2013;14(8):15312–15329. doi: 10.3390/ijms140815312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Israelachvili J, Wennerström H. Role of hydration and water structure in biological and colloidal interactions. Nature. 1996;379(6562):219–225. doi: 10.1038/379219a0. [DOI] [PubMed] [Google Scholar]
- 79.Urbanija J, Tomsic N, Lokar M, et al. Coalescence of phospholipid membranes as a possible origin of anticoagulant effect of serum proteins. Chem Phys Lipids. 2007;150(1):49–57. doi: 10.1016/j.chemphyslip.2007.06.216. [DOI] [PubMed] [Google Scholar]
- 80.Lokar M, Urbanija J, Frank M, et al. Agglutination of like-charged red blood cells induced by binding of beta2-glycoprotein I to outer cell surface. Bioelectrochemistry. 2008;73(2):110–116. doi: 10.1016/j.bioelechem.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 81.Schousboe I. Binding of beta-2-glycoprotein I to platelets: effect of adenylate cyclase activity. Thromb Res. 1980;19(1–2):225–237. doi: 10.1016/0049-3848(80)90421-1. [DOI] [PubMed] [Google Scholar]
- 82.Polz E, Kostner GM. Binding of beta 2-glycoprotein I to human serum lipoproteins: distribution among density fractions. FEBS Lett. 1979;102(1):183–186. doi: 10.1016/0014-5793(79)80955-2. [DOI] [PubMed] [Google Scholar]
- 83.Kobayashi K, Kishi M, Atsumi T, et al. Circulating oxidized LDL forms complexes with beta2-glycoprotein I: implication as an atherogenic autoantigen. J Lipid Res. 2003;44(4):716–726. doi: 10.1194/jlr.M200329-JLR200. [DOI] [PubMed] [Google Scholar]
- 84.Wang SX, Cai GP, Sui SF. The insertion of human apolipoprotein H into phospholipid membranes: a monolayer study. Biochem J. 1998;335(Pt 2):225–232. doi: 10.1042/bj3350225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kertesz Z, Yu BB, Steinkasserer A, Haupt H, Benham A, Sim RB. Characterization of binding of human beta 2-glycoprotein I to cardiolipin. Biochem J. 1995;310(Pt 1):315–321. doi: 10.1042/bj3100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bouma B, de Groot PG, van den Elsen JM, et al. Adhesion mechanism of human beta(2)-glycoprotein I to phospholipids based on its crystal structure. EMBO J. 1999;18(19):5166–5174. doi: 10.1093/emboj/18.19.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kilár F, Simon I, Lakatos S, Vonderviszt F, Medgyesi GA, Závodszky P. Conformation of human IgG subclasses in solution. Small-angle X-ray scattering and hydrodynamic studies. Eur J Biochem. 1985;147(1):17–25. doi: 10.1111/j.1432-1033.1985.tb08712.x. [DOI] [PubMed] [Google Scholar]
- 88.Chiba T, Chang MY, Wang S, et al. Serum amyloid A facilitates the binding of high-density lipoprotein from mice injected with lipopolysaccharide to vascular proteoglycans. Arterioscler Thromb Vasc Biol. 2011;31(6):1326–1332. doi: 10.1161/ATVBAHA.111.226159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kulkarni M, Patil-Sen Y, Junkar I, Kulkarni CV, Iglic A. Wettability Studies of Topologically Distinct Titanium Surfaces. 2014. In press. [DOI] [PubMed] [Google Scholar]
- 90.Park J, Bauer S, Schmuki P, Schlegel K, Neukam F, von der Mark K. Nanotube diameter directs stem cell fate. J Stem Cells Regen Med. 2007;2(1):168. [PubMed] [Google Scholar]
- 91.Park J, Bauer S, Schmuki P, von der Mark K. Narrow window in nanoscale dependent activation of endothelial cell growth and differentiation on TiO2 nanotube surfaces. Nano Lett. 2009;9(9):3157–3164. doi: 10.1021/nl9013502. [DOI] [PubMed] [Google Scholar]
- 92.Yang XM, Li M, Lin X, et al. Enhanced in vitro biocompatibility/bioactivity of biodegradable Mg-Zn-Zr alloy by micro-arc oxidation coating contained Mg2SiO4. Surf Coat Tech. 2013;233:65–73. [Google Scholar]
- 93.Caliskan N, Bayram C, Erdal E, Karahaliloğlu Z, Denkbaş EB. Titania nanotubes with adjustable dimensions for drug reservoir sites and enhanced cell adhesion. Mater Sci Eng C Mater Biol Appl. 2014;35:100–105. doi: 10.1016/j.msec.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 94.Zhang HZ, Sun Y, Tian A, et al. Improved antibacterial activity and biocompatibility on vancomycin-loaded TiO2 nanotubes: in vivo and in vitro studies. Int J Nanomedicine. 2013;8:4379–4389. doi: 10.2147/IJN.S53221. [DOI] [PMC free article] [PubMed] [Google Scholar]